
Knowledge of Midwives toward Misopristol in the Prevention of Postpartum Hemorrhages at Community Level in Khartoum State in Sudan
*Corresponding Author(s):
Elkheir HAOmdorman Maternity Hospital, Central Laboratories, Khartoum, Sudan
Tel:+249 913364999,
Email:akramalk@yahoo.com
Abstract
Keywords
INTRODUCTION
Variety of case reports and Randomized Controlled Trials (RCTs) found that 600 microgram orally is optimum for prevention and treatment of PPH. It was found to be associated with fewer side effects compared to other routes. RCTs of Misopristol usage found a 38% reduction in maternal deaths due to PPH in resource-poor communities [5]. In Sudan, home- delivery is still practiced, for, hospital- delivery hindrances such as; the wrong public-views toward hospital- delivery, poverty, illiteracy and ignorance, and difficult access to hospitals. The preventable and even treatable PPH is still one of the major leading causes of maternal morbidity and mortality. So far, it is such a big challenge for us to distribute Misopristol at community level, as well as, minimizing the deviated usage of it for illegal- pregnancy abortions.
Furthermore, both prevention and treatment depend- basically- on inject able uterotonics, which are seldom available for births outside the health system. For these reasons, the use of Misopristol to prevent or treat postpartum hemorrhage has attracted considerable attention. "Misopristol" is an inexpensive and stable prostaglandin E1 analogue that has been shown to stimulate uterine contractility in early pregnancy and at term. Administered orally or vaginally, it is effective for inducing abortion and labor, though it poses certain risks [6]. Administration of this drug on a wide scale at community level to prevent and treat PPH is of major public health significance. Misopristol has been widely recommended to prevent PPH when other methods are not available; however, large-scale implementation projects are currently in progress. Caution has been urged, because side-effects may occasionally be life-threatening [7]. A principal reduction in PPH and other complications were obtained with Misopristol, 600 µg orally used alone (i.e., without other components of the active management of the third stage of labor - umbilical cord clamping and controlled cord traction) [8] WHO has developed guidelines supporting the use of a uterotonic when the full package of active management of the third stage of labor is not practiced, which can be either oxytocin, 10 IU administered parenterally, or Misopristol, 600 µg administered orally [9].
The drug can be safely used at community level through either administration by health providers or distribution by Community Health Workers (CHWs) (including Traditional Birth Attendants [TBAs]) directly to pregnant women for self-administration at home. Sutherland et al., noted that such an intervention is particularly cost effective [10]. Rajbhandari et al., concluded that the largest gains in protection against PPH were realized by the poor, the illiterate and those who live in remote areas [11]. Misopristol has been widely recommended to prevent PPH when other methods are not available [12], however, large-scale implementation projects are currently in progress. Caution has been urged, because side-effects may occasionally be life-threatening [10].
METHODOLOGY
Study design
Study area
Study population
Inclusion criteria
Exclusion criteria
Study period
Sample size
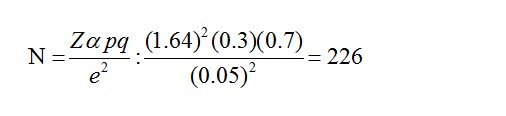
Sampling
Participants were randomly selected from the list of the midwives from each cluster. Total number of community midwives in Khartoum State was 1700: Khartoum locality: 868, Omdurman locality: 632, Bahri locality: 200. Accordingly selected numbers in the three localities was 115, 84 and 27 respectively.
Data collection
Data analysis
• Verbal consent was obtained from participants after declaring the purpose of the study
• Privacy of participants’ data was considered
RESULTS
|
Demographics |
Frequency |
Percent |
|
Area |
||
|
Khartoum |
115 |
50.9 |
|
Omdurman |
84 |
37.2 |
|
Bahri |
27 |
11.9 |
|
Total |
226 |
100 |
|
Age group |
||
|
20-30 |
12 |
5.3 |
|
31-40 |
44 |
19.5 |
|
41-50 |
96 |
42.5 |
|
51-60 |
74 |
32.7 |
|
Total |
226 |
100 |
|
Level of education |
||
|
Illiterate |
46 |
20.4 |
|
Primary school |
105 |
46.5 |
|
Intermediate |
30 |
13.3 |
|
Secondary school |
45 |
19.9 |
|
Total |
226 |
100 |
|
Years of experience |
||
|
1-10 Years |
60 |
26.5 |
|
11-20 Years |
92 |
40.7 |
|
21-30 Years |
40 |
17.7 |
|
31-40 Years |
34 |
15 |
|
Total |
226 |
100 |
Midwives who found to receive training course were 69(30.5%), out of which 47(68.1%) had one course only, 16 (23.2%) had two courses, 5(7.2%) had 3 courses and one (1.4%) had 4 courses (Table 2). Among midwives who received training courses, 44(63.8%) had 2-5 years since the last training course, 20(29%) had 6-9 years since the last training and 5(7.2%) more than 10 years since their last training course.
