
Cannabidiol and Contributions of Major Hemp Phytocompounds to the “Entourage Effect”; Possible Mechanisms
*Corresponding Author(s):
Gerhard NahlerClinical Investigation Support Gmbh, Kaiserstrasse 43, 1070 Wien, Austria
Tel:+43 15234015,
Email:cis-qa@aon.at
Abstract
Cannabidiol (CBD) is the primary cannabinoid in “fibre-type” cannabis chemovars (hemp) and the subject of intensive research. In addition to cannabinoids, Cannabis sativa species produce several hundreds of other phytocompounds such as terpenes and phenolic compounds that can produce a wide variety of effects and are claimed to modify the action of CBD, in extracts and other derivatives (“entourage effect”). Different hemp varieties demonstrate a large genetic variability of the profile of phytocompounds which is further modified by agroclimatic factors and processes after harvest such as extraction. Overall, the pharmacological effects of hemp extracts, particularly when grown in outdoor culture, are likely to vary due to the variable profile of their phytocompounds. Consequently, a generalization of the medicinal properties of different extracts is therefore not possible. This review summarizes the complex interaction of CBD with its main targets, possible modulatory effects by other phyto- and endocannabinoids and the possible impact of concomitant phytosubstances in hemp extracts such as terpenes and flavonoids. For a better understanding of the “entourage effect” and to enable a systematic investigation of differences between chemovars and derivatives, future clinical studies need to ensure proper characterisation of phytocompounds content beyond the concentration of CBD and THC.
Keywords
INTRODUCTION
Cannabis cultivars are often characterised by their THC/CBD ratio but a variety of terpenes have also been described as characteristic, additional markers among a number of other phytocompounds that vary considerably between chemical varieties or “chemovars” [3,4].
The term “strain” is commonly misapplied to chemovars of Cannabis in common parlance, but is properly pertinent to bacteria and viruses, but not plants [5-8]. In fact, neither CBD nor THC is formed enzymatically by the plant. Both substances are the decarboxylated form of Cannabidiolic Acid (CBDA) and delta-9-Tetrahydrocannabinolic Acid (THCA) respectively, induced in nature by slowly aging (mainly by light), or in post-harvest processing e.g., by heating. About 20 to 50-times higher concentrations of acid cannabinoid (THCA) are found in dried but otherwise unheated Cannabis plants [9].
Cannabigerolic Acid (CBGA) is the precursor of CBDA, THCA and cannabichromenic acid, CBCA. CBGA is rapidly converted to the a.m. acids CBDA, CBCA, THCA or decarboxylated to Cannabigerol (CBG). Unsurprisingly, whilst CBD is usually the primary constituent, CBG predominant fibre hemp varieties also exist (e.g., the French Santhica-23, Santhica-27) without notable concentrations of THC (THCA) and CBD (CBDA) [5,10].
Each of these phytocannabinoids has its own individual pharmacologic effects and interacts with various targets, including but not limited to the so called Endocannabinoid System (ECS) of humans as will be discussed in this text.
Since its discovery in the 1990s, the ECS emerged as one of the largest signalling and regulatory network of the organism, consisting basically of ligands, namely endocannabinoids, receptors and enzymes which are responsible for their formation and deactivation. The ECS owes his discovery largely to the historic research on the mechanisms of the psychotropic THC. After THC, CBD is the next most widely investigated phytocannabinoid.
CBD, isolated first in 1940, was given to human subjects already in 1972 to study antiepileptic effects [7,11]. From 1998 onwards, CBD was subject of an intensive development program of GW Pharmaceuticals, together with its combination with THC (“nabiximols”, US adopted name). Public interest increased tremendously in 2013/2014, when several media (e.g., CNN,https://edition.cnn.com/2013/08/07/health/charlotte-child-medical-marijuana/index.html) reported the case of a little girl suffering from Dravet syndrome, a type of rare, in-born form of epilepsy, that was successfully treated with a whole plant extract high in CBD (~ 17% CBD and 0.3% to 0.5% THC). The reduction of seizures from over 40 per day to 2-4 per month was so spectacular that many other families with similar cases as well as physicians became highly interested in CBD; at this time, anti-epileptic properties of CBD had already been reported, and larger prospective clinical trials were underway [7,12-14]. In June 2018 purified CBD (>98%) derived from Cannabis (Epidiolex®, GW Pharmaceuticals) received marketing authorisation as orphan drug by the US Food and Drug Authority for treatment of Dravet- and Lennox Gastaut - syndrome.
Cannabidiol (CBD) is a major non-intoxicating component of hemp. Reports have suggested that CBD possesses anti-epileptic, anxiolytic, anti-inflammatory, anti-psychotic and anti-hyperalgesic properties [8,15,16].
CBD acts on the canonical G-protein coupled receptor CB1 as a negative allosteric modulator; this alters the signalling of agonists such as delta-9-Tetrahydrocannabinol (THC) but also of antagonists such as rimonabant [17]. At CB2 receptor, CBD is a partial agonist [18,19]. Therefore, treatment with therapeutic doses of CBD does not cause the common side effects of CB1-agonists, such as psychotomimetic effects, increase of appetite and anxiety, nor those of antagonists, such as weight loss and depression. Instead, CBD targets a number of other receptors and ion-channels, as well as enzymes and other constituents within the endocannabinoid system as will be described later. However, the mechanism of action of CBD in producing such effects is still insufficiently known.
In this article, selected phytocompounds of hemp (Cannabis) are briefly discussed as well as their putative targets, in order to give an overview on the extremely complex interaction commonly called as “entourage effect”; lacking more systematic studies particularly in man, a number of mechanisms must remain assumptions. It is beyond the scope of this article to summarise all experimental work that has been published on specific ligands, receptors and ion channels. Primacy is given to most recent articles and reviews rather than to original papers.
THE COMPOSITION OF PHYTOCOMPOUNDS OF HEMP (CANNABIS) DEMONSTRATES A LARGE VARIABILITY
Terpenes, nature’s largest group of phytocompounds, are important components also in Cannabis and can represent about 3% to 10% of the trichome content by weight [20,21]. More than 150 were identified in Cannabis; their profile contributes to the characteristic of a specific cultivar. Only a few terpenes are more prominent and found in a majority of cultivars; monoterpenes such as myrcene (prominent also in hop), alpha-pinene, D-limonene, trans-ocimene and alpha-terpinolene make up to 28.3 mg/g or 0.7% of flower dry weight [3,4,22-26]. Limonene and perillyl alcohol, a precursor of limonene, demonstrated anticancer effects in phase I/II clinical studies [27,28]. Other major terpenes are sesquiterpenes, particularly ß-caryophyllene that occurs also in rosemary, or alpha-humulene, occurring in hops. Some terpenes are reported to interact directly or indirectly with the ECS as will be outlined later. Their use is considered as safe; numerous of those found in Cannabis are listed in the database on “food flavourings” of the EU (https://webgate.ec.europa.eu/foods_system/main/) and/or the US “GRAS-List” (https://www.femaflavor.org). Myrcene and caryophyllene, the main terpenes in essential oil from Cannabis, demonstrated measurable effects on autonomic nervous system parameters with beneficial subjective effects in volunteers [29]. Terpenes’ medicinal properties are supported by numerous in vitroand in vivo studies that used human cells, and show anti-inflammatory, antioxidant, analgesic, anticonvulsive, antidepressant, anxiolytic, anticancer, neuroprotective, anti-mutagenic, anti-allergic, antibiotic and anti-diabetic effects [21,30,31].
Flavonoids are less numerous in Cannabis than terpenes. Among the over 6,000 flavonoids (a subgroup of phenolics) known, 26 have been identified in Cannabis so far. The content of flavonoids in Cannabis is highly variable and seems to be influenced more by growth conditions than by genetics [32]. The major flavonoids present in the leaves and flowers of the hemp cultivars Felina and Futura given as examples are orientin, vitexin, luteolin-7-O-beta-D-glucuronide, apigenin-7-O-beta-D-glucuronide [33] and cannflavin A; cannflavins are unique for Cannabis and can make up to over 400 mcg/g hemp inflorescence [26]. Flavonoids have their own pharmacological effects. A meta-analysis suggested that consumption of dietary flavonoids and subtypes (isoflavones, flavonols) has protective effects such as against ovarian and colorectal cancer [34,35]; luteolin as example, demonstrated excellent anti-inflammatory properties in man [36,37]. Although phenolics are considered as valuable components in maintaining health, little is actually known about contributions to the “entourage effect”.
