
Colonic Infarction Following Open and Endovascular Abdominal Aortic Aneurysm Repair in Patients with Dolicosigma-2 Case Reports and Revision of Literature
*Corresponding Author(s):
Luca GarriboliDepartment Of Vascular And Endovascular Surgery, IRCCS Sacred Heart Hospital, Negrar, Verona, Italy
Tel:+39 3388433121,
Email:luca.garriboli@gmail.com
Abstract
Background
Colonic ischemia is a major adverse event after abdominal aortic aneurysm repair, both in open surgical or endovascular procedures, with poor prognosis and high mortality rates.
Case Report
We report two different cases of colonic infarction, the first following a standard EVAR procedure and the second following open abdominal aortic aneurysm repair. Both patients had a patent Inferior Mesenteric Artery (IMA), intact patent iliac hypogastric arteries pre and post-operatively and had in common a dolicosigma as additional anatomical finding. Informed consent for aneurysm repair and publishing of our case studies was obtained for both patients.
Discussion
Colon ischemia accompanying aortic surgery may be an intra-operative finding or a postoperative diagnosis and may be due to several causative factors. The impact of IMA exclusion on colonic perfusion has been largely evaluated, as well as the importance in maintaining adequate blood supply with the preservation of at least one hypogastric artery in case of chronic occlusion of the iliac arteries or distal aorta. Laboratory and clinical parameters may heighten suspicion of bowel ischemia, but they don’t have high enough sensitivity and therefore can’t be considered the only diagnostic modality. Colonoscopy still remains the gold standard for documenting ischemic bowel after aneurysm repair.
Conclusion
Early diagnosis is an essential aspect when colonic ischemia occurs as an adverse event after abdominal aortic surgery. Colonscopy has to be performed as early as possible for a certain diagnosis, while clinical parameters and radiological exams, even useful, sometimes may represent confusing factors that could delay the exact diagnosis. Angio-CT scan may be helpful as well in identifying patients with predisposing factors or anatomical variants that can increase the risk of colon ischemia.
Keywords
INTRODUCTION
Colonic Ischemia (CI) is a major adverse event both after open or endovascular Abdominal Aortic Aneurysm (AAA) repair, with a current incidence ranging from 1.5% to 3% during elective surgery [1-13]. Presumed causes of CI are non-occlusive ischemia due to shock or vasopressive drugs, inferior mesenteric artery and/or internal iliac arteries occlusion and atheroembolization [2, 14-16].
We report two different cases of colonic infarction, the first following a standard EVAR procedure and the second following open abdominal aortic aneurysm repair, in patients with a patent Inferior Mesenteric Artery (IMA) and intact patent iliac hypogastric arteries pre and post-operatively; both patients had in common a dolicosigma.
MATERIALS AND METHODS
Patient 1
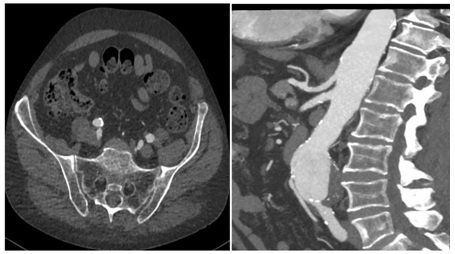 Figure 1: Pre-operative Spiral Computed Tomography (CTA) demonstrates regular patency of visceral vessels, Inferior Mesenteric Artery (IMA) and both hypogastric arteries.
Figure 1: Pre-operative Spiral Computed Tomography (CTA) demonstrates regular patency of visceral vessels, Inferior Mesenteric Artery (IMA) and both hypogastric arteries.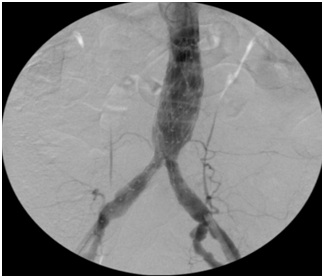 Figure 2: Final intra-operative angiography reveals regular deployment of the graft with preservation of both hypogastric arteries.