|
Training after graduate |
Frequency |
Percent |
|
|
Training |
Yes |
69 |
30.5 |
|
|
No |
157 |
69.5 |
|
|
Total |
226 |
100 |
|
|
|
Frequency |
Percent |
|
If yes |
1 Course |
47 |
68.1% |
|
|
2 courses |
16 |
23.2% |
|
|
3 Courses |
5 |
7.2% |
|
|
4 courses |
1 |
1.4% |
|
|
Total |
69 |
100% |
|
Duration since last training |
|
Frequency |
Percent |
|
Year |
2.00 - 5.00 |
44 |
63.8% |
|
|
6.00 - 9.00 |
20 |
29% |
|
|
10.00+ |
5 |
7.2% |
|
|
Total |
69 |
100% |
In regard to other uterotonic drugs used were syntocinon and ergometrine which was used by 186(82.3%), syntocinon only used by 24(10.6%) and ergometrine used by 16(7.1%) (Figure 1).
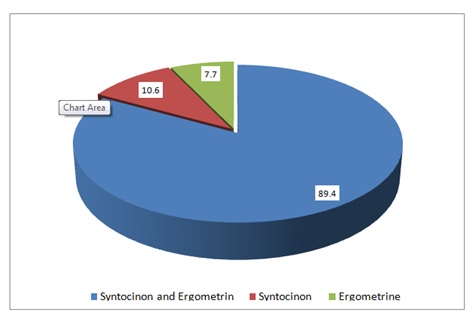
Figure 1: Midwives’ knowledge towards other uterotonic drugs used in PPH.
Midwives who reported that, uterotonic drugs is always effective in PPH were 95(42%), those who said it is effective in most of the times were 87(38.5%), while 44(19.5%) denied their effectiveness (Figure 2).
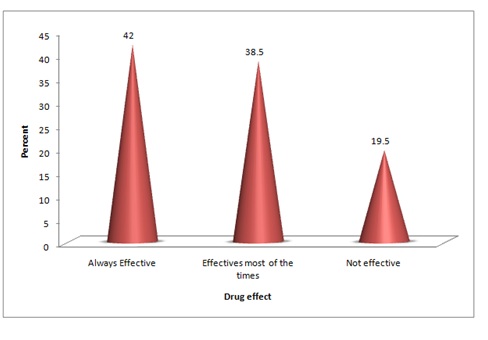
Figure 2: Midwives’ knowledge towards uterotonicdrugs effectiveness.
When midwives inquired about misoprostol, 181(80.1%) answered positively, 27(11.9%) said to some extent and 18(8%) didn’t know it (Figure 3).

Figure 3: Midwives’ knowledge towards misoprostol.
Out of 208 midwives who knew misoprostol, 107(51.4%) answered that it is used for PPH, 153(73.6%) said it is used for induction of labour and 125(60.1%) said it is used for termination of pregnancy (Table 3).
|
PPH |
Induction of labour |
Termination of pregnancy |
||||
|
|
Frequency |
Percent |
Frequency |
Percent |
Frequency |
Percent |
|
Yes |
107 |
51.4 |
153 |
73.6 |
125 |
60.1 |
|
No |
101 |
48.6 |
55 |
26.4 |
83 |
39.9% |
|
Total |
208 |
100 |
208 |
100 |
208 |
100 |
Inquiring midwives about misoprostol formula showed that, 169(74.8%) said it is presented as tablets, 19(8.4%) said it is presented as pessaries, 16(7.1%) said it is presented as suppositories, one participant said it is presented as tablets and suppositories and one other midwife said it is a pessaries and suppositories (Table 4).
|
Misoprostol formula |
Frequency |
Percent |
|
Tab |
169 |
74.8 |
|
Injection |
2 |
0.9 |
|
Pessaries |
19 |
8.4 |
|
Suppositories |
16 |
7.1 |
|
Tab and suppositions |
1 |
0.4 |
|
Pessaries and Suppositions |
1 |
0.4 |
|
Total |
208 |
100 |
Midwives who said that, misoprostol are administered orally were 46(22.1%), who said it is administered sublingually were 138(66.3%), who said it is administered vaginally were 115(55.3%) and who said it is taken by rectal were 110(52.9%) (Table 5).