The most important (agroclimatic) factors influencing the profile of phytocompounds are summarised in table 1 below, although differences between cultivars exist.
|
Choice of the cultivar |
The profile of cannabinoids and terpenes is mainly genetically determined [38]; however, THC contents may vary considerably between individual plants of the same field/culture [39] |
|
Light |
Light influences the composition (e.g., LED increases CBGA [40]; abundant light is necessary to achieve sufficient photosynthesis; an increase of exposure of two hours/day doubled THC levels [41]; UV-B radiation increases THC content in drug-type, but not in fiber-type Cannabis [42-44] |
|
Average temperature in the entire growing period |
Temperature has a positive influence on the content of CBD and THC [45] |
|
Growing Season precipitation |
A wet growing season with a lot of precipitations has a negative influence on the content of CBD and THC (CBD is more affected [45,46] |
|
Air humidity |
Moderate air humidity has a positive influence on the content of CBD and THC (THC is more affected) [45,46] |
|
Soil temperature |
Soil temperature at 5 cm has a positive influence on the content of CBD [46] |
|
Sowing time |
Sowing one month later (Fibranova) significantly increased the antimicrobial activity of the essential oil and affected the content of specific terpenes such as nerolidol and ocimene [47] |
|
Time of harvest, stage of plant development |
The content of both, cannabinoids and terpenes, increases during growth up to the end of flowering, but tends to decrease and to fluctuate in the last stages of vegetation in drug-type Cannabis [1,10] |
|
Herbal material collected |
The content of both, cannabinoids and terpenes decreases from top to bottom of the plant; older (lower) leaves contain less than younger (upper) leaves; flowers have the highest content |
|
Fertilization |
For CBD, nitrogen + potassium had a positive, the addition of phosphate a negative influence [48-50]; phosphate + potassium had a positive, phosphate + nitrogen a negative influence on THC |
|
Environmental stress |
Under stress (e.g., drought, direct sunlight, salinity, mineral/ organic nutrients, difference day-night temperature) the plant increases the concentration of (protective) phenolic compounds (e.g., flavonoids) |
In a field experiment that examined eight industrial hemp varieties over six years, the THC content in Futura 77 varied by a factor of twenty two (between 0.045 % and 1.000 %), the CBD content varied by a factor of three (between 1.011 % and 3.261 %). Overall, agroclimatic conditions seem to affect in general the content of THC more than of CBD, as the variability was distinctly higher in six of eight cultivars (variation of max. /min. concentration: THC between 4.2 and 25, CBD between 2.5 and 5.7 [46]. Unsurprisingly, a marked regional influence has been observed as well. When cultures of Futura 75, another hemp variety authorized by the European Community, was grown in parallel in four different regions of Morocco, cultures of the region of Agadir had 50% higher concentrations of THC than those of Beni-Mellal (0.035% vs 0.021%. [51].
The profile of phytochemicals originally present in the plant is considerably modified by processes after harvest as summarised below (Table 2), particularly by drying and extraction [52].
|
Transport and storage of fresh herbal material |
Fresh material can be contaminated between harvest and drying, particularly by growth of bacteria and molds, and the subsequent formation of toxins |
|
Herbal material used |
Concentration of cannabinoids is about ten times higher in flowers than in leaves [53] and decreases down the stem; the profile of terpenes varies between upper/younger leaves and older leaves; extracts derived from all parts of the plant will have a markedly different composition compared to extracts made from flowers alone [48]. |
|
Drying of raw herbal material |
Drying at temperatures above ambient and exposure to sunlight accelerates aging/decarboxylation of cannabinoid acids, and the transformation of THC to CBN; fresh material has a higher content of terpenes [54]. |
|
Solvent used for extraction |
The nature of the solvent used for extraction distorts the profile: lipophilic solvents dissolve lipophilic, hydrophilic solvents hydrophilic substances, and small changes of the solvent polarity influence the extraction efficiency |
|
Extraction process |
The solubility of cannabinoids in supercritical CO2 increases at 52.9°C in the order: THC < CBG < CBD < CBN [55]. |
|
Heating during extraction |
Decarboxylation (140-170°C) of cannabinoid acids is higher in solvents and is markedly accelerated at temperatures above 100°C [56]; in very mildly heated extracts (below 50°C), the content of CBDA and THCA exceeds the decarboxylated forms by a factor of ten or more; heat treatment reduces the content of (volatile) terpenes [54], and some polyphenols (orientin, quercetin) are lost [57]; heat induces also structural changes in flavonoids [58]. |
|
Transport and storage of the final product |
Aging is accelerated by increases of the storage temperature and exposure to light [20]. |
As can be seen, a number of pre-harvest factors, post-harvest conditions and processes influence the final profile of phytocompounds, among which the selection of the strain, the conditions of growth and harvest of the herbal material, drying and extraction is likely to have the greatest influence. Drying at temperatures below 50°C yielded the highest amount of total phenolics [59]; temperatures above 100°C decrease not only the content of phenolics and volatile terpenes but also of cannabinoid acids such as CBDA and THCA. The nature of the solvent distorts the genuine profile further; already small changes of the solvent polarity influence the extraction efficiency, e.g., ethanol (marginally less polar than methanol) extracts the cannabinoids slightly better than methanol (THC>THCA>CBDA>CBD [60]. When investigating supercritical CO2-extracts, cannabinoid potency increased by factors of 3.2 for THC but 4.0 for CBD in concentrates compared to flowers, sesquiterpenes up to a factor of 8.9 [61].
In summary, a large number of factors, many of which are not usually standardised, influence the final content of cannabinoids and other phytosubstances in dried flowers and leaves. Processing differences from manufacturer to manufacturer, changes the profile even further. Products may thus be very different in composition, from batch to batch and from different suppliers.
DIFFERENT CANNABIS CULTIVARS HAVE DIFFERENT EFFECTS
Cannabis consumer claim that cultivars not only differ in their “characters” in terms of smell and flavour but also in terms of biological effects, despite that the “potency” (THC-content) cannot be perceived by smelling [62]. It is not fully clear yet how such popular claims can be linked to differences in chemical composition and therapeutic effects, but volatile terpenes likely play a role.
Cannabis consists only of a single species Cannabis sativa L.; the ratio between CBD and THC is often taken as a further characteristic [38,63]. Notable differences in effects related to large differences concerning the THC/CBD ratio are summarised in table 3, although it has for many years been argued that effects cannot be accounted for in terms of the THC and/or CBD content alone [64,65].
|
THC:CBD |
Effects |
|
1:0 |
Synthetic THC/dronabinol; decreases nausea, pain, spasm, increases appetite, mood; very high psychoactive effects, uplifted emotions, uncontrollable laugh, increases anxiety (higher doses), dry mouth, transient cognitive deficits, (short term) impairment of cognition and memory, sedation, dejection; prominent side effects for novice users; long term exposure to high doses induces down-regulation of cannabinoid receptor CB1 [66]. |
|
2:1 *) |
Corresponds to the ratio in Cannador; decreases pain, spasms; in general milder side effects than with pure THC: poor concentration, laugh and euphoria with calmer thoughts, attention reduced, dry mouth; a cultivar with approx. a 1:2 ratio is Harlequin. |
|
1:1 |
Corresponds to the ratio in nabiximols (Sativex™); CBD counteracts some of the negative effects of THC; side effects on emotional processing and working memory are almost absent; less sedation. Cultivars with approx. a 1:1 ratio are Bediol, FM2 or ARGYLE™ |
|
1:2 |
Corresponds to the ratio in the cultivar Cannatonic; used for pain, headaches, migraine, stress; weak euphoric effects. (https://www.prufcultivar.com/products/cannatonic) |
|
0:1 |
Highly purified phyto-CBD or synthetic CBD; antipsychotic, antidepressant, anxiolytic, relaxing effects, antiepileptic/ anticonvulsant; improvement of sleep, mood, alertness, cognition, pain; no “high” effect, no appetite stimulation. |
Compiled from [67-71].
*CBD may potentiate some of the THC side effects when the CBD:THC ratio is around 1.8:1; a much higher ratio of 20:1 blocks THC effects [71,72]; psychoactive effects are mediated via CB1 receptors which are dose- and time-dependently down regulated by THC [66].
According to a meta-analysis, no significant differences have been observed in outcomes on patients treated for chronic pain, either with THC or CBD; both treatments reduced pain significantly when compared to placebo but were less effective than a THC: CBD buccal spray [73].
Effects related to other phytocompounds than CBD and THC are therefore of interest. In a study in which seven different Cannabis cultivars were administered to 442 participants, two cultivars, Bubba Kush and Chocolope, both with approximately the same content of THC (17-22%) and CBD (0.1%), rated at the extremes concerning their influence on anxiety; Bubba Kush was the most effective and Chocolope the least [20]. After quantification of 29 terpenes and four Cannabinoids (CBD, THC, CBG, CBC), most striking differences were found between the contents of trans-nerodiol (already known to reduce anxiety as well as to enhance bioavailability) and guaiol, gamma-terpinene and sabinene hydrate (having the least effect on anxiety). According to other investigations, eucalyptol (1,8-cineole) has also been reported to reduce anxiety [74]. Further examples, linked to improvement of sleeping disorders and social anxiety, are caryophyllene, linalool and myrcene which are terpenes that widely occur in Cannabis and hemp [31].
