
Endometriosis - Pathogenesis and Sequelae
*Corresponding Author(s):
Razan AsallyDiscipline Of Obstetrics, Gynaecology And Neonatology, The University Of Sydney, New South Wales, Medical Foundation Building, Australia
Tel:+61 0290363129,
Fax:+61 0290363188
Email:razan2022@hotmail.com
Abstract
Keywords
ENDOMETRIOSIS: DEFINITION AND BACKGROUND
Endometriosis is considered as a highly variable condition in terms of symptoms, anatomical extent of the disease, rate of progression, incidence of infertility, response to treatment and likelihood of recurrence [3]. Endometriotic lesions can be spread through many areas, and are usually multiple. They are usually found in pelvic peritoneal and visceral surfaces, ovaries and rectovaginal septum [4]. They also can be rarely found in the pancreas, pleura, lung, umbilicus, on sciatic nerve roots, fallopian tubes, thorax and the kidneys, the vertebrae, the extremities or even the brain [5,6].
There are several factors that have been suggested that play a role in the establishment and development of endometriosis. These factors could be related to the genetic profile, inflammation, immune dysfunction, oxidative stress, hormonal activity, menstrual cyclicity, burden and immunological factors [7,8]. There are three symptomatic groups that are usually compared; these are asymptomatic patients, symptomatic (with pain) and infertile patients [9]. As a result the accurate, true prevalence and incidence of endometriosis in the general female population remains unclear, as some women with the disease can be completely asymptomatic, allied with the difficulty of a confirming diagnosis of the disease, which can only be reliably done by laparoscopy together with histological confirmation [3].
THEORIES OF ENDOMETRIOSIS
Retrograde menstruation/implantation theory
Coelomic metaplasia theory
Induction theory
SYMPTOMS OF ENDOMETRIOSIS
PELVIC PAIN
INFERTILITY
|
Ovarian-Tubal Dysfunction |
|
Anatomical distortion of ovary and tube |
|
Ovulation failure |
|
Hyperprolactinemia |
|
LUF (Luteinized Un-ruptured Follicle) |
|
Abnormal follicle development |
|
Reduced follicle development |
|
Decreased estrogen production |
|
Increased apoptosis of granulosa cells |
|
Immunological disorder |
|
Anti-endometrial antibody |
|
Abnormal peritoneal environment |
|
Increased peritoneal fluids and high concentration of cytokines |
|
Activated macrophage |
|
Dysregulated endometrial function |
APPEARANCE OF ENDOMETRIOSIS
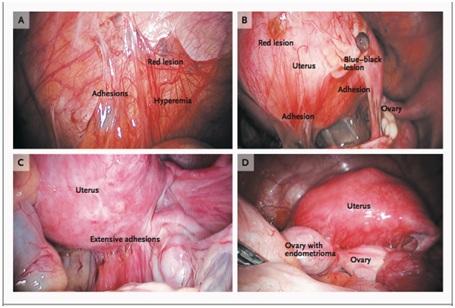 Figure 1: Peritoneal lesions and an ovarian endometrioma due to endometriosis.
Figure 1: Peritoneal lesions and an ovarian endometrioma due to endometriosis.Panel A shows an endometriotic implant (red lesion), adhesions, and hyperemia in the peritoneum. Panel B shows peritoneal implants, including red and blue-black lesions and adhesions. Panel C shows extensive adhesions distorting the normal pelvic anatomy. Panel D shows an endometrioma adherent to the posterior uterus and distending the ovarian capsule.
Superficial endometriosis
Deep infiltrating endometriosis
Ovarian endometriomas
Diagnosis of endometriosis
GOLD STANDARD: INVASIVE DIAGNOSTIC TECHNIQUE
Non-Invasive diagnostic imaging
The need for biomarkers for the non-invasive diagnosis of endometriosis
MANAGEMENT OF ENDOMETRIOSIS
Surgical management of endometriosis
Laparotomy (open surgery) was considered the standard method for surgical therapy of endometriosis prior to the improvements in laparoscopic techniques [30]. In severe endometriosis, it was found that laparotomy and laparoscopy perform equally in treating pain and infertility problems, yet there was a trend towards a higher pregnancy rate and lower dyspareunia recurrence rate after laparotomy compared with laparoscopy in the 1990s [64]. This appeared to have been improved with developments in laparoscopic equipment, techniques and skill.
Laparoscopy is considered as the gold standard for treating mild and moderate stages of endometriosis [65]. Laparoscopy offers several advantages when compared with laparotomy procedures including decreased recovery time and cost [66]. Surgical procedures involve excision, fulguration, or laser ablation of endometriotic implants on the peritoneum, excision, drainage or ablation of endometriomas, resection of rectovaginal nodules, lysis of adhesions and interruption of nerve pathways [1]. Resection is preferred and is usually more effective in healing both pain and infertility. There are risks will all surgery, with laparoscopy, problems are rare, however can be severe.
Radical surgery involves total abdominal hysterectomy with or without bilateral oophorectomy [67]. This option may suit women with chronic pelvic pain with endometriosis who have completed their families and have undergone other medical and or conservative surgical treatments without complete symptom relief. However, this approach may not always alleviate pain symptoms especially in deep endometriotic disease [67].