|
|
Oral |
Sublingual |
Vaginal |
Rectal |
others |
||||
|
|
Frequency |
Percent |
Frequency |
Percent |
Frequency |
Percent |
Frequency |
Percent |
|
|
Yes |
46 |
22.1 |
138 |
66.3 |
115 |
55.3 |
110 |
52.9 |
|
|
No |
162 |
77.9 |
70 |
33.7 |
93 |
44.7 |
98 |
47.1 |
|
|
Total |
208 |
100 |
208 |
100 |
208 |
100 |
208 |
100 |
|
Midwives who answered correctly about dose of misoprostol tablet were 9(4.3%), while 199(95.7%) mentioned other wrong weight (Figure 4).
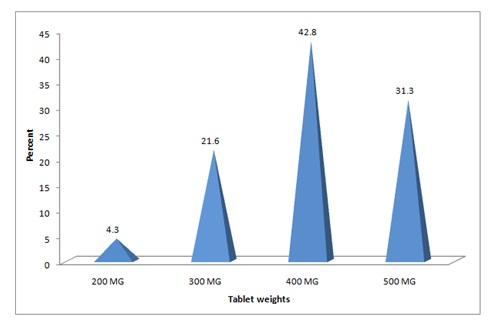
Figure 4: Midwives’ knowledge towards misoprostol tablets dose.
Midwives who found to have good knowledge about misoprostol complications were 171(82.2%), those who had little knowledge were 33(15.9%), while others; 4(1.9%) don’t know its complications (Figure 5, Table 6).
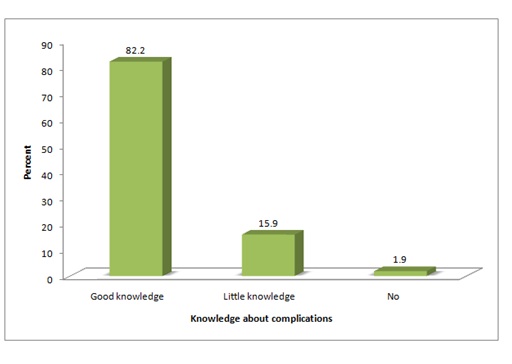
Figure 5: Midwives knowledge towards misoprostol complications.
|
Criteria of knowledge |
% /Mean |
|
Knowledge towards other uterotonic drugs used in PPH |
89.40% |
|
Knowledge towards uterotonicdrugs effectiveness |
80.50% |
|
Knowledge towards misoprostol |
92% |
|
Indications of using misoprostol |
61.70% |
|
Misoprostol formula |
74.80% |
|
Route of misoprostol administration |
49.20% |
|
Misoprostol tablets weight |
4.30% |
|
Dose of misoprostol to prevent PPH |
40.90% |
|
Misoprostol complications |
82.2 |
|
Types of complications of misoprostol |
58.30% |
|
Side effects of misoprostol |
25.9 |
|
Total Mean of knowledge |
59.90% |
Regard to midwives’ knowledge about complications following misoprostol usage in pregnant ladies, 140 (67.3%) mentioned miscarriage, 129(62%) mentioned uterine rupture, 146(70.2%) mentioned fetal distress and 70(33.7%) mentioned fetal and/or maternal death (Table 7).
|
|
Miscarriage |
Rupture uterus |
Fetal distress |
Fetal and mother death |
||||
|
|
Frequency |
Percent |
Frequency |
Percent |
Frequency |
Percent |
Frequency |
Percent |
|
Yes |
140 |
67.3 |
129 |
62 |
146 |
70.2 |
70 |
33.7 |
|
No |
68 |
32.7 |
79 |
38 |
62 |
29.8 |
138 |
66.3 |
|
Total |
208 |
100 |
208 |
100 |
208 |
100 |
208 |
100 |
When midwives inquired about side effects of misoprostol, 173(83.2%) mentioned sweating, 28(13.5%) mentioned diarrhea, 10(4.8%) mentioned shivering and 4(1.9%) mentioned abdominal pain, some midwives mentioned more than one side effect (Figure 6).