Phenolic compounds are likely contributing as well. They have been isolated and detected from flowers, leaves, stem, roots, seeds, twigs and pollen and can be found in hemp-flower tee [75]. They are not only potent antioxidants; many of them, including apigenin, kaempferol, luteolin, naringin and quercetin have anxiolytic properties that may have influenced the results of the study mentioned above [33,76].
EFFECTS OF CBD ARE COMPLEX AND MODIFIED BY A LARGE NUMBER OF CONCOMITANT PHYTOCOMPOUNDS
Inhibition of metabolising liver enzymes such as CYP3A4 (by terpenes or sesquiterpenes e.g., ß-caryophyllene) or CYP3A5 (by luteolin), CYP2D6 (by naringenin) or CYP2C9, CYP2C19 (by quercetin), all of which are found in Cannabis, is another mechanism that increases the bioavailability of various substances, particularly of CBD [81]. Such very complex mixtures in extracts have thus a broad range of overlapping effects that may vary with their composition and concentration. Unfortunately, the composition of Cannabis products included in clinical studies and for products available on the market is insufficiently characterised; systematic investigations are missing. Overall effects of extracts rich in CBD thus differ from those observed with pure CBD [82].
Out of the numerous and varying phytocompounds of Cannabis, CBD is not only considered as the main cannabinoid of hemp and primary substance responsible for the overall effects but it has also been investigated more thoroughly than the large number of non-cannabinoids and minor cannabinoids, except THC. Studies on isolated substances, although necessary, cannot foresee effects of natural extracts where many compounds interact. Of approximately 65 targets that have been reported for CBD [83], many of them function like in a network; an overview on selected targets that may be relevant for effects as well as potential interactions are given in figure 1.
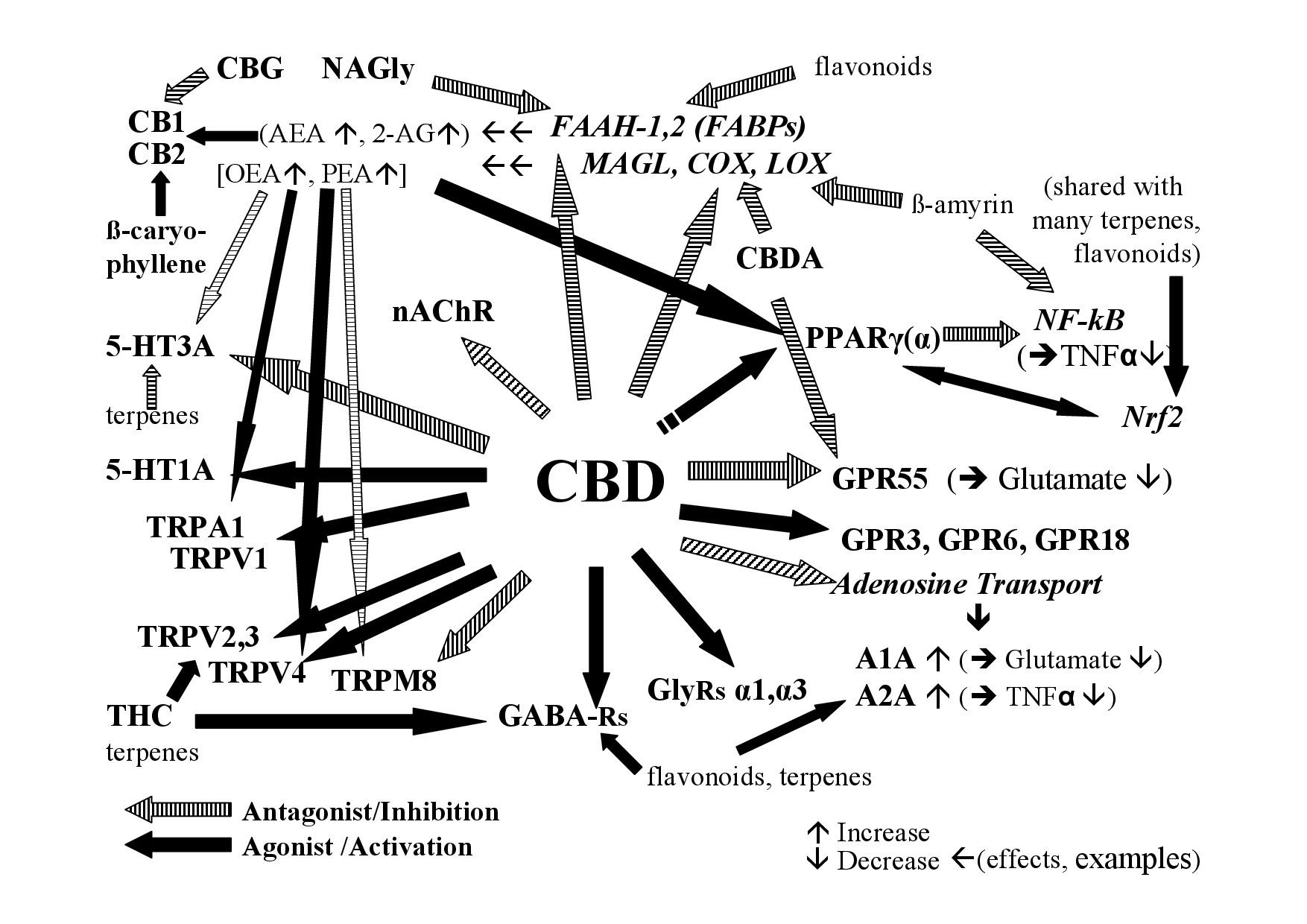 Figure 1: Examples of the interaction of CBD and non-cannabinoids in hemp with the endocannabinoid system.
Figure 1: Examples of the interaction of CBD and non-cannabinoids in hemp with the endocannabinoid system.A1A, A2A - Adenosine receptor 1A, 2A; AEA - Anandamide; 2-AG - 2-Arachidonoylglycerol; CB1- Cannabinoid receptor 1; CB2- Cannabinoid receptor 2; COX-Cyclooxigenase; FAAH – Fatty Acid Amid Hydrolase; FABP - Fatty Acid Binding Protein; GABA Rs - Gamma Aminobutyric Acid Receptors; GlyRs - Glycine receptors; GPR3, 6, 18, 55 - G-Protein-Coupled Receptor 3, 6, 18, 55; 5-HT - 5-Hydroxy-Tryptamin receptor; LOX - Lipoxygenases; MAGL - Monoacylglycerol-Lipase; nAChR - nicotinic Acetylcholine Receptor; NAGly - N-Arachidonoyl Glycine; NF-kB - Nuclear Factor Kappa B; Nrf2-Nuclear factor erythroid derived 2; PPAR- Peroxisome Proliferator-Activated Receptor (g-gamma, a-alpha); TNFa - Tumor Necrosis Factor Alpha; TRP - Transient Receptor Potential [V-Vanilloid; A-Ankyrin repeats; M-Melastatin-type] [84]; see text for more details.
As many conditions are multifactorial in nature, multitarget treatments such as with CBD should therefore have therapeutic advantages. Interactions may occur through various mechanisms including:
• Bioavailability (example: inhibition/induction of metabolism)
• Interference with cellular transport processes (example: inhibition of anandamide uptake/transport/degradation by CBD)
• Activation of pro-drugs or deactivation of active compounds to inactive metabolites; alteration of phytocompounds via bacterial fermentation in the intestine
• Action of synergistic partners at different points of the same signalling cascade (multi-target effects)
• Inhibition of binding to target proteins (example: reduction of psychotropic effects of THC by CBD that acts as a negative allosteric modulator of CB1 receptors)
• Indirectly increasing/decreasing ligands such as Anandamide (AEA) or 2-Arachidonoylglycerol (2-AG) (example: modulation of the endocannabinoid tone by nutritional supply of omega-3 / omega-6 fatty acids and/or inhibiting the deactivation and/or modulating the synthesis of endocannabinoids)
When interactions (synergism) between a number of phyto substances such as in extracts is possible, effective concentrations of substances can be much lower, but one can only speculate as respective systematic studies are missing.
The theoretical mechanisms of action of CBD and of other ligands seem to be very complex whereby the overwhelming amount of data still comes from experimental studies. Although it is often argued that effects in vivo need concentrations in a magnitude as observed under experimental conditions, correlation between binding studies with pure substances on one hand and the situation in the human organism, particularly in diseased conditions on the other, is often poor. As an example, experimental Inhibition (IC50) of various cytochrome P450 liver enzymes (CYP2C19, 3A4, 2D6, 2C9) by CBD is in the range between 0.8 mcM for CYP2C19 and 5.6 mcM for CYP2C9 [84], concentrations that are too high to be achieved via oral route. Nonetheless, CBD can increase the plasma levels of drugs administered concomitantly via inhibition of their metabolisation. Examples are warfarin S (metabolised by CYP 2C9) or N-desmethylclobazam (metabolised by CYP 2C19) where CBD increases the respective plasma levels despite that repeated doses of 700mg CBD result in average plasma concentrations of only 6 to 11 ng/ml (0.019 to 0.035 nM) which are roughly 100.000 times lower than the IC50 values [85].