MEDICAL MANAGEMENT OF ENDOMETRIOSIS
Oral contraceptives
Progestogens
GnRH agonists
Danazol
Aromatase inhibitors
Analgesics
Antioxidants
ENDOMETRIOSIS AND PAIN MECHANISMS
Prostaglandins (PGs) have an important role in the pathogenesis of the symptom of pain in endometriosis due to an excess in the amount of PGs secreted from the endometrium during menstruation. Among PGs, PGFα is considered the most potent causal factor of pain [92]. Chronically elevated catecholamine levels are associated with pain and inflammatory disease, both often are associated with endometriosis [93-96]. Medina and Lebovic reported that Substance P (SP) and Calcitonin Gene-Related Peptide (CGRP) were both involved in the inflammatory and pain responses and both were present in the myometrial layer, this was reported as being indicative of the occurrence of sensory C and Aδ fibres [97]. Bulletti observed a greater contractility at the time of menstruation in endometriosis subjects in comparison with control subjects, this was postulated as the possible roles of SP and CGRP nerve fibres in relation to the generation of pain in endometriosis and dysmenorrhea [98].
NEUROGENESIS AND PAIN PERCEPTION
|
Type of Fibers |
Function |
Axonal Diameter (μm) |
Conduction Velocity (m/s) |
Direction of Conduction |
|
|
A (Myelinated) |
α |
Proprioception, muscle contraction |
12-22 |
70-120 |
Afferent and efferent |
|
β |
Touch-Pressure, vibration |
6-12 |
80 |
Afferent and efferent |
|
|
γ |
Intrafusal fibers contraction |
4-8 |
15-30 |
Efferent |
|
|
δ |
Pain (acute, shallow), temperature |
1-5 |
4-30 |
Afferent |
|
|
B (Myelinated) |
Preganglionic fibers of ANS |
1-3 |
3-15 |
Efferent |
|
|
C (Unmyelinated) |
Fibers of dorsal roots |
Pain (chronic, deep), temperature (warmth receptors), touch-pressure (tactile or mechanoreceptors) |
0.2-1.5 |
0.5-2 |
Afferent |
|
Postganglionic fibers of sympathetic nerves |
0.2-1.5 |
0.5-2 |
Efferent |
Neurotrophins and their receptors role in neurogenesis and pain
The biological effects of neurotrophins are facilitated through two major receptors including neurotrophic tyrosine kinase (Trks) receptors and the pan neurotrophin receptor at 75 kDa (p75NTR) [105]. Each neurotrophins binds with a specific Trk receptor in particular, NGF binds with TrkA, BDNF and NT-4 bind with TrkB, and NT-3 to TrkC [105]. NT-3 also binds with TrkA and TrkB however, with lower affinities and all four mature NTs bind with similar affinity to the p75NTR [106,107] (Figure 2).
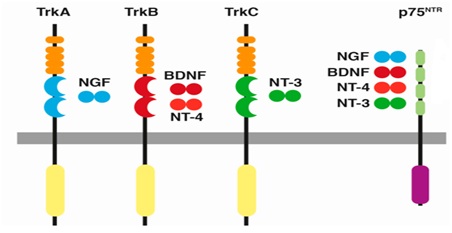 Figure 2: Neurotrophins and their receptors. Each neurotrophin binds with a specific Trk receptor. NGF binds with TrkA, BDNF and NT-4 bind with TrkB, and NT-3 to TrkC. Trk: Tropomyosin Related Kinase; NGF: Neuronal Growth Factor; BDNF: Brain-Derived Neurotrophic Factor; NT: Neurotrophin. Source: Arevalo et al., [107] and Sanchez-Sanchez et al., [108].
Figure 2: Neurotrophins and their receptors. Each neurotrophin binds with a specific Trk receptor. NGF binds with TrkA, BDNF and NT-4 bind with TrkB, and NT-3 to TrkC. Trk: Tropomyosin Related Kinase; NGF: Neuronal Growth Factor; BDNF: Brain-Derived Neurotrophic Factor; NT: Neurotrophin. Source: Arevalo et al., [107] and Sanchez-Sanchez et al., [108].In addition, Glial cell line-Derived Neurotrophic Factor (GDNF) family, which includes GDNF, Neurturin, Artemin and Persephin and other neurotrophic factors that are crucial for the development, survival of the nervous system [109,110]. The activation of all GDNF family members are facilitated by binding with multicomponent GDNF Family ligand Receptor (GFR) α [110]. GDNF binds to GFRα1 and also interacts with GFRα2 and GFRα3 at lower affinities, Neurturin to GFRα2, Artemin to GFRα3 and Persephin to GFRα4 [110].
There is strong evidence that neurotrophins, specially NGF and BDNF, act as mediators and modulators of pain in different conditions [111]. Nociceptors express NGF receptors activating NGF that leads to the sensitisation of these nociceptors. In addition, BDNF is released from the spinal terminals of activated nociceptors. BDNF modulate and alter the effectiveness of the central nociceptive signals. NT3 and NT-4/5 has been shown to play reasonably modest roles in pain processing in most conditions [111]. Moreover, almost one-half of the nociceptor population has receptor components for GDNF, suggesting an important role for GDNF in the activation and maintenance of nociceptors [112,113].