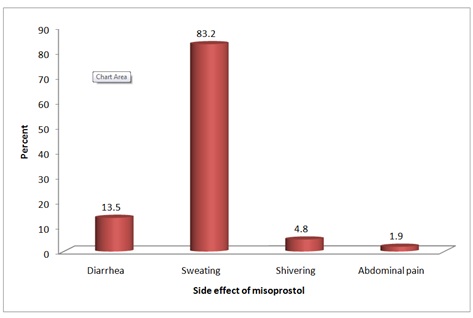
Figure 6: Midwives knowledge towards misoprostol complications.
DISCUSSION
The basic data about participants showed that, most of midwives in the study were above 40 years (75.2%), and the most common midwives' ages were 41-50 years representing 96(42.5%), followed by midwives had ages of 51-60 years representing 74(32.7%). Pertaining level of education; Low education predominated among the studied group, as, 151 (66.9%) were found to have either primary education or illiterate. Low education might have a passive impact upon gaining further and updated knowledge. The general evaluation of midwives in the current study was estimated by choosing mean of correct answer among respondents; the mean of knowledge toward using "Misopristol" in the prevention of PPH; a vast majority of community midwives mentioned their knowledge about "Misopristol", but 8% denied their knowledge and other 11.9% hesitated. The dominant range of experience years in midwifery was 11-20 years, and it represented (40.7%), followed by experience of less than 10 years.
Moreover, the study revealed shortness in training which received by, approximately, one third of midwives (30.5%), most of them (91.3%) had either one course or two courses, with percentages of(68.1%) and (23.2%) respectively. Despite Sudan was having an early history of midwifery services, when "Miss ME Wolf" arrived to Sudan from Egypt to establish midwifery training in 1920 [14], but the current facts might indicate instability of training programs which might lack updating. When midwives were being inquired about other uterotonic drugs used in PPH, a vast majority (89.4%) mentioned both "Syntocinon", and "ergometrin". Most midwives (80.5%) whom were inquired believed that uterotonic drugs were always/most of time effective but still nearly fifth of them didn't believe in such an issue.
A majority of participants (73.6%), when we’re being inquired about the indications of using Misopristol, they were found to have better knowledge about its usage for induction of labor compared to other indications of using the drug, in terms of, termination of pregnancy and PPH, with percentages of 60.1% and 51.4% respectively; however, mean of knowledge was 61.7%.Indeed, it was quite compatible with the community-based study that was conducted by "Ranneberg LB", who reported that, induction of labor was the most common indication of use among midwives and doctors, however, treatment of incomplete and missed abortions were the most prevalent indications [5]. The right formula of "Misopristol" was mentioned correctly by most participants (74.8%), while the rest mentioned incorrect formula.
Mean of knowledge about routes of administration of Misopristol was 49.2%; however, a majority of participants (66.3%) knew a sublingual route of administration, followed by those who knew a vaginal route of administration (55.3%) and rectal route (52.9%), while oral route was the less to be mentioned (22.1%); however, some midwives mentioned more than one route including up to the four routes. But very few knew its weight, only 4.3% mentioned the correct weight available in Sudan.
The right preventive dose of Misopristol was mentioned correctly by less than a half of participants (40.9%) “Which is 3 tablets equivalent to 600 microgram orally as recommended by WHO”; (In the absence of personnel to offer active management of the third stage of labor, it is recommended that the trained health worker should offer "Misopristol" 600 micrograms orally immediately after birth (Table 8). In such cases no active intervention to deliver the placenta should be carried out) [15]. Another important concern about use of "Misopristol" at community level was reported by Prata N et al., which is the risk associated with using Misopristol for induction of labor. The dosage required for induction of labor is only a fraction of a Misopristol tablet and a higher dose could result in serious adverse events to both mother and fetus. For this reason, published guidelines and IEC materials related to "Misopristol" for PPH prevention in home births includes the warning that "Misopristol" should never be taken before delivery of the baby. Any training related to Misopristol must strongly reiterate this warning [16]. Good knowledge about complications of "Misopristol" was found among most of midwives (82.2%), followed by other who either had little knowledge or they didn't know (15.9% and 1.9% respectively).
|
Preventive dose |
Frequency |
Percent |
|
1 tab |
40 |
19.2 |
|
2 tabs |
46 |
22.1 |
|
3 tabs |
85 |
40.9 |
|
4 tabs |
35 |
16.8 |
|
1 or 2 tabs |
1 |
0.5 |
|
1,2, or 3 tabs |
1 |
0.5 |
|
Total |
208 |
100 |
Mean of knowledge toward types of complications of "Misopristol" was 58.3%; fetal distress and miscarriage were the most complications of "Misopristol" reported correctly by community-midwives with percentages of 70.2% and 67.3% respectively, followed by 62% who mentioned "a rupture of uterus", and a less percentage mentioned fetal and maternal death. Regarding the drug side-effects, "Sweating" was reported correctly by a vast majority of midwives (83.2%), followed by few who mentioned diarrhea, shivering and abdominal pain. However, the study of N Mobeen et al., [11] reported that, shivering and chills were significantly more common with Misopristol [17].