CBD treatment (800mg CBD per day) was also accompanied by a significant increase in serum AEA as well as of other substrates of Fatty Acid Amid Hydrolase (FAAH), the enzyme responsible for the degradation of AEA in man, suggestive for an inhibition of the uptake/transport or of the enzyme [86], despite that CBD does not inhibit human FAAH in vitro in ligand assays [87]. Similar, effects of terpenes (citrus fragrance) have been observed at very low, nearly undetectable serum concentrations in animals and depressive subjects [31,88]. Consequently, components in concentrations as low as 0.05% have been considered of pharmacological interest [6].
Whilst preclinical studies, including in rodents, can provide valuable insight into possible performance in humans, caution must always be shown in extrapolation of such data to humans.
Experience has demonstrated that the predictability of rodent models for toxicity in man fails in 57% [89]. This is even more important for extracts where individual substances do not act in isolation but together with many other compounds.
In view of these limitations of any extrapolation of preclinical effects to effects in man, comparisons between Cannabis chemovars, between genuine extracts and between well defined extracts and pure substances would be of particular interest. Not only such studies are almost missing, extracts lack unfortunately characterisation beyond the content of CBD and THC and therefore also comparability between extracts.
In the following, effects of selected hemp (Cannabis) components on the endocannabinoid system and related targets are summarised as an illustration of the complexity of the numerous mechanisms and interactions that, in theory, are possible; many signalling systems seem to be “redundant” and act in parallel. To note, observations mainly result from preclinical studies. In the absence of systematic studies in man, the contribution of individual compounds to overall effects (“entourage effect”) must remain assumptions.
THE CANONICAL ENDOCANNABINOID RECEPTORS CB1, CB2 AND GPR55
Little is known about the respective acids, CBDA and THCA which are the main cannabinoids in unheated flowers and extracts. CBDA has weak activity at CB1 and CB2 receptors; THCA is thermal unstable which complicates investigations about its binding to cannabinoid receptors [93,94]. As THCA-A (and also CBDA) shows anticonvulsant activities in vivo they likely passed the blood-brain barrier in case of disease-related impairment [95]. CBG binds to CB1 (antagonist) and CB2 and is an antagonist to GPR55 [96].
CB1 and CB2 are targeted also by some flavonoids, the largest group of phenolics, such as quercetin, and terpenes notably the sesquiterpene ß-caryophyllene which is a selective agonist of CB2; it is widely found in hemp [97-99].
The only naturally occurring CB1- / CB2-antagonist detected so far is THCV, a D9-THC homologue with a shorter side-chain [100]. It is a neutral antagonist at CB1, in contrast to rimonabant (SR141716A), but an agonist at high doses [101,102].
The CB1 receptor influences (among other) appetite, memory and learning, addiction disorders, motor dysfunction, schizophrenia, depression, bipolar- and anxiety disorders. Beyond the brain, CB1 receptors also function in liver and adipose tissues, vascular as well as cardiac tissue, reproductive tissues and bone; its expression is increased in most tumours including leukaemia, probably as a protective mechanism as loss of CB1 accelerates tumour growth [103,104].
CB2 is involved in immune regulation; in the brain, CB2 receptors are found on microglial cells and are transiently elevated in traumatic brain injury [105]. CBD at nanomolar concentrations was able to significantly reduce the effect of a selective CB2R agonist, whereas it is also described as partial agonist [19]. Activation of CB2 stimulates bone formation, represses bone resorption and mediates renal fibrosis [106,107].
GPR55 is widely expressed in the central nervous system, glial cells, caudate, putamen, frontal cortex, striatum, hypothalamus, as well as in dorsal root ganglia neurons and adipocytes, chondrocytes and the gastrointestinal tract. It is upregulated in cancer, inflammation, pain and possibly regulates bone formation, blood pressure and insulin secretion [108,109]. At present, little is known about functional ligands other than the endogenous Lysophosphatidyl-inositol (LPI).
Other receptors such as GPR3, GPR6 and GPR12 have also been described as being activated by CBD as inverse agonist but research is still at the beginning [110].
ACETYLCHOLINE RECEPTORS (ACHRS)
CBD is a reversible inhibitor of n-AChRs and has been shown to increase dose-dependently the extracellular levels of ACh and wakefulness in rats [111]. In addition, terpenes such as 1,8-cineole, ß-caryophyllene, linalool, (+), (-)-alpha-pinene, ß-myrcene and (+)-3-carene that occur in hemp are also known as effective inhibitors of AChE, in vitro and in vivo, increasing the concentration of this neurotransmitter [112,113]; synergistic inhibition has been described between caryophyllene oxide and 1,8-cineole [114]. Phenolics such as luteolin, naringenin or quercetin are active as well although less [115]. Endogenous inhibitors of n-AChRs are AEA, 2-Arachidonoylglycerol (2-AG) and Arachidonic Acid (AA) which is a metabolite of AEA and 2-AG, but also of omega-6-phospholipids [116]. AEA inhibits the function of n-AChRs in thalamic synaptosomes via a non-competitive, cannabinoid-independent mechanism.
n-AChR receptors are found throughout the peripheral and central nervous system but also on cancer cells and are involved in reward-circuits, appetite, stress-related behaviours, neuronal development and neurodegenerative disorders. In muscle, receptors mediate the neuromuscular transmission [117]; this may play a role in motor neuron diseases such as Amyotrophic Lateral Sclerosis (ALS).
5-HYDROXYTRYPTAMINE RECEPTORS (5-HT, SEROTONIN RECEPTORS)
CBD is an agonist of 5-HT1A and increased 5-HT firing via desensitization of 5-HT1A receptors in rats in doses of 5mg/kg/day [118,119]. It is an agonist of 5-HT2A, and an antagonist of 5-HT3A; CBG is an antagonist to 5-HT1A [120].
Therefore, moderate doses of CBG and CBD may oppose one another at the 5-HT1A receptor, at least in the regulation of nausea and vomiting [121]. Other agonists on 5-HT1A are CBDA (110-times more potent), and THCV [90,122]. Agonists of 5-HT1A and 5-HT2A are anxiolytic, antidepressant, attenuate nausea, vomiting, motor- and cognitive impairment, cerebral infarction/ hypoxic brain damage (stroke), stress and mechanical allodynia. Serotonin agonists are effective in treatment of migraine and cluster headache.
On 5-HT3A, CBD acts, similar to THC and AEA, as non-competitive allosteric inhibitor [123-126]. 5-HT3 receptors are further inhibited by eucalyptol (1,8-cineole) and linalool, terpenes that occur also in hemp [127]. CBDA binds weakly to 5-HT5A that mediates psychiatric effects [128].
ADENOSINE RECEPTORS
Adenosine A1 receptor (A1A)
Agonists protect against reperfusion injury, are neuro- and cardioprotective/ anti-arrhythmic, protect against ischemia/ reperfusion-induced ventricular arrhythmias following coronary artery occlusion and decrease hypoxic brain damage after occlusion (stroke). Further on, activation reduces glutamate release (as in seizures, excitotoxicity and oxidative stress). A1A is involved in wound healing, anti-nociception and fibrosis [101,131]. Both, A1A and A2A, cross-talk with CB2.
Adenosine A2 receptor (A2A)
A2A receptors mediate glutamate uptake and neuropathic pain; they are neuroprotective, antinociceptive/ anti-inflammatory; reduce glutamate-, IL-6, TNFa-, COX-2, iNOS-release and attenuate brain damage in neurodegenerative disorders, inflammation of lung and retina, in vitro and in vivo. Agonists promote also angiogenesis, macrophage expression, collagen production and downregulate matrix metalloproteinase (MMP 9, 2, 14), which are involved in collagen breakdown (important in cancer), and increase Hepatic Stellate Cell (HSC) proliferation [132-135].
GAMMA AMINO BUTYRIC ACID RECEPTORS (GABARS)
The level of enhancement seen with either CBD or 2-AG was maximal on α2-containing GABAA receptor subtypes.
GLYCINE RECEPTORS (GLYRS)
Drugs that activate glycinergic signalling by potentiating glycine receptor activity or inhibiting transporter activity are anti-inflammatory, neuroprotective and contribute to analgesia [141,142].
TRANSIENT RECEPTOR POTENTIAL CATION CHANNELS (TRPS)
Ankrin type (TRPA1)
TRPA1 is activated and desensitized by a number of cannabinoids (rank order of potency: CBC > CBD > CBN > CBDV > CBG > THCV > CBGV >THCA > CBDA > CBGA > THCVA, but also by Arachidonic Acid (AA), as well as by hemp flavonoids such as quercetin, gingerol and eugenol [125,148,149]. The presence of such phytocompounds, in addition to cannabinoids, enhances their effects on TRPA1 channels [150]. Examples for antagonists are camphor, thymol, menthol, 1,8-cineole, which can attenuate nociception, neurogenic inflammation in skin, non-histaminergic pruritus, colitis and release of vasoactive substances [151-153].