Neuronal guidance molecules and their receptors role in neurogenesis and pain
These neuronal guidance molecules are activated through binding to their specific receptors to trigger the intracellular signalling cascades, which cause axon routing through stimulation of changes in the growth-cone cytoskeleton [114,115]. Semaphorin family, which have attractant and repellent effects, bind to receptors from the plexin and neuropilin protein families [117]. Netrin molecules are activated through binding to DCC receptors and UNC-5 receptors to produce attractive and repulsive effects respectively [118]. Slit molecules have repellent effects which prevent axons re-crossing and through binding to the roundabout (Robo) family receptors [119]. RGMs have also repellent affects that signal through binding to Neogenin receptor [120]. While tethered to the membrane Ephrin A molecules interact with class A Eph receptors, the transmembrane Ephrin B interact with class B Eph receptors and both play a well-known role in organising axon connections between the eye and the brain [121] (Figure 3).
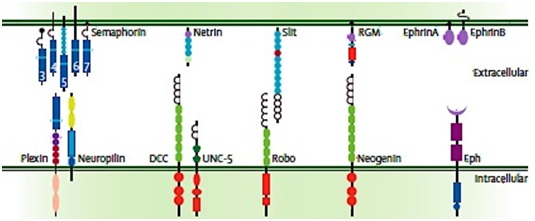 Figure 3: Five families of identified neuronal guidance molecules (top) and their binding receptors (bottom). Semaphorins binds to receptors from the Plexin and neuropilin protein families; Netrin molecules binds to DCC receptors and UNC-5 receptors; Slit molecules binds to the roundabout (Robo) family receptors, Repulsive Guidance Molecules (RGMs) binds to their receptors Neogenin; Ephrin A molecules interact with class A Eph receptors and Ephrin B interacts with class B Eph receptors. Source: Van Battum et al., [114].
Figure 3: Five families of identified neuronal guidance molecules (top) and their binding receptors (bottom). Semaphorins binds to receptors from the Plexin and neuropilin protein families; Netrin molecules binds to DCC receptors and UNC-5 receptors; Slit molecules binds to the roundabout (Robo) family receptors, Repulsive Guidance Molecules (RGMs) binds to their receptors Neogenin; Ephrin A molecules interact with class A Eph receptors and Ephrin B interacts with class B Eph receptors. Source: Van Battum et al., [114].NEUROGENESIS AND ENDOMETRIOSIS
In addition, some studies have also investigated the effect of hormonal treatment on nerve fibre density. Tokushige reported a decrease in the density of nerve fibres stained with PGP 9.5 in peritoneal endometriotic lesions of hormone-treated women in comparison to endometriotic lesion of untreated women [126]. However, Wang et al., showed no differences in the density of nerve fibres stained with NF or PGP 9.5 through different stages of the menstrual cycle [127].
The results of previous studies have supported using endometrial nerve fibres as potential biomarkers for endometriosis [61]. Al-Jefout et al., was the first study to investigate this hypothesis. In this study nerve fibers were stained with PGP 9.5 and were reportedly detected with a specificity and sensitivity of 100% in the endometrial biopsies [128]. A further double-blinded study assessed efficacy of the detection of nerve fibres stained with PGP 9.5 in endometrial biopsies compared to laparoscopically verified endometriosis. This result demonstrated a specificity and sensitivity of 83% and 98%, respectively, with a positive predictive value of 91% and negative predictive value of 96% [133]. In addition, women with endometriosis and pain symptoms showed a significantly higher nerve fibre density compared to women with endometriosis and without [133]. However, the following studies have contradicted these encouraging results and it remains unclear why recent studies have disproved previous studies. Possible explanations could be the heterogeneity of the endometriosis, variability in tissue collection and processing protocols [134-138]. To find definite answers to the usefulness of the presence of nerve fibres in eutopic endometrium as a biomarker for endometriosis, the standardisation of these variable factors in large Randomized Controlled Trials (RCTs) with specific emphasis on minimal and mild endometriosis is needed [43,139].
NEUROTROPHINS AND ENDOMETRIOSIS
Barcena de Arellano also showed elevated levels of NGF, NT-3, but none in BDNF in the PF of women with peritoneal endometriosis compared with adenomyosis, adhesions or asymptomatic controls [144]. However, the concentrations of these neurotrophins did not show a correlation with the pain symptoms in any of the groups. Kajitani et al., demonstrated contrasting results, with a significant correlation between high NGF levels and the severity of dysmenorrhoea and moderate or severe dyspareunia in women with peritoneal or ovarian endometriosis [145]. Moreover, women with endometriosis showed elevated BDNF in their plasma [146]. These results are suggestive that neurotrophins may stimulate a differential nerve fibre growth pattern in women with endometriosis and potentially contributing to pain mechanisms associated with endometriosis.
NEURONAL GUIDANCE MOLECULES AND ENDOMETRIOSIS
CONCLUSION
DECLARATION OF CONFLICTING INTERESTS
REFERENCES
- Giudice LC (2010) Endometriosis. N Engl J Med 362: 2389-2398.
- Eskenazi B, Warner ML (1997) Epidemiology of endometriosis. Obstet and Gynecol Clin North Am 24: 235-258.
- Fraser IS (2008) Recognising, understanding and managing endometriosis. J Hum Reprod Sci 1: 56-64.
- Olive DL, Pritts EA (2001) Drug therapy: Treatment of endometriosis. New England Journal of Medicine 345: 266-275.