Figure 2: Final intra-operative angiography reveals regular deployment of the graft with preservation of both hypogastric arteries.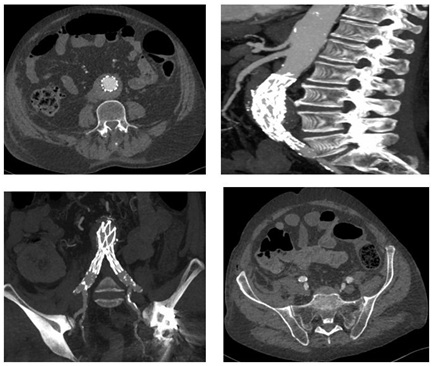 Figure 3: Spiral Computed Tomography (CTA) on 1st post-operative day reveals correct deployment of the graft with patency of main visceral vessel, thrombosis of the IMA secondary to graft deployment and patency of both hypogastric arteries.
Figure 3: Spiral Computed Tomography (CTA) on 1st post-operative day reveals correct deployment of the graft with patency of main visceral vessel, thrombosis of the IMA secondary to graft deployment and patency of both hypogastric arteries.On the third-fourth day his symptoms progressed and patient’s condition significantly deteriorated, the lower abdominal pain worsened with tenderness, (no signs of peritonism were present), as well as biochemical exams, with rise of creatinine values (265mmol/L), LDH (630U/L) and ions (potassium of 5.3mmol/L); white blood cell count was still normal. He developed also acute atrial fibrillation. The general surgeon positioned a naso-gastric tube (that didn’t reveal pathologic findings), the patient repeated a computed tomography of abdomen which revealed a paralytic ileus. With a suspicious diagnosis of colon ischemia as the only cause of clinical deterioration, the patient firstly underwent colonscopy (which confirmed the diagnosis) and subsequent underwent laparotomy which showed transmural ischemia and infarction of the upper rectum, sigmoid, descending colon and the splenic flexure. A colonic resection with formation of Hartmann’s pouch and tansverse colostomy was performed.
Following colonic resection, he was transferred to Intensive Care Unit where he had significant respiratory problems. His general condition progressively worsened, he refused several times tracheostomy and unfortunately he died after 12 days of hospitalization.
Patient 2
DISCUSSION
Colon ischemia due to aortic surgery may be an intraoperative finding or a postoperative diagnosis, depending on several causes such as embolization of aneurysm contents, decreased perfusion from hypotension, ischemia-reperfusion injury, colonic distention, mesenteric interruption (by previous partial colectomy) or congenitally inadequate mesenteric collaterals [17-49].
A study of J-P. Becquemin et al. [18], found that the type of treatment, rupture, duration of operation, renal disease, pulmonary dysfunction, blood loss, femoral anastomosis and hypogastric artery loss were statistically associated with the onset of CI.
The ischemic damage of colon could be divided in three different anatomical stages. The first stage is characterized by a transient low-degree damage of mucosal and sub mucosal tunic with high treatment rates and low mortality, while the second by intermediate grade damage including the muscle coat. Both of them are reversible and are normally managed with a conservative treatment, even if damage can lead to fibrosis and stricture of the intestine after healing. In the last stage, transmural damage leads to gangrene and perforation and the surgical treatment is the only choice even if with poor prognosis and a high mortality rates.
Colonic infarction secondary to aortic reconstruction is associated with a nearly 90% mortality. The primary cause in conventional surgery is the generalized hypoperfusion and reperfusion injury resulting from aortic cross clamping and/or hemorrhage [19]. Rare cases of intestinal embolization have been documented after open [20] as well as endovascular AAA repair, [21-23] but IMA occlusion or IIA thrombosis is more commonly associated with bowel ischemia.
The impact of IMA exclusion on colonic perfusion has been largely evaluated. Velazquez et al., reported no episodes of colonic ischemia in patients with a pre-operatively patent IMA that had subsequently thrombosed after endovascular aortic aneurysm repair [24]. However IMA is located between two nutrient systems that can provide collaterality in case of occlusion; the cephalic system involves the SMA with the Riolan and inter-mesenteric arches, while the caudal system involves the IIA via the middle rectal arteries anastomosed with the superior rectal ones. Iliopoulos et al., stated that the cephalic system was a major source of collaterality but that the caudal system was not sufficiently developed to be effective [25]; however, in case of chronic occlusion of the iliac artery or the distal aorta, the IIA may become a determining factor in maintaining adequate blood supply and in these cases anastomoses between the IIA and IMA can be important [26-27]. Some authors have recommended reconstruction of at least one IIA to improve vascularization of the descending colon after AAA repair [28-29], as well as others have suggested tactical reimplantation of a patent IMA to prevent intestinal necrosis [30,31-49]. It is common opinion that maintenance of IMA perfusion has to be taken into consideration if patients have an occlusion of the celiac trunk, SMA or if both hypogastric arteries are occluded; in literature there are several case reports describing endovascular preservation of IMA perfusion to prevent colon ischemia [32-34].