Melastatin type (TRPM8, “menthol receptor”)
Vanilloid type (TRPV), TRPV1 (Type 1, “capsaicin receptor”)
TRPV2 is activated at temperatures over 52°C. A number of cannabinoids show agonist activity (rank order of potency THC > CBD > CBGV > CBG > THCV > CBDV > CBN [125]. TRPV2 is also activated by THCA [90,94]. Similar to TRPM8, TRPV2 was found over-expressed in cancer cell lines [155].
TRPV3 is activated by temperature of 33°C to 39°C. It is activated / rapidly desensitized by CBD, but also by THC, THCV and CBC [90,100,160,161]. CBDA is a very weak activator. A number of monoterpenes also can activate TRPV3 such as (+) borneol, thymol, camphor, eugenol, menthol and carvacrol [151,162]. TRPV3 is predominantly expressed in skin and can form hetero-tetrameric channels with TRPV1; it is upregulated in the skin of patients with atopic dermatitis, chronic itch (e.g., Olmsted syndrome) and hair loss [163].
TRPV4 is activated at temperatures between 27°C and 42°C, mechanical forces (e.g., cell swelling), but also by THCV (THCV> CBD), CBG, CBGA, CBGV, THC and CBN, by AA, some endocannabinoids (AEA, 2-AG) and by apigenin [100,151]. It is a crucial regulator in the progression of fibrosis including myocardial fibrosis, cystic-, pulmonary-, hepatic- and pancreatic fibrosis [164]. Further on, it plays a role in tumour angiogenesis, inflammation of airways and vascular relaxation induced by P450 epoxygenase-dependent metabolites of endocannabinoids [165,166].
Interestingly, TRPV2, TRPV3 and TRPV4 are so far the only TRP channels that THC is able to activate.
NUCLEAR TRANSCRIPTION FACTOR KAPPA B (NF-KB), NUCLEAR FACTOR ERYTHROID-DERIVED 2 (NRF2)
Concentrations of PPARg agonists such as CBD matters: low concentrations promote cell survival, whereas high increase cell loss. Beneficial effects have been reported in a number of animal brain-inflammation models, including ischemic stroke [169,170].
In these respects, CBD differs from THC: in vitro, CBD altered the expression of over ten times more genes than THC. From the 1298 transcripts found to be differentially regulated by the treatments, 680 gene probe sets were up-regulated by CBD, only 58 by THC. On the other hand, 524 gene products were down-regulated by CBD but only 36 by THC. CBD-specific gene expression profile showed changes associated particularly with oxidative stress [171].
NF-κB regulates the transcription of genes involved in inflammation and pain (expression of genes of pro-inflammatory cytokines such as IL-1b, IL-6, TNFa, expression of inflammatory mediators such as COX-2, inducible nitric oxide synthase (iNOS) but also in lipid transport/storage and cell proliferation [172,173]. NF-κB is induced by harmful stimuli including high glucose levels, and is commonly elevated in older subjects. Suppression has therefore a high potential for the prevention and management of chronic diseases.
NF-κB is inhibited, directly or indirectly via agonists of PPARg, such as CBD, CBC, CBG, THCA, THC (but not THCV), endogenous compounds such as AEA, 2-AG, PEA, AA, as well as by a number of terpenes that occur in hemp such as a/ß-amyrin, ß-caryophyllene, a-pinene, borneol, eugenol, a-humulene, D-limonene, ß-myrcene, nerolidol, and hemp-flavonoids such as apigenin, genistein, kaempferol, luteolin, myricetin, naringenin, orientin, rutin, vitexin and quercetin [90,98,173,174]. Other activation-inhibitors are vitamin E and linoleic acid. Quercetin has specific protective effect on Chronic Prostatitis/Chronic Pelvic Pain Syndrome (CP/CPPS), a common disease of urology, which is mediated by anti-inflammation, anti-oxidation, and at least partly through NF-κB and MAPK signalling pathways in vivo [175].
Nrf2 is another important transcription factor that is targeted by CBD and other hemp components; it controls genes encoding proteins formed in response to oxidative stress such as in stroke, cardio-vascular disorders, chronic liver disease or excito-neurotoxicity, in contrast to NF-kB that controls mainly inflammatory proteins. Nrf-2 and PPARg regulate each other but also the nuclear factors, NF-kB and Nrf2, seem to interact [131]. Nrf2 controls phase II detoxifying enzymes and transporters [176]. Nrf2 is over-expressed in several types of human cancer, notably of the lung, breast, ovary, prostate, pancreas, colon and skin [177].
Agonists such as CBD enhance the expression of Nrf2 target genes. A number of polyphenols/ flavonoids that occur in hemp are also agonists, e.g., apigenin, genistein, kaempferol, luteolin, lutein, naringenin, quercetin [178].
ENDOCANNABINOIDS
FATTY ACID AMID HYDROLASE (FAAH)
FAAH inhibition increases the basal levels of AEA and of other ligands and therefore an interaction with targets such as CB1, GABAA, TRPV1 and PPARgamma; it also antagonises the function of AChRs and 5-HT1A. It may be speculated that this adds to restore indirectly a dysregulated endocannabinoid system, thereby enhancing hippocampal neurogenesis, possibly also learning and memory [100]. In addition, inhibition of FAAH has anxiolytic and neuroprotective effects; it is anti-convulsant, anti-inflammatory and anti-nociceptive (through interaction of a.m. ligands with CB1 and PPARs). Interestingly, FAAH levels are altered in obese subjects [185].
Further to FAAH, Lipoxygenases (LOX) are able to degrade AEA as well. Their inhibition (e.g., by CBD, THC) also enhances the interaction of AEA and of other endocannabinoids with a large number of targets. Lipoxygenases (5-LOX, 12-LOX, 15-LOX) are known to be crucial enzymes in the biosynthesis of pro-inflammatory prostaglandin E2 and leukotrienes using not only AEA and 2-AG but also AA as substrate [186]. Some of the degradation products of AEA have biological activities of their own, but their impact on biological processes within the ECS is actually not sufficiently known.
Other enzymes that degrade AEA are Cyclooxygenase-2 (COX-2) and some Cytochrome P450 (Cyt P450) enzymes (CYP3A4). COX-2 is inhibited by Cannabidiolic Acid (CBDA), the parent compound of CBD, but not by CBD itself; MAGL inactivates AEA also to some extent [96].
CBD is a moderate inhibitor of FAAH but more potent than other phytocannabinoids. Of 11 pure cannabinoids tested (CBC, CBD, CBG, CBN, CBDA, CBGA, CBDV, CBGV, THCA, THCV, THCVA) CBD was relatively the most potent inhibitor [101,150]. It is also an inhibitor of AEA-uptake/transport. A number of flavonoids that occur in hemp, also inhibit FAAH (genistein, kaempferol > apigenin ≈ quercetin > luteolin) [187].
MONOACYL GLYCEROLLIPASE (MAGL)
So far, of the cannabinoids tested, only THCV inhibits 2-AG degradation by MAGL. CBD inhibits 2-AG degradation via LOX but not via COX or MAGL. Other Cannabinoids (CBG, CBC and THCA) exert effects only at high concentrations [100,101,125].
Among terpenes, only the triterpenes pristimerin, a-amyrin, b-amyrin and euphol have been reported to inhibit 2-AG hydrolysis by MAGL; in addition, ß-amyrin can inhibit ABHD6 and ABHD12 [97,183].
Inhibition of MAGL is neuroprotective, attenuates neuro-inflammation, lowers amyloid-β levels and plaques (with a potential use in Alzheimer’s disease); this results in reduced activity in the prostaglandin-mediated pro-inflammatory signalling cascades, and improves learning and memory deficits. MAGL is over-expressed in aggressive human cancer cells. MAGL levels decrease with age and are, similar to FAAH, down regulated in obese subjects [194,195].
In conclusion, a countless number of interactions probably occur after administration of Cannabis (hemp) products. Some but not all phytocompounds may act synergistically; the overall effect on the organism depends on the profile of these compounds and their concentrations but also on the health state of the organism. Likely the “entourage effect” is therefore not always the same and depends primarily on the profile of phytocompounds. Whereas the pharmacological effects of pure CBD have been extensively studied and are well described with generally consistent results, effects observed with genuine hemp (Cannabis) extracts vary and lack systematic investigations. In consequence, pharmacological and clinical effects are not predictable as the profile as well as the concentration of phytocompounds markedly differs and is rarely sufficiently characterised. In fact, each hemp (Cannabis) product must be considered to be unique. Research so far fails in most cases adequately to characterise the study material(s) beyond the content of the decarboxylated main cannabinoids CBD and THC. Unfortunately, investigations on well characterised cultivars as well as genuine extracts are still in their infancy, such that systematic comparisons between different, well characterised products and pure cannabinoids are missing. For research purposes it is absolutely necessary to more fully characterise the phytocompound constituents of formulations and extracts, rather than simply list their CBD- and THC-concentrations. As a minimum, the percentages of the respective cannabinoid acids, eventually including the content of Cannabigerol (CBG) as the precursor substance of CBD and THC, as well as an analytical profile of the most prominent terpenes and phenolic compounds should be included in order to enable comparisons and improve further research. With a better understanding it might be possible to select cultivars and/or extracts for specific clinical indications or health benefits.