- Jubanyik KJ, Comite F (1997) Extrapelvic endometriosis. Obstet Gynecol Clin North Am 24: 411-440.
- Saad A (2008) Endometriosis. Obstet Gynaecol amp; Reprod Med 18: 126-133.
- Mu F, Rich-Edwards J, Rimm EB, Spiegelman D, Missmer SA (2016) Endometriosis and risk of coronary heart disease. Circ Cardiovasc Qual Outcomes 9: 257-264.
- Sourial S, Tempest N, Hapangama DK (2014) Theories on the pathogenesis of endometriosis. International Journal of Reproductive Medicine 179515.
- McLeod BS, Retzloff MG (2010) Epidemiology of endometriosis: An assessment of risk factors. Clin Obstet Gynecol 53: 389-396.
- Sampson JA (1927) Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol 14: 422-469.
- Sasson IE, Taylor HS (2008) Stem Cells and the Pathogenesis of Endometriosis. Ann N Y Acad Sci 1127: 106-115.
- Ebert AD, Fuhr N, David M, Schneppel L, Papadopoulos T (2009) Histological con?rmation of endometriosis in a 9-year-old girl suffering from unexplained cyclic pelvic pain since her eighth year of life. Gynecol Obstet Invest 67: 158-161.
- Marsh EE, Laufer MR (2005) Endometriosis in premenarcheal girls who do not have an associated obstructive anomaly. Fertil Steril 83: 758-760.
- Díez García R, Prieto Díez M, Aguilar Tremoya F (1996) Neonatal ovarian endometriosis. Its conservative treatment. An Esp Pediatr 44: 397-398.
- Balci O, Karatayli R, Capar M (2008) An incidental coexistence of Mayer-Rokitansky-Kuster-Hauser syndrome with pelvic ectopic kidney and perirenal endometrioma. Saudi Med J 29: 1340-1341.
- Bulun SE (2009) Endometriosis. N Engl J Med 360: 268-279.
- Metzger DA, Haney AF (1989) Etiology of endometriosis. Obstet Gynecol Clin North Am 16: 1-14.
- Clark AH (1948) Endometriosis in a young girl. J Am Med Assoc 136: 690.
- Schifrin BS, Erez S, Moore JG (1973) Teen-age endometriosis. Am J Obstet Gynecol 116: 973-980.
- EL-Mahgoub S, Yaseen S (1980) A positive proof for the theory of coelomic metaplasia. Am J Obstet Gynecol 137: 137-140.
- Hobbs JE, Bortnick AR (1940) Endometriosis of the lungs: An experimental and clinical study. Am J Obstet Gynecol 40: 832-843.
- Cassina PC, Hauser M, Kacl G, Imthurn B, Schröder S, et al. (1997) Catamenial hemoptysis - Diagnosis with MRI. Chest 111: 1447-1450.
- Seli E, Berkkanoglu M, Arici A (2003) Pathogenesis of endometriosis. Obstet Gynecol Clin North Am 30: 41-61.
- Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, et al. (2007) Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod 22: 266-271.
- Fraser IS (2010) Mysteries of endometriosis pain: Chien-Tien Hsu Memorial Lecture 2009. J Obstet Gynaecol Res 36: 1-10.
- Sinaii N, Plumb K, Cotton L, Lambert A, Kennedy S, et al. (2008) Differences in characteristics among 1,000 women with endometriosis based on extent of disease. Fertil Steril 89: 538-545.
- Markham R (2002) Endometriosis symptoms in australian women (phd thesis). The university of Sydney, Austraila.
- Evans S, Moalem-Taylor G, Tracey DJ (2007) Pain and endometriosis. Pain 132: 22-25.
- D'Hooghe TM, Debrock S, Hill JA, Meuleman C (2003) Endometriosis and subfertility: is the relationship resolved? Semin Reprod Med 21: 243-254.
- Candiani GB, Fedele L, Vercellini P (1989) Laparotomy versus laparoscopy in the surgical treatment of endometriosis: the end of an era? Acta Eur Fertil 20: 163-166.
- Khine YM, Taniguchi F, Harada T (2016) Clinical management of endometriosis-associated infertility. Reprod Med Biol 15: 217-225.
- Amer S (2008) Endometriosis. Obstet Gynecol Reprod Med 18: 126-133.
- Nisenblat V, Farquhar C, Akoum A, Fraser I, Bossuyt PMM (2012) Non?invasive tests for the diagnosis of endometriosis. Cochrane Database of Systematic Reviews, John Wiley & Sons, New Jersey, United States.
- Gupta S, Harlev A, Agarwal A, Ellis-Kahana J, Cirenza C (2015) Diagnosis of endometriosis. Endometriosis: A Comprehensive update. Cham: Springer International Publishing, Switzerland.
- Manderson L, Warren N, Markovic M (2008) Circuit breaking: pathways of treatment seeking for women with endometriosis in australia. Qual Health Res18: 522-534.
- Somigliana E, Vercellini P, Vigano' P, Benaglia L, Crosignani PG, et al. (2010) Non-invasive diagnosis of endometriosis: the goal or own goal? Hum Reprod 25: 1863-1868.
- Mehedintu C, Plotogea M N, Ionescu S, Antonovici M (2014) Endometriosis still a challenge. J Med Life 7: 349-357.