Also collateral pathways between Superior Mesenteric Artery (SMA) and IMA are an important aspect to take into consideration. In Dadian’s series [13] between eight patients with colonic ischemia following EVAR one patient had a previous colectomy and Maldonado et al., [15] reported a series of seven patients with pelvic ischemia, four of whom had colonic ischemia and between them one had a previous colectomy.
The arterial supply of internal abdominal organs is characterized by a high degree of variation in origin, trajectory and branching patterns; the greater part of descriptions in literature relate to different variations of vascularization due to anomalies of the superior mesenteric artery and these variations frequently cause difficulties even for experienced specialists during surgical and diagnostic procedures. There are different case reports describing abnormalities of colon vascularization due to the presence of an aberrant “middle” mesenteric artery supplying the distal segment of the ascending and transverse colon [35,36], as well as the presence of an inferior mesenteric artery supplying the entire colon vascularization [37] or an inferior mesenteric artery arising direct from the superior mesenteric artery instead of abdominal aorta [38]. M. C. Rusu et al., described the presence of an Accessory Aberrant Colic Artery (LAACA) originating from the superior mesenteric artery and anastomosed with the middle colic and proper left colic arteries [39]. Furthermore, J. launge et al., proposed an interesting review on Riolano’s arch, analyzing the literature related to the vasculature of the colon to emphasize its clinical importance [40]. All these examples are useful because demonstrate the extreme variability in colon vascularization and the fact that a case of necrosis following aortic surgery can be attributed to underdeveloped collaterals.
There are different methods that a vascular interventionist can employ to predict ischemia, including measurement of IMA stump pressure and sigmoid intramucosal pH, both of which have proven to be valuable predictors of colonic ischemia [41-42]. Lebuffe et al., suggested that non-invasive regional and automated capnometry by using semi-continuous sigmoid-to-arterial [P(r-a) CO2] PCO2 gap monitoring may be used to detect preoperative intestinal ischemia in aortic surgery [43]. Another study of M. Poeze et al. [44], investigated the value of D- or L-lactate serum concentration after acute aneurysm repair in prediction of colonic ischemia; results indicated that blood lactate levels provide a marker of metabolism as well as tissue perfusion and that D-lactate may be a more specific marker for colonic ischemia after surgery than L-lactate (which has a raised concentration in other critical illnesses). However, laboratory and clinical parameters may heighten suspicion for ischemia, but have not demonstrated high enough sensitivity or specificity to be used as the only diagnostic modality.
Colonoscopy remains the gold standard for documenting an ischemic bowel and this exam has to be performed as soon as possible when a suspicious diagnosis of colon ischemia is present [45-46]; a retrospective study of Levison et al., recommended selective sigmoidoscopy to detect ischemia when more than two perioperative risk factors were present [47]. BJ. Champagne et al., instituted an aggressive approach with routine colonoscopy after ruptured abdominal aortic aneurysm repair to identify the true incidence of postoperative bowel ischemia and to reduce overall mortality through early treatment, demonstrating that routine surveillance colonoscopy can be safely used as a valid method in detecting early colon ischemia [48].
Our patients had in common a dolicosigma with a pre-operatively patent IMA that subsequently thrombosed after EVAR and that was not reimplanted after open surgery (due to good intraoperative vitality of the bowel evaluated before closure of the abdomen), which could have predisposed him to colonic ischemia. These case reports show the importance of performing colonscopy as soon as possible even if biochemical exams and/or patient’s clinic are not diriment for colon ischemia. Moreover, it’s always useful the meticulous examination of all anatomical aspects of the CT-exam, with technical focus not only on the aorta and its main branches. In these cases probably the presence of a dolicosigma has been a fundamental aspect causing the absence of vascular compensation from SMA and hypogastric arteries due to sudden thrombosis of IMA. The exact pre-operative knowledge of these anatomical variants could permit tactical surgical reimplantation of a patent IMA to prevent intestinal necrosis even with the absence of intra-operative signs of bowel ischemia, as well as trying to perform IMA endovascular savage when EVAR has been chosen as the repair modality.