REFERENCES
- Aizpurua-Olaizola O, Soydaner U, Öztürk E, Schibano D, Simsir Y, et al. (2016) Evolution of cannabinoid and terpene content during the growth of Cannabis sativa plants from different chemotypes. J Nat Prod 79: 324-331.
- Calvi L, Pentimalli D, Panseri S, Giupponi L, Gelmini F, et al. (2018) Comprehensive quality evaluation of medical Cannabis sativa L. inflorescence and macerated oils based on HS-SPME coupled to GC-MS and LC-HRMS (q-exactive orbitrap®) approach. J Pharm Biomed Anal 150: 208-219.
- Fischedick JT, Hazekamp A, Erkelens T, Choi YH, Verpoorte R (2010) Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes. Phytochemistry 71: 2058-2073.
- Mediavilla V, Steinemann S (1997) Essential oil of Cannabis sativa L. strains. J Int Hemp Association 4: 80-82.
- de Meijer EPM, Hammond KM (2005) The inheritance of chemical phenotype in Cannabis sativa L. (II): Cannabigerol predominant plants. Euphytica 145: 189-198.
- Adams TB, Taylor SV (2010) Safety evaluation of essential oils: a constituent-based approach. In: Baser KHC, Buchbauer G (eds). Handbook of Essential Oils: Science, Technology, and Applications. CRC Press: Boca Raton, FL, pp. 185-208.
- Carlini EA, Cunha JM (1981) Hypnotic and antiepileptic effects of cannabidiol. J Clin Pharmacol 21: 417-427.
- Fasinu PS, PhillipsS, Elsohly MA, Walker LA (2016) Current status and prospects for cannabidiol preparations as new therapeutic agents. Pharmacotherapy 36: 781-796.
- Aizpurua-Olaizola O, Omar J, Navarro P, Olivares M, Etexbarria N, et al. (2014) Identification and quantification of cannabinoids in Cannabis sativa L. plants by high performance liquid chromatography-mass spectrometry. Anal Bioanal Chem 406: 7549-7560.
- Fournier G, Beherec O, Bertucelli S (2004) Santhica 23 et 27: deux variétés de chanvre (Cannabis sativa L.) sans Δ-9-THC. [Santhica 23 and 27: two varieties of hemp (Cannabis sativa L.) without Δ-9-THC.]. Annales de Toxicologie Analytique 16: 128-132.
- Adams R, Hunt M, Clark JH (1940) Structure of cannabidiol, a product isolated from the marihuana extract of Minnesota wild hemp. J Am Chem Soc 62: 196-200.
- Cunha JM, Carlini EA, Pereira AE, Pimentel C, Gagliardi R, et al. (1980) Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology 21: 175-185.
- Jones NA, Hill AJ, Smith I, Bevan SA, Williams CM, et al. (2010) Cannabidiol displays antiepileptiform and antiseizure properties in vitro and in vivo. J Pharmacol Exp Ther 332: 569-577.
- Whalley BJ, Wilkinson JD, Williamson EM, Constanti A (2004) A novel component of Cannabisextract potentiates excitatory synaptic transmission in rat olfactory cortex in vitro. Neurosci Lett 365: 58-63.
- Iffland K, Grotenhermen F (2017) An update on safety and side effeccts of cannabidiol: a review of clinical data and relevant animal studies. Cannabis Cannabinoid Res 2: 139-154.
- Zhornitsky S, Potvin S (2012) Cannabidiol in humans-The quest for therapeutic targets. Pharmaceuticals 5: 529-552.
- Laprairie RB, Bagher AM, Kelly ME, Denovan-Wright EM (2015) Cannabidiol is a negative allosteric modulator of the type 1 cannabinoid receptor. Br J Pharmacol 172: 4790-4805.
- Martinez-Pinilla E, Varani K, Reyes-Resina I, Angelats E, Vincenzi F, et al. (2017) Binding and signalling studies disclose a potential allosteric site for cannabidiol in cannabinoid CB2 receptors. Front Pharmacol 8: 744.
- Tham M, Yilmaz O, Alaverdashvili M, Kelly MEM, Denovan-Wright EM, et al. (2018) Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors. Br J Pharmacol 176: 1455-1469.
- Kamal BS, Kamal F, Lantela DE (2018) Cannabis and the anxiety of fragmentation-A systems approach for finding an anxiolytic Cannabis chemotype. Front Neurosci 12: 730.
- Nuutinen T (2018) Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus. Eur J Med Chem 157: 198-228.
- Russo EB (2019) The case for the entourage effect and conventional breeding of clinical Cannabis: no “strain,” no gain. Front Plant Sci 9: 1969.
- Andre CM, Hausman J-F, Guerriero G (2016) Cannabis sativa: The plant of the thousand and one molecules. Front Plant Sci 7: 19.
- Casano S, Grassi G, Martini V, Michelozzi M (2011) Variations in terpene profiles of different strains of Cannabis sativa L. Acta Horticulturae 925: 115-121.
- Lynch RC, Vergara D, Tittes S, White K, Schwartz CJ, et al. (2016) Genomic and chemical diversity in Cannabis. Critical Reviews in Plant Sciences 35: 349-363.
- Pellati F, Brighenti V, Sperlea J, Marchetti L, Bertelli B, et al. (2018) New Methods for the Comprehensive Analysis of Bioactive Compounds in Cannabis sativa L. (hemp). Molecules 23: 2639.
- da Fonesca CO, Simao M, Lins IR, Caetano RO, Futuro D, et al. (2011) Efficacy of monoterpene perillyl alcohol upon survival rate of patients with recurrent glioblastoma. J Cancer Res Clin Oncol 137: 287-293.
- Shojaei S, Kiumarsi A, Moghadam AR, Alizadeh J, Marzban H, et al. (2014) Perillyl alcohol (monoterpene alcohol), limonene. Enzymes 36: 7-32.
- Gulluni N, Re T, Loiacono I, Lanzo G, Gori L, et al. (2018) Cannabis essential oil: a preliminary study for the evaluation of the brain effects. Evidence-Based Complementary and Alternative Medicine 2018: 1709182.
- Cho KS, Lim YR, Lee K, Lee J, Lee JH, et al. (2017) Terpenes from forests and human health. Toxicol Res 33: 97-106.
- Russo EB (2011) Taming THC: potential Cannabis synergy and phytocannabinoid-terpenoid entourage effects. Brit J Pharmacol 163: 1344-1364.
- Clark MN (1978) A study of infraspecific flavonoid variation of Cannabis sativa L. (cannabaceae). Thesis University of British Columbia, Vancouver, Canada, pg no: 94.
- Vanhoenacker G, Van Rompaey P, De Keukeleire D, Sandra P (2002) Chemotaxonomic features associated with flavonoids of cannabinoid-free Cannabis (Cannabis sativa subsp. sativa L.) in relation to hops (Humulus lupulus L.). Nat Prod Lett 16: 57-63.
- Alam MN, Almoyad M, Huq F (2018) Polyphenols in Colorectal Cancer: Current State of Knowledge including Clinical Trials and Molecular Mechanism of Action. Biomed Res Int 2018: 29.
- Hua X, Yu L, You R, Yang Y, Liao J, et al. (2016) Association among Dietary Flavonoids, Flavonoid Subclasses and Ovarian Cancer Risk: A Meta-Analysis. PLoS one 11: 0151134.
- Aziz N, Kim MY, Cho JY (2018) Anti-inflammatory effects of luteolin: a review of in vitro, in vivo, and in silico studies. J Ethnopharmacol 225: 342-358.
- Fernandez-Rojas B, Gutierrez-Venegas G (2018) Flavonoids exert multiple periodontic benefits including anti-inflammatory, periodontal ligament-supporting, and alveolar bone-preserving effects. Life Sci 209: 435-454.
- Hillig KW (2004) A chemotaxonomic analysis of terpenoid variation in Cannabis. Biochemical Systematics and Ecology 32: 875-891.
- Mechtler K, Bailer J, de Hueber K (2004) Variations of Δ 9THC content in single plants of hemp varieties. Industrial Crops and Products 19: 19-24.
- Namdar D, Charuv D, Ajjampura A, Mazuz M, Ion A, et al. (2019) LED lighting affects the composition and biological activity of Cannabis sativa secondary metabolites. Industrial Crops and Products 132: 177-185.
- Valle JR, Vieira JE, Aucelio JG, Valio IF (1978) Influence of photoperiodism on cannabinoid content of Cannabis sativa L. Bull Narc 30: 67-68.