- Van den Bosch T, Van Schoubroeck D (2018) Ultrasound diagnosis of endometriosis and adenomyosis: State of the art. Best Pract Res Clin Obstet Gynaecol 1521.
- Brosens I, Puttemans P, Campo R, Gordts S, Kinkel K (2004) Diagnosis of endometriosis: pelvic endoscopy and imaging techniques. Best Pract Res Clin Obstet Gynaecol 18: 285-303.
- Seckin B, Oruc AS, Turkcapar F, Ugur M (2013) The relation of pelvic pain and dense adhesions to Doppler ultrasound findings in patients with ovarian endometriomas. Arch Gynecol Obstet 287: 723-728.
- Ahn SH, Singh V, Tayade C (2017) Biomarkers in endometriosis: challenges and opportunities. Fertil Steril 107: 523-532.
- Fassbender A, Burney RO, O DF, D'Hooghe T, Giudice L (2015) Update on Biomarkers for the Detection of Endometriosis. Biomed Res Int 2015: 130854.
- Fassbender A, Vodolazkaia A, Saunders P, Lebovic D, Waelkens E, et al. (2013b) Biomarkers of endometriosis. Fertil Steril 100: 20-20.
- Fassbender A, Vodolazkaia A, Saunders P, Lebovic D, Waelkens E, et al. (2013a) Biomarkers of endometriosis. Fertil Steril 99: 1135-1145.
- Gupta D, Hull ML, Fraser I, Miller L, Bossuyt PM, et al. (2016) Endometrial biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev 4: 012165.
- Nisenblat V, Bossuyt PM, Shaikh R, Farquhar C, Jordan V, et al. (2016) Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev 1: 012179.
- Nisenblat V, Prentice L, Bossuyt PM, Farquhar C, Hull ML, et al. (2016) Combination of the non-invasive tests for the diagnosis of endometriosis. Cochrane Database Syst Rev 7: 012281.
- Reis FMD, Monteiro CDS, Carneiro MM (2017) Biomarkers of Pelvic Endometriosis. Rev Bras Ginecol Obstet 39: 091-093.
- Worku DA (2017) The Role of Biomarkers in the Early Diagnosis of Endometriosis. Invest Gynecol Res Women’s Health 1: 1-4.
- Johnson NP, Hummelshoj L (2013) Consensus on current management of endometriosis. Hum Reprod 28: 3163-3164.
- Johnson NP, Hummelshoj L; World Endometriosis Society Montpellier Consortium (2013) Consensus on current management of endometriosis. Hum Reprod 28: 1552-1568.
- Nisenblat V, Bossuyt PM, Farquhar C, Johnson N, Hull ML (2016) Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev 2: 009591.
- Johnson NP, Hummelshoj L, Nisenblat V, Bush D, Kiesel L, et al. (2014) 12th world congress on endometriosis. World endometriosis society, Sao paulo, Brazil.
- Joshi SG, Zamah NM, Raikar RS, Buttram VC Jr, Henriques ES, et al.(1986) Serum and peritoneal fluid proteins in women with and without endometriosis. Fertil Steril 46: 1077-1082.
- Kingsmore SF (2006) Multiplexed protein measurement: technologies and applications of protein and antibody arrays. Nat Rev Drug Discov 5: 310-320.
- El-Kasti MM, Wright C, Fye HK, Roseman F, Kessler BM, et al. (2011) Urinary peptide profiling identifies a panel of putative biomarkers for diagnosing and staging endometriosis. Fertility and sterility 95: 1-6.
- Tokushige N, Markham R, Crossett B, Ahn SB, Nelaturi VL, et al (2011) Discovery of a novel biomarker in the urine in women with endometriosis. Fertil Steril 95: 46-49.
- Cho S, Choi YS, Yim SY, Yang HI, Jeon YE, et al. (2012) Urinary vitamin D-binding protein is elevated in patients with endometriosis. Hum Reprod 27: 515-22.
- Yun BH, Lee YS, Chon SJ, Jung YS, Yim SY, et al. (2014) Evaluation of elevated urinary enolase I levels in patients with endometriosis. Biomarkers 19: 16-21.
- Rogers PA, D'Hooghe TM, Fazleabas A, Giudice LC, Montgomery GW (2013) Defining future directions for endometriosis research: workshop report from the 2011 World Congress of Endometriosis In Montpellier, France. Reprod Sci 20: 483-499.
- May KE, Conduit-Hulbert SA, Villar J, Kirtley S, Kennedy SH, et al. (2010) Peripheral biomarkers of endometriosis: A systematic review. Hum Reprod Update 16: 651-674.
- Hirsch M, Duffy JMN, Davis CJ, Plana MN, Khan KS, et al. (2016) Diagnostic accuracy of cancer antigen 125 for endometriosis: A systematic review and meta-analysis. BJOG 123: 1761-1768.
- Platteeuw L1 D'Hooghe T (2014) Novel agents for the medical treatment of endometriosis. Curr Opin Obstet Gynecol 26: 243-252.
- Crosignani PG, Vercellini P, Biffignandi F, Costantini W, Cortesi I , et al. (1996) Laparoscopy versus laparotomy in conservative surgical treatment for severe endometriosis. Fertil Steril 66: 706-711.
- Afors K, Murtada R, Centini G, Fernandes R, Meza C, et al. (2014) Employing laparoscopic surgery for endometriosis. Women's Health 10: 431-443.