CONCLUSION
Colonic necrosis still remains a major adverse event with poor prognosis and a high mortality rates. These case reports show the importance of performing an early diagnosis based on high suspicious findings and highlights that colonscopy is the best resource that the surgeon has to perform an early diagnosis of colon ischemia with subsequent immediate open surgical repair. Pre-operative angio-CT scan may be helpful in identifying patients with predisposing factors that increase the risk of colon ischemia; however, it is important not to forget that clinical parameters and radiological exams, even if useful in heighten suspicion for ischemia, may be sometimes confusing factors that delay the exact diagnosis. In conclusion, clinical suspect of colon ischemia after abdominal aortic aneurysm repair must be always high especially during first post-operative days.
CONFLICT OF INTERESTS
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
FUNDING
None.
AUTHOR’S CONTRIBUTION
Each author participated to the elaboration of the manuscript.
REFERENCES
- Brewster DC, Franklin DP, Cambria RP, Darling RC, Moncure AC, et al. (1991) Intestinal ischemia complicating abdominal aortic surgery. Surgery 109: 447-454.
- Bjorck M, Bergqvist D, Troeng T (1996) Incidence and clinical presentation of bowel ischaemia after aortoiliac surgery--2930 operations from a population-based registry in Sweden. Eur J Vasc Endovasc Surg 12: 139-144.
- Van Damme H, Creemers E, Limet R (2000) Ischaemic colitis following aortoiliac surgery. Acta Chir Belg 100: 21-27.
- Chen JC1, Hildebrand HD, Salvian AJ, Taylor DC, Strandberg S, et al. (1996) Predictors of death in nonruptured and ruptured abdominal aortic aneurysms. J Vasc Surg 24: 614-620.
- Bjorck M, Troeng T, Bergqvist D (1997) Risk factors for intestinal ischaemia after aortoiliac surgery: A combined cohort and case-control study of 2824 operations. Eur J Vasc Endovasc Surg 13: 531-539.
- Lederle FA, Kane RL, MacDonald R, Wilt TJ (2007) Systematic review: Repair of unruptured abdominal aortic aneurysm. Ann Intern Med 146: 735-741.
- Franks SC, Sutton AJ, Bown MJ, Sayers RD (2007) Systematic review and meta-analysis of 12 years of endovascular abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg 33: 154-171.
- Alsac JM, Desgranges P, Kobeiter H, Becquemin JP (2005) Emergency endovascular repair for ruptured abdominal aortic aneurysms: Feasibility and comparison of early results with conventional open repair. Eur J Vasc Endovasc Surg 30: 632-639.
- Arya N, Makar RR, Lau LL, Loan W, Lee B, et al. (2006) An intention-to-treat by endovascular repair policy may reduce overall mortality in ruptured abdominal aortic aneurysm. J Vasc Surg 44: 467-471.
- Coppi G, Silingardi R, Gennai S, Saitta G, Ciardullo AV (2006) A single-center experience in open and endovascular treatment of hemodynamically unstable and stable patients with ruptured abdominal aortic aneurysms. J Vasc Surg 44: 1140-1147.
- Mialhe C, Amicabile C, Becquemin JP (1997) Endovascular treatment of infrarenal abdominal aneurysms by the Stentor system: Preliminary results of 79 cases. Stentor Retrospective Study Group. J Vasc Surg 26: 199-209.
- Maldonado TS, Rockman CB, Riles E, Douglas D, Adelman MA, et al. (2004) Ischemic complications after endovascular abdominal aortic aneurysm repair. J Vasc Surg 40: 703-709.
- Dadian N, Ohki T, Veith FJ, Edelman M, Mehta M, et al. (2001) Overt colon ischemia after endovascular aneurysm repair: The importance of microembolization as an etiology. J Vasc Surg 34: 986-996.
- Mehta M, Veith FJ, Ohki T, Cynamon J, Goldstein K, et al. (2001) Unilateral and bilateral hypogastric artery interruption during aortoiliac aneurysm repair in 154 patients: A relatively innocuous procedure. J Vasc Surg 33: 27-32.