CONCLUSION
REFERENCES
- Tenore JL (2003) Methods for cervical ripening and induction of labor. Am Fam Physician 15: 2123-2128.
- WHO (2013) WHO Model List of Essential Medicines. WHO, Geneva, Switzerland.
- Høj L, Cardoso P, Nielsen BB, Hvidman L, Nielsen J, et al. (2005) Effect of sublingual misoprostol on severe postpartum haemorrhage in a primary health centre in Guinea-Bissau: Randomised double blind clinical trial. BMJ 331: 723.
- Hashima-E-Nasreen, Nahar S, Al Mamun M, Afsana K, Byass P (2011) Oral misoprostol for preventing postpartum haemorrhage in home births in rural Bangladesh: how effective is it? Glob Health Action (Vol 4).
- Hofmeyr GJ, Milos D, Nikodem VC, de Jager M (1998) Limb reduction anomaly after failed misoprostol abortion. S Afr Med J 88: 566-567.
- WHO (2007) WHO recommendations for the management of the third stage of labor and community perceptions and actions on postpartum haemorrhage: Findings from a national survey in Ethiopia. WHO, Geneva, Switzerland.
- Davies NM, Longstreth J, Jamali F (2001) Misoprostol therapeutics revisited. Pharmacotherapy 21: 60-73
- SC M (2006) Inadequate reporting of safety issues from clinical trials in academic journals. BMJ.
- Smith JM, Gubin R, Holston MM, Fullerton J, Prata N (2013) Misoprostol for postpartum hemorrhage prevention at home birth: an integrative review of global implementation experience to date. BMC Pregnancy and Childbirth 13: 44.
- Sutherland T, Meyer C, Bishai DM, Geller S, Miller S (2010) Community-based distribution of misoprostol for treatment or prevention of postpartum hemorrhage: Cost-effectiveness, mortality, and morbidity reduction analysis. Int J Gynaecol Obstet 108: 289-294.
- Mobeen N, Durocher J, Zuberi N, Jahan N, Blum J, et al. (2011) Administration of misoprostol by trained traditional birth attendants to prevent postpartum haemorrhage in homebirths in Pakistan: A randomised placebo-controlled trial: a randomized placebo-controlled trial. BJOG 118: 353-361.
- Sanghvi H, Ansari N, Prata NJ, Gibson H, Ehsan AT (2010) Prevention of postpartum hemorrhage at home birth in Afghanstan. Int J Gynaecol Obstet 108: 276-281.
- Tang OS, Gemzell-Danielsson K, Ho PC (2007) Misoprostol: Pharmacokinetic profiles, effects on the uterus and side-effects. Int J Gynaecol Obstet 99: 160-167.
- Schaff EA, DiCenzo R, Fielding SL (2005) Comparison of misoprostol plasma concentrations following buccal and sublingual administration. Contraception 71: 22-25.
- Meckstroth KR, Whitaker AK, Bertisch S, Goldberg AB, Darney PD (2006) Misoprostol administered by epithelial routes: Drug absorption and uterine response. Obstet Gynecol 108: 582-590.
- Bhattacharjee N, Saha SP, Ganguly RP, Patra KK, Jha T, et al. (2012) A randomized comparative study on vaginal administration of acetic acid-moistened versus dry misoprostol for mid-trimester pregnancy termination. Arch Gynecol Obstet 285: 311-316.
- Pongsatha S, Tongsong T (2011) Randomized controlled study comparing misoprostol moistened with normal saline and with acetic acid for second-trimester pregnancy termination. Is it different? J Obstet Gynaecol Res 37: 882-886.
Citation: Kunna A, Musa IDM, Ismail KS, Elkheir HA, Enour S, et al. (2019) Knowledge of Midwives toward Misopristol in the Prevention of Postpartum Hemorrhages at Community Level in Khartoum State in Sudan. J Reprod Med Gynecol Obstet 4: 022.
Copyright: © 2019 Kunna A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