- Duffy JM, Arambage K, Correa FJ, Olive D, Farquhar C, et al. (2014) Laparoscopic surgery for endometriosis. Cochrane Database of Syst Rev 011031.
- Al-Jefout M (2011) Brief update on endometriosis treatment. Middle East Fertility Society Journal 16: 167-174.
- Dunselman GA, Vermeulen N, Becker C, Calhaz-Jorge C, D'Hooghe T, et al. (2014) ESHRE guideline: management of women with endometriosis. Hum Reprod 29: 400-412.
- Muzii L, Panici PB (2010) Combined technique of excision and ablation for the surgical treatment of ovarian endometriomas: the way forward? Reprod Biomed Online 20: 300-302.
- Somigliana E , Vigano P , Barbara G , Vercellini P (2009) Treatment of endometriosis-related pain: options and outcomes. Frontiers in Bioscience (Elite Edition) 1: 455-465.
- Vercellini P, Somigliana E, Viganò P, Abbiati A, Daguati R, et al. (2008) Endometriosis: current and future medical therapies. Best Pract Res Clin Obstet Gynaecol 22: 275-306.
- Sulak PJ, Cressman BE, Waldrop E, Holleman S, Kuehl TJ (1997) Extending the duration of active oral contraceptive pills to manage hormone withdrawal symptoms. Obstet Gynecol 89: 179-183.
- Coffee AL, Sulak PJ, Kuehl TJ (2007) Long-term assessment of symptomatology and satisfaction of an extended oral contraceptive regimen. Contraception 75: 444-449.
- Practice Committee of The American Society for Reproductive (2006) Treatment of pelvic pain associated with endometriosis. Fertil Steril 86: 18-27.
- Olive DL (2003) Medical therapy of endometriosis. Semin Reprod Med 21: 209-222.
- Gupta S, Harlev A, Agarwal A, Rakhit M, Ellis-Kahana, et al. (2015b) Management of endometriosis. Endometriosis: A comprehensive update. Cham: Springer International Publishing, Switzerland.
- Hornstein MD, Gibbons WE (2018) Endometriosis: Long-term treatment with gonadotropin-releasing hormone agonists. Wolters Kluwer, Alphen aan den Rijn, The Netherlands.
- Selak V, Farquhar C, Prentice A, Singla A (2007) Danazol for pelvic pain associated with endometriosis. Cochrane Database Syst Rev 17: 000068.
- Velasco I, Rueda J, Acien P (2006) Aromatase expression in endometriotic tissues and cell cultures of patients with endometriosis. MHR: Basic science of reproductive medicine 12: 377-381.
- Pavone ME, Bulun SE(2012) Aromatase Inhibitors For The Treatment Of Endometriosis: A Review. Fertil Steril 98: 1370-1379.
- Ailawadi RK, Jobanputra S, Kataria M, Gurates B, Bulun SE (2004) Treatment of endometriosis and chronic pelvic pain with letrozole and norethindrone acetate: a pilot study. Fertil Steril 81: 290-296.
- Remorgida V, Abbamonte HL, Ragni N, Fulcheri E, Ferrero S (2007 Letrozole and norethisterone acetate in rectovaginal endometriosis. Fertil Steril 88: 724-726.
- Hamann GO (1980) Severe, primary dysmenorrhea treated with naproxen. A prospective, double-blind, crossover investigation. Prostaglandins 19: 651-657.
- Hanson FW, Izu A, Henzl MR (1978) Naproxen sodium in dysmenorrhea. Its influence in allowing continuation of work/school activities. Obstet Gynecol 52: 583-587.
- Zito G, Luppi S, Giolo E, Martinelli M, Venturin, et al. (2014) Medical treatments for endometriosis-associated pelvic pain. Biomed Res Int 2014: 191967.
- Brown J, Crawford TJ, Allen C, Hopewell S, Prentice A (2009) Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst Rev 1: 00487.
- Sinha A, Gupta S (2017) The Role of Antioxidant Supplementation in Endometriosis Therapy. J Gynecol Women’s Health 3: 1-3.
- Santanam N, Kavtaradze N, Murphy A, Dominguez C, Parthasarathy S (2013) Antioxidant supplementation reduces endometriosis-related pelvic pain in humans. Transl Res 161: 189 - 195.
- Howard FM (2009) Endometriosis and mechanisms of pelvic pain. J Minim Invasive Gynecol 16: 540-550.
- Vercellini P, Fedele L, Molteni P, Arcaini L, Bianchi S, et al. (1990) Laparoscopy in the diagnosis of gynecologic chronic pelvic pain. Int J Gynaecol Obstet 32: 261-265.
- Whiteside JL, Falcone T (2003) Endometriosis-related pelvic pain: what is the evidence? Clin Obstet Gynecol 46: 824-830.
- Harada T (2013) Dysmenorrhea and endometriosis in young women. Yonago Acta Med 56: 81-84.
- Chrousos GP (2009) Stress and disorders of the stress system. Nat Rev Endocrinol 5: 374-381.
- Huether G, Doering S, Rüger U, Rüther E, Schüssler G (1996) [Psychological stress and neuronal plasticity. An expanded model of the stress reaction process as the basis for understand central nervous system adaptation processes]. Z Psychosom Med Psychoanal 42: 107-127.