- Lee C, Dougherty M, Calligaro K (2006) Concomitant unilateral internal iliac artery embolization and endovascular infrarenal aortic aneurysm repair. J Vasc Surg 43: 903-907.
- Arko FR, Lee WA, Hill BB, Fogarty TJ, Zarins CK (2004) Hypogastric artery bypass to preserve pelvic circulation: Improved outcome after endovascular abdominal aortic aneurysm repair. J Vasc Surg 39: 404-408.
- Mitchell KM, Valentine RJ (2002) Inferior mesenteric artery reimplantation does not guarantee colon viability in aortic surgery. J Am Coll Surg 194: 151-155.
- Becquemin JP, Majewski M, Fermani N, Marzelle J, Desgrandes P, et al. (2008) Colon ischemia following abdominal aortic aneurysm repair in the era of endovascular abdominal aortic repair. J Vasc Surg. 47: 258-263.
- Tollefson DF, Ernst CB (1991) Colon ischemia following aortic reconstruction. Ann Vasc Surg 5: 485-489.
- Bayne SR, Donovan DL, Henthorne WA (1994) A rare complication in elective repair of an abdominal aortic aneurysm: Multiple transmural colonic infarcts secondary to atheroemboli. Ann Vasc Surg 8: 290-295.
- Jaeger HJ, Mathias KD, Gissler HM, Neumann G, Walther LD (1999) Rectum and sigmoid colon necrosis due to cholesterol embolization after implantation of an aortic stent-graft. J Vasc Interv Radiol 10: 751-755.
- Sandison AJ, Edmondson RA, Panayiotopoulos YP, Reidy JF, Adam A, et al. (1997) Fatal colonic ischaemia after stent graft for aortic aneurysm. Eur J Vasc Endovasc Surg 13: 219-220.
- Thomas SM, Gaines PA, Beard JD (1999) Vascular surgical society of great britain and ireland: RETA: The registry of endovascular treatment of abdominal aortic aneurysms. Br J Surg 86: 711.
- Velazquez OC, Baum RA, Carpenter JP, Golden MA, Cohn M, et al. (2000) Relationship between preoperative patency of the inferior mesenteric artery and subsequent occurrence of type II endoleak in patients undergoing endovascular repair of abdominal aortic aneurysms. J Vasc Surg 32: 777-788.
- Iliopoulos JI, Pierce GE, Hermreck AS, Haller CC, Thomas JH (1990) Hemodynamics of the inferior mesenteric arterial circulation. J Vasc Surg 11: 120-126.
- Hassen-Khodja R, Batt M, Michetti C, Le Bas P (1987) Radiologic anatomy of the anastomotic systems of the internal iliac artery. Surg Radiol Anat 9: 135-140.
- Courbier R, Jausseran JM, Reggi M. L’arcade de Riolan (1975) Signification hemodynamique. Deductions therapeutiques. In: Courbier R (ed.). Chirurgie des Arteriopathies Digestives. Paris: Expansion Scientifique Francaise, Pg No: 63-71.
- Smith RF, Szilagyi DE (1960) Ischemia of the colon as a complication in the surgery of the abdominal aorta. AMA Arch Surg 80: 806-821.
- Batt M, Ricco JB, Staccini P (2001) Do internal iliac arteries contribute to vascularization of the descending colon during abdominal aortic aneurysm surgery? An intraoperative hemodynamic study. Ann Vasc Surg 15: 171-174.
- Senekowitsch C, Assadian A, Assadian O, Hartleb H, Ptakovsky H, et al. (2000) Replanting the inferior mesentery artery during infrarenal aortic aneurysm repair: Influence on postoperative colon ischemia. J Vasc Surg 43: 689-694.
- Shunei Saito S, Shirota K, Nakamura H, et al. (2007) Two cases of 265 colonic necrosis following aortoiliac surgery due to coronary induced cardiogenic shock. Gen Thorac Cardiovasc Surg 55: 208-211.
- Igari K, Kudo T, Mori K, Oonuki M, Hirooka K, et al. (2013) Two cases of successful inferior mesenteric artery preservation with bare metal stent in endovascular iliac artery aneurysm repair. Ann Vasc Dis 6: 674-677.