- Lydon J, Teramura AH, Coffman CB (1987) UV-B radiation effects on photosynthesis, growth and cannabinoid production of two Cannabis sativa chemotypes. Photochem Photobiol 46: 201-206.
- Potter DJ (2014) A review of the cultivation and processing of Cannabis (Cannabis sativa L.) for production of prescription medicines in the UK. Drug Test Anal 6: 31-38.
- Vanhove W (2014) The agronomy and economy of illicit indoor Cannabis cultivation. Ghent University, Ghent, Belgium.
- Calzolari D, Magagnini G, Lucini L, Grassi G, Appendino GB, et al. (2017) High added-value compounds from Cannabis threshing residues. Indust Crops & Products 108: 558-563.
- Sikora V, Berenji J, Latkovic D (2011) Influence of agroclimatic conditions on content of main cannabinoids in industrial hemp (Cannabis sativa L.). Genetika 43: 449 -456.
- Nissen L, Zatta A, Stefanini I, Grandi S, Sgorbati B, et al. (2010) Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 81: 413-419.
- Bocsa I, Mathé P, Hangyel L (1997) Effect of nitrogen on tetrahydrocannabinol (THC) content in hemp (Cannabis sativa L.) leaves at different positions. J Int Hemp Association 4: 80-81.
- Coffman CB, Gentner WA (1975) Cannabinoid profile and elemental uptake of Cannabis sativa L. as influenced by soil characteristics. Agronomy J 67: 491-497.
- Hanus L, Dostalova M (1994) The effect of soil fertilization on the formation and the amount of cannabinoid substances in Cannabis sativa L. in the course of one vegetation period. Acta Univ Palacki Olomuc Fac Med 138: 11-15.
- Taoufik B, Hamid S, Aziz EB, Abdellah F, Seddik S, et al. (2017) Comparative study of three varieties of Cannabis sativa L. cultivate in different region of Morocco. Int J Pharmacognosy Phytochem Res 9: 643-653.
- Pavlovic R, Nenna G, Calvi L, Panseri S, Borgonovo G, et al. (2018) Quality Traits of “Cannabidiol Oils”: Cannabinoids Content, Terpene Fingerprint and Oxidation Stability of European Commercially Available Preparations. Molecules 23: 1230.
- Richins RD, Rodriguez-Uribe L, Lowe K, Ferral R, O’Connell MA (2018) Accumulation of bioactive metabolites in cultivated medical Cannabis. PLoS one 13: 0201119.
- Ross SA, ElSohly MA (1996) The volatile oil composition of fresh and air-dried buds of Cannabis sativa. J Nat Prod 59: 49-51.
- Perrotin-Brunel H, Kroon MC, van Roosmalen MJE, van Spronsen J, Peters CJ, et al. (2010) Solubility of non-psychoactive cannabinoids in supercritical carbon dioxide and comparison with psychoactive cannabinoids. J Supercritical Fluids 55: 603-608.
- Wang M, Wang YH, Avula B, Radwan MM, Wanas AS, et al. (2016) Decarboxylation study of acidic cannabinoids: A novel approach using ultra-high-performance supercritical fluid chromatography/photodiode array-mass spectrometry. Cannabis Cannabinoid Res 1: 262-271.
- Lewis MM, Yang Y, Wasilewski E, Clarke HA, Kotra LP (2017) Chemical profiling of medical Cannabisextracts. ACS Omega 2: 6091-6103.
- El Gueder D, Maatouk M, Kalboussi Z, Daouefi Z, Chaaban H, et al. (2018) Heat processing effect of luteolin on anti-metastasis activity of human glioblastoma cells U87. Environ Sci Pollut Res Int 25: 36545-36554.
- Asif M, Khodadadi E (2013) Medicinal uses and chemistry of flavonoid contents of some common edible tropical plants. J Paramedical Sciences 4: 119-138.
- Krizek T, Bursova M, Horsley R, Kucha M, Tuma P, et al. (2018) Menthol-based hydrophobic deep eutectic solvents: Towards greener and efficient extraction of phytocannabinoids. J Cleaner Production 193: 391-396.
- Sexton M, Shelton K, Haley P, West M (2018) Evaluation of Cannabinoid and Terpenoid Content: Cannabis Flower Compared to Supercritical CO2 Concentrate. Planta Med 84: 234-241.
- Gilbert AN, DiVerdi JA (2018) Consumer perceptions of strain differences in Cannabis aroma. PLos One 13: 0192247.
- Hazekamp A, Tejkalova K, Papadimitriou S (2016) Cannabis: From cultivar to chemovar II-A metabolomics approach to Cannabis classification. Cannabis and Cannabinoid Research 1: 202-215.
- Carlini EA, Karniol IG, Renault PF, Schuster CR (1974) Effects of marihuana in laboratory animals and in man. Br J Pharmacol 50: 299-309.
- Pickens JT (1981) Sedative Sedative activity of Cannabis in relation to its delta’-trans-tetrahydrocannabinol and cannabidiol content. Br J Pharmacol 72: 649-656.
- Bloomfield MAP, Hindocha C, Green SF, Wall MB, Lee R, et al. (2019) The neuropsychopharmacology of Cannabis: A review of human imaging studies. Pharmacol Ther 195: 132-161.
- Atakan Z (2012) Cannabis, a complex plant: different compounds and different effects on individuals. Ther Adv Psychopharmacol 2: 241-254.
- Brunt TM, van Genugten M, Höner-Snoeken K, van de Velde MJ, Niesink RJ (2014) Therapeutic satisfaction and subjective effects of different strains of pharmaceutical-grade Cannabis. J Clin Psychopharmacol 34: 344-349.
- Colizzi M, Bhattacharyya S (2017) Does Cannabis Composition Matter? Differential Effects of Delta-9-tetrahydrocannabinol and Cannabidiol on Human Cognition. Curr Addict Rep 4: 62-74.
- Corral VL (2001) Differential effects of medical marijuana based on strain and route of administration: a three-year observational study. J Cannabis Therapeutics 1: 43-59.
- Solowij N, Broyd S, Greenwood LM, van Hell H, Martelozzo D, et al. (2019) A randomised controlled trial of vaporised Δ9-tetrahydrocannabinol and cannabidiol alone and in combination in frequent and infrequent Cannabis users: acute intoxication effects. Eur Arch Psychiatry Clin Neurosci 269: 17-35.
- Zuardi AW, Hallak JE, Crippa JA (2012) Interaction between cannabidiol (CBD) and D9-tetrahydrocannabinol (THC): In?uence of administration interval and dose ratio between the cannabinoids. Psychopharmacology (Berl) 219: 247-249.
- Iskedjian M; Bereza B, Gordon A, Piwko C, Einarson TR (2007) Meta-analysis of Cannabis based treatments for neuropathic and multiple sclerosis-related pain. Curr Med Res Opin 23: 17-24.
- Ceremuga TE, McClellan CB, Green XC, Heber BE, Jolly ML, et al. (2017) Investigation of the anxiolytic and antidepressant effects of eucalyptol (1,8-cineole), a compound from eucalyptus, in the adult male Sprague-Dawley rat. AANA Journal 85: 277-284.
- Choudhary N, Siddiqui MB, Bi S, Khatoon S (2014) Variation in preliminary phytochemicals screening of Cannabis sativa L. leaf, stem and root. Int J Pharmacognosy 1: 516-519.
- Karim N, Khan I, Khan H, Ayub B, Abdel-Halim H et al. (2018) Anxiolytic potential of natural flavonoids. SM J Steroids Horm 1-10.
- Eichler M, Spinedi L, Unfer-Grauwiler S, Bodmer M, Surber C, et al. (2012) Heat exposure of Cannabis sativa extracts affects the pharmacokinetic and metabolic profile in healthy male subjects. Planta Med 78: 686-691.
- Keserwani K, Gupta R, Mukerjee A (2013) Bioavailability enhancers of herbal origin: an overview. Asian Pac J Trop Biomed 3: 253-266.
- Chavhan SA, Shinde SA, Gupta HN (2018) Current trends on natural bioenhancers: a review. Int J Pharmacogn Chinese Med 2: 1-13.
- Anjali MR, Chandran M, Krishnakumar K (2017) Piperine as a bioavailability enhancer: a review. Asian J Pharmaceutical Analysis and Medicinal Chemistry 5: 44-48.
- Domitrovic R, Potocnjak I (2015) A comprehensive overview of hepatoprotective natural compounds: mechanism of action and clinical perspectives. Arch Toxicol 90: 39-79.
- Nahler G, Jones TM (2018) Pure cannabidiol versus cannabidiol-containing extracts: distinctly different multi-target modulators. Altern Complement Integr Med 4: 048.
- Bih CI, Chen T, Nunn AVM, Bazelot M, Dallas M, et al. (2015) Molecular targets of Cannabidiol in Neurological Disorders. Neurotherapeutics 12: 699-730.