- Lundberg U (2002) Psychophysiology of work: stress, gender, endocrine response, and work-related upper extremity disorders. Am J Ind Med 41: 383-392.
- Paiva S1, Carneiro MM (2013) Complementary and Alternative Medicine in the Treatment of Chronic Pelvic Pain in Women: What Is the Evidence?. ISRN Pain 2013: 469575.
- Medina MG, Lebovic DI (2009) Endometriosis-associated nerve fibers and pain. Acta Obstet Gynecol Scand 88: 968-975.
- Bulletti C, De Ziegler D, Polli V, Del Ferro E, Palini S, et al. (2002) Characteristics of uterine contractility during menses in women with mild to moderate endometriosis. Fertil Steril 77: 1156-1161.
- Yan D, Liu X, Guo SW (2017) Nerve fibers and endometriotic lesions: partners in crime in inflicting pains in women with endometriosis. Eur J Obstet Gynecol Reprod Biol 209: 14-24.
- Laverdet B, Danigo A, Girard D, Magy L, Demiot C, et al. (2015) Skin innervation: important roles during normal and pathological cutaneous repair. Histol Histopathol 30: 875-892.
- Purves D (2012) Neuroscience, Sunderland (Massachusetts), Sinauer Associates.
- Ceni C, Unsain N, Zeinieh MP, Barker PA (2014) Neurotrophins in the regulation of cellular survival and death. Handb Exp Pharmacol 220: 193-221.
- Skaper SD (2012) The neurotrophin family of neurotrophic factors: an overview. Methods Mol Biol 846: 1-12.
- Davies AM (2008) Neurotrophins giveth and they taketh away. Nature Neuroscience 11: 627-628.
- Patapoutian A, Reichardt LF (2001) Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol 11: 272-280.
- Davies AM, Minichiello L, Klein R (1995) Developmental changes in NT3 signalling via TrkA and TrkB in embryonic neurons. EMBO J 14: 4482-4489.
- Arévalo JC, Wu SH (2006) Neurotrophin signaling: many exciting surprises! Cell Mol Life Sci 63: 1523-1537.
- Sánchez-Sánchez J, Arévalo JC (2017) A Review on Ubiquitination of Neurotrophin Receptors: Facts and Perspectives. Int J Mol Sci 18: 630.
- Saarma M, Sariola H (1999) Other neurotrophic factors: glial cell line-derived neurotrophic factor (GDNF). Microsc Res Tech 45: 292-302.
- Airaksinen MS, Saarma M (2002) The GDNF family: Signalling, biological functions and therapeutic value. Nat Rev Neurosci 3: 383-394.
- Pezet S, McMahon SB (2006) Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci 29: 507-538.
- Snider W D, Mcmahon S B (1998) Tackling pain at the source: new ideas about nociceptors. Neuron 20: 629-632.
- Salio C, Ferrini F (2016) BDNF and GDNF expression in discrete populations of nociceptors. Ann Anat 207: 55-61.
- Van Battum EY, Brignani S, Pasterkamp RJ (2015) Axon guidance proteins in neurological disorders. Lancet Neurol 14: 532-546.
- Kolodkin AL, Tessier-Lavigne M (2011) Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harb Perspect Biol 3: 001727.
- Pasterkamp RJ, Kolodkin AL (2013) SnapShot: Axon Guidance. Cell 153: 494e1-2.
- Pasterkamp RJ (2012) Getting neural circuits into shape with semaphorins. Nat Rev Neurosci 13: 605-618.
- Lai Wing Sun K, Correia JP, Kennedy TE (2011) Netrins: versatile extracellular cues with diverse functions. Development 138: 2153-2169.
- Ypsilanti AR, Zagar Y, Chédotal A (2010) Moving away from the midline: new developments for Slit and Robo. Development 137: 1939-1952.
- Severyn CJ, Shinde U, Rotwein P (2009) Molecular biology, genetics and biochemistry of the repulsive guidance molecule family. Biochem J 422: 393-403.
- Klein R (2012) Eph/ephrin signalling during development. Development 139: 4105-4109.
- Tokushige N, Markham R, Russell P, Fraser IS (2006) Nerve fibres in peritoneal endometriosis. Hum Reprod 21: 3001-3007.
- Mechsner S, Schwarz J, Thode J, Loddenkemper C, Salomon DS, et al. (2007) Growth-associated protein 43-positive sensory nerve fibers accompanied by immature vessels are located in or near peritoneal endometriotic lesions. Fertil steril, 88: 581-7.
- Mechsner S, Kaiser A, Kopf A, Gericke C, Ebert A, et al. (2009) A pilot study to evaluate the clinical relevance of endometriosis-associated nerve fibers in peritoneal endometriotic lesions. Fertil Steril, 92: 1856-1861.
- Tulandi T, Felemban A, Chen MF (2001) Nerve fibers and histopathology of endometriosis-harboring peritoneum. J Am Assoc Gynecol Laparosc 8: 95-98.
- Tokushige N, Markham R, Russell P, Fraser IS (2009). Effect of progestogens and combined oral contraceptives on nerve fibers in peritoneal endometriosis. Fertil Steril 92: 1234-1239.
- Wang G, Tokushige N, Fraser IS (2011) Nerve fibers and menstrual cycle in peritoneal endometriosis. Fertil Steril 95: 2772-2774.