- Donas KP, Torsello G, Bisdas T, Austermann M, Stavroulakis K, et al. (2014) Novel indication for chimney graft placement in the inferior mesenteric artery in AAA patients with coexistent bilateral internal iliac artery occlusion. J Endovasc Ther 21: 548-552.
- Pfister K, Kasprzak PM, Apfelbeck H, Kopp R, Janotta M, et al. (2016) Inferior mesenteric artery side branch for selected patients with endovascular aortic aneurysm repair. EJVES Short Reports 31: 1-5.
- Milnerowicz S, Milnerowicz A, Tabo?a R (2012) A middle mesenteric artery. Surg Radiol Anat 34: 973-975.
- Kawai K, Koizumi M, Honma S, Tokiyoshi A, Kodama K (2006) A case of nonrotation of the midgut with a middle mesenteric artery. Ann Anat 188: 13-17.
- Covan?ev S, Mazuruc N1, Belic O (2017) An unusual case of colon vascularization by the inferior mesenteric artery. J Vasc Bras 16: 52-55.
- Kitamura S, Nishiguchi T, Sakai A, Kumamoto K (1987) Rare case of the inferior mesenteric artery arising from the superior mesenteric artery. The Anatomical Record 217: 99-102.
- Rusu MC, Vlad M, Voinea LM, Curc? GC, Si?u AM (2008) Detailed anatomy of a left accessory aberrant colic artery. Surg. Radiol Anat 30: 595-599.
- Lange JF, Komen N, Akkerman G, Nout E, Horstmanshoff H, et al. (2007) Riolan’s arch: Confusing, misnomer, and obsolete. A literature survey of the connection(s) between the superior and inferior mesenteric arteries. Am J Surg 193: 742-748.
- Elmarasy NM, Soong CV, Walker SR, Macierewicz JA, Yusuf SW, et al. (2000) Sigmoid ischemia and the inflammatory response following endovascular abdominal aortic aneurysm repair. J Endovasc Ther 7: 21-30.
- Ernst CB, Hagihara PF, Daugherty ME, Griffen WO Jr (1978) Inferior mesenteric artery stump pressure: A reliable index for safe IMA ligation during abdominal aortic aneurysmectomy. Ann Surg 187: 641-646.
- Lebuffe G, Decoene C, Raingeval X, Lokey JS, Pol A, et al. (2001) Pilot study with air-automated sigmoid capnometry in abdominal aortic aneurysm surgery. Eur J Anaesthesiol 18: 585-592.
- Poeze M, Froon AH, Greve JW, Ramsay G (1998) D-lactate as an early marker of intestinal ischaemia after ruptured abdominal aortic aneurysm repair. Br J Surg 85: 1221-1224.
- Fanti L, Masci E, Mariani A, Chiesa R, Jannello A, et al. (1997) Is endoscopy useful for early diagnosis of ischaemic colitis after aortic surgery? Results of a prospective trial. Ital J Gastroenterol Hepatol 29: 357- 360.
- Houe T, Thorböll JE, Sigild U, Liisberg-Larsen O, Schroeder TV (2000) Can colonoscopy diagnose transmural ischaemic colitis after abdominal aortic surgery? An evidence based approach. Eur J Vasc Endovasc Surg 19: 304-307.
- Levison JA, Halpern VJ, Kline RG, Faust GR, Cohen JR (1999) Perioperative predictors of colonic ischemia after abdominal aortic aneurysm. J Vasc Surg 29: 40-45.
- Champagne BJ, Darling RC 3rd, Daneshmand M, Kreienberg PB, Lee EC, et al. (2004) Outcome of aggressive surveillance colonoscopy in ruptured abdominal aortic aneurysm. J Vasc Surg 39: 792-796.
- Kotsis T, Christoforou P, Asaloumidis N, Papaconstantinou I (2017) Delayed sigmoid ischemic rupture following open repair abdominal aortic aneurysm. Vasc Endovascular Surg 51: 413-416.
Citation: Pruner G, Garriboli L, Miccoli T, Jannello AM (2019) Colonic Infarction Following Open and Endovascular Abdominal Aortic Aneurysm Repair in Patients with Dolicosigma-2 Case Reports and Revision of Literature. J Surg Curr Trend Innov S1: 001.
Copyright: © 2019 Gianguido Pruner, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