- Al-Jefout M, Andreadis N, Tokushige N, Markham R, Fraser I (2007) A pilot study to evaluate the relative efficacy of endometrial biopsy and full curettage in making a diagnosis of endometriosis by the detection of endometrial nerve fibers. Am J Obstet Gynecol 197: 1-4.
- Atwal G, du Plessis D, Armstrong G, Slade R, Quinn M (2005) Uterine innervation after hysterectomy for chronic pelvic pain with, and without, endometriosis. Am J Obstet Gynecol 193: 1650-1655.
- Tokushige N, Markham R, Russell P, Fraser IS (2006) High density of small nerve fibres in the functional layer of the endometrium in women with endometriosis. Hum Reprod 21: 782-787.
- Tokushige N, Markham R, Russell P, Fraser IS (2007) Different types of small nerve fibers in eutopic endometrium and myometrium in women with endometriosis. Fertil Steril 88: 795-803.
- Tokushige N, Markham R, Russell P, Fraser IS (2008) Effects of hormonal treatment on nerve fibers in endometrium and myometrium in women with endometriosis. Fertil Steril 90: 1589-1598.
- Al-Jefout M, Dezarnaulds G, Cooper M, Tokushige N, Luscombe GM, et al. (2009) Diagnosis of endometriosis by detection of nerve fibres in an endometrial biopsy: a double blind study. Hum Reprod 24: 3019-3024.
- Zhang X, Lu B, Huang X, Xu H, Zhou C, et al. (2009) Endometrial nerve fibers in women with endometriosis, adenomyosis, and uterine fibroids. Fertil Steril 92: 1799-1801.
- Zhang X, Yao H, Huang X, Lu B, Xu H, et al. (2010) Nerve fibres in ovarian endometriotic lesions in women with ovarian endometriosis. Hum Reprod 25: 392-397.
- Leslie C, Ma T, McElhinney B, Leake R, Stewart CJ (2013) Is the detection of endometrial nerve fibers useful in the diagnosis of endometriosis? Int J Gynecol Pathol 32: 149-55.
- Newman TA, Bailey JL, Stocker LJ, Woo YL, Macklon NS, et al. (2013) Expression of neuronal markers in the endometrium of women with and those without endometriosis. Hum Reprod 28: 2502-2510.
- Morotti M, Vincent K, Brawn J, Zondervan KT, Becker CM (2014) Peripheral changes in endometriosis-associated pain. Hum Reprod Update 20: 717-736.
- D'Hooghe TM, Mihalyi AM, Simsa P, Kyama CK, Peeraer K, et al. (2006) Why we need a noninvasive diagnostic test for minimal to mild endometriosis with a high sensitivity. Gynecol Obstet Invest 62: 136-138.
- Anaf V, Simon P, El Nakadi I, Fayt I, Simonart T, et al. (2002) Hyperalgesia, nerve infiltration and nerve growth factor expression in deep adenomyotic nodules, peritoneal and ovarian endometriosis. Hum Reprod 17: 1895-1900.
- Tokushige N, Russell P, Black K, Barrera H, Dubinovsky S, et al. (2010) Nerve fibers in ovarian endometriomas. Fertil Steril 94: 1944-1947.
- Barcena de Arellano ML, Arnold J, Sacher F, Blöchle M, Staube M, et al. (2012) Eutopic endometrium from women with endometriosis does not exhibit neurotrophic properties. J Neuroimmunol 249: 49-55.
- Browne AS, Yu J, Huang RP, Francisco AM, Sidell N, et al. (2012) Proteomic identification of neurotrophins in the eutopic endometrium of women with endometriosis. Fertil Steril 98: 713-719.
- Barcena de Arellano ML, Arnold J, Lang H, Vercellino GF, Chiantera V, et al. (2013) Evidence of neurotrophic events due to peritoneal endometriotic lesions. Cytokine 62: 253-261.
- Kajitani T, Maruyama T, Asada H, Uchida H, Oda H, et al. (2013) Possible involvement of nerve growth factor in dysmenorrhea and dyspareunia associated with endometriosis. Endocr J 60: 1155-64.
- Wessels JM, Kay VR, Leyland NA, Agarwal SK, Foster WG (2016) Assessing brain-derived neurotrophic factor as a novel clinical marker of endometriosis. Fertil Steril 105: 119-128.
- Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, et al. (2003) Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology 144: 2870-2881.
- Liang Y, Wang W, Huang J, Tan H, Liu T, et al. (2015) Potential role of semaphorin 3a and its receptors in regulating aberrant sympathetic innervation in peritoneal and deep infiltrating endometriosis. Plos one 10: 0146027.
- Scheerer C, Frangini S, Chiantera V, Mechsner S (2017) Reduced Sympathetic Innervation in Endometriosis is Associated to Semaphorin 3C and 3F Expression. Mol Neurobiol 54: 5131-5141.
- Shen F, Liu X, Geng JG, Guo SW (2009) Increased immunoreactivity to SLIT/ROBO1 in ovarian endometriomas: a likely constituent biomarker for recurrence. Am J Pathol 175: 479-488.
Citation: Asally R, Markham R, Manconi F (2018) Endometriosis - Pathogenesis and Sequelae. J Reprod Med Gynecol Obstet 3: 010.
Copyright: © 2018 Razan Asally, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