- Zendulka O, Dovrtelová G, Noskova K, Turjap K, Sulcova M, et al. (2016) Cannabinoids and cytochrome P450 interactions. Current Drug Metabolism 17: 206-226.
- Consroe P, Kennedy K, Schram K (1991) Assay of plasma cannabidiol by capillary gas chromatography/ion trap mass spectroscopy following high-dose repeated daily oral administration in humans. Pharmacol Biochem Behav 40: 517-522.
- Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, et al. (2012) Cannabidiol enhances anandamide signalling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry 2: 94.
- Elmes MW, Kaczocha M, Berger WT, Leung KN, Ralph PB, et al. (2015) Fatty acid binding proteins (FABPs) are intracellular carriers for D9-tetrahydrocannabinol (THC) and cannabidiol (CBD). J Biol Chem 290: 8711-8721.
- Komori T, Fujiwara R, Tanida M, Nomura J, Yokoyama MM (1995) Effects of citrus fragrance on immune function and depressive states. Neuroimmunomodulation 2: 174-180.
- Olson H, Betton G, Robinson D, Thomas K, Monro A, et al. (2000) Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul Toxicol Pharmacol 32: 56-67.
- Morales P, Hurst DP, Reggio PH (2017) Molecular Targets of the Phytocannabinoids: A Complex Picture. Prog Chem Org Nat Prod 103: 103-131.
- Blair RE, Deshpande LS, Sombati S, Elphick MR, Martin BR, et al. (2009) Prolonged exposure to WIN55,212-2 causes downregulation of the CB1 receptor and the development of tolerance to its anticonvulsant effects in the hippocampal neuronal culture model of acquired epilepsy. Neuropharmacology 57: 208-218.
- Laurikainen H, Tuominen L, Tikka M, Merisaari H, Armio RL, et al. (2019) Sex difference in brain CB1 receptor availability in man. Neuroimage 184: 834-842.
- McPartland JM, MacDonald C, Young M, Grant PS, Furkert DP, et al. (2017) Affinity and efficacy studies of tetrahydrocannabinolic acid A at cannabinoid receptor types one and two. CannabisCannabinoid Res 2: 87-95.
- Moreno-Sanz G (2016) Can you pass the acid test? Critical review and novel therapeutic perspectives of D9-tetrahydrocannabinolic acid A. Cannabis Cannabinoid Res 1: 124-130.
- Russo EB (2018) Cannabis therapeutics and the future of neurology. Front Integr Neurosci 12:51.
- Anavi-Goffer S, Baillie G, Irving AJ, Gertsch J, Greig IR, et al. (2012) Modulation of L-α-lysophosphatidylinositol/GPR55 mitogen-activated protein kinase (MAPK) signaling by cannabinoids. J Biol Chemistry 287: 91-104.
- Chicca A, Marazzi J, Gertsch J (2012) The antinociceptive triterpene β-amyrin inhibits 2-arachidonoylglycerol (2-AG) hydrolysis without directly targeting cannabinoid receptors. Br J Pharmacol 167: 1596-608.
- da Silva KA, Paszcuk AF, Passos GF, Silva ES, Bento AF, et al. (2011) Activation of cannabinoid receptors by the pentacyclic triterpene α,β-amyrin inhibits inflammatory and neuropathic persistent pain in mice. Pain 152: 1872-1887.
- Russo EB (2016) Current Therapeutic Cannabis Controversies and Clinical Trial Design Issues. Front Pharmacol 7: 309.
- Di Marzo V, Piscitelli F (2015a) The endocannabinoid system and its modulation by phytocannabinoids. Neurotherapeutics 12: 692-698.
- McPartland JM, Duncan M, Di Marzo V, Pertwee RG (2015) Are cannabidiol and Δ(9) -tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review A systematic review. Br J Pharmacol 172: 737-753.
- Pertwee RG (2008) The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br J Pharmacol 153: 199-215.
- Javid FA, Phillips RM, Afshinjavid S, Verde R, Ligresti A (2016) Cannabinoid pharmacology in cancer research: A new hope for cancer patients? European J Pharmacol 775: 1-14.
- Velasco G, Hernandez-Tiedra S, Davila D, Lorente M (2016) The use of cannabinoids as anticancer agents. Prog Neuropsychopharmacol Biol Psychiatry 64: 259-266.
- Ligresti A, De Petrocellis L, Di Marzo V (2016) From phytocannabinoids to cannabinoid receptors and endocannabinoids: pleiotropic physiological and pathological roles through complex pharmacology. Physiol Rev 96: 1593-1659.
- Zhou L, Zhou S, Yang P, Tian Y, Feng Z, et al. (2018) Targeted inhibition of the type 2 cannabinoid receptor is a novel approach to reduce renal fibrosis. Kidney Int 94: 756-772.
- Zimmer A (2016) A collaboration investigating endocannabinoid signalling in brain and bone. J Basic Clin Physiol Pharmacol 27: 229-235.
- Liu B, Song S1 Ruz-Maldonado I, Pingitore A, Huang GC, et al. (2016) GPR55-dependent stimulation of insulin secretion from isolated mouse and human islets of Langerhans. Diabetes Obes Metab 18: 1263-1273.
- Tuduri E, Lopez M, Dieguez C, Nadal A, Nogueiras R (2017) GPR55 and the regulation of glucose homeostasis. Int J Biochem Cell Biol 88: 204-207.
- Morales P, Isawi I, Reggio PH (2018) Towards a better understanding of the cannabinoid-related orphan receptors GPR3, GPR6, and GPR12. Drug Metab Rev 50: 74-93.
- Murillo-Rodriguez E, Arankowsky-Sandoval G, Rocha NB, Amante RB, et al. (2018) Systemic Injections of Cannabidiol Enhance Acetylcholine Levels from Basal Forebrain in Rats. Neurochem Res Jun 43: 1511-1518.
- Miyazawa M, Yamafuji C (2005) Inhibition of acetylcholinesterase activity by bicyclic monoterpenoids. J Agric Food Chem 53: 1765-1768.
- Zarrad K, Laarif A, Ben Hamouda A, Chaieb I, Jemaa MB (2017) Anticholinesterase potential of monoterpenoids on the whitefly Bemisia tabaci and their kinetic studies. J Agr Sci Tech 19: 643-652.
- Howes M-JR, Houghton PJ (2009) Acetylcholinesterase inhibitors of natural origin. Int J Biomedical Biopharmaceutical Sciences 67-86.
- Dos Santos TC, Gomes TM, Pinto BAS, Camara AL, Paes AMA (2018) Naturally occurring acetylcholinesterase inhibitors and their potential use for Alzheimer's disease therapy. Front Pharmacol 9:1192.
- Butt C, Alptekin A, Shippenberg T, Oz M (2008) Endogenous cannabinoid anandamide inhibits nicotinic acetylcholine receptor function in mouse thalamic synaptosomes. J Neurochem 105: 1235-1243.
- Mahgoub M, Keun-Hang SY, Sydorenko V, Ashoor A, Kabbani N, et al. (2013) Effects of cannabidiol on the function of α7-nicotinic acetylcholine receptors. Eur J Pharmacol 720: 310-319.
- Campos AC, Fogaca MV, Scarante FF, Joca SRL, Sales AJ, et al. (2017) Plastic and neuroprotective mechanisms involved in the therapeutic effects of cannabidiol in psychiatric disorders. Front Pharmacol 8: 269.
- De Gregorio D, McLaughlin RJ, Posa L, Ochoa-Sanchez R, Enns J, et al. (2019) Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain 160: 136-150.
- Cascio MG, Gauson LA, Stevenson LA, Ross RA, Pertwee RG (2010) Evidence that the plant cannabinoid cannabigerol is a highly potent a2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist. Br J Pharmacol 159: 129-141.
- Rock EM, Goodwin JM, Limebeer CL, Breuer A, Pertwee RG et al. (2011) Interaction between non-psychotropic cannabinoids in marihuana: effect of cannabigerol (CBG) on the anti-nausea or anti-emetic effects of cannabidiol (CBD) in rats and shrews. Psychopharmacology (Berl) 215: 505-512.
- Bolognini D, Rock EM, Cluny NL, Cascio MG, Limebeer CL, et al. (2013) Cannabidiolic acid prevents vomiting in Suncus murinus and nausea-induced behaviour in rats by enhancing 5-HT1A receptor activation. Br J Pharmacol 168: 1456-1470.
- Davies PA (2011) Allosteric modulation of the 5-HT3 receptor. Curr Opin Pharmacol 11: 75-80.
- Russo EB, Burnett A, Hall B, Parker KK (2005) Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res 30: 1037–1043.
- Soderstrom K, Soliman E, Van Dross R (2017) Cannabinoids Modulate Neuronal Activity and Cancer by CB1 and CB2 Receptor-Independent Mechanisms. Frontiers in Pharmacol 8: 720.
- Yang KH, Galadri S, Isaev D, Petroianu G, Shippenberg TS, et al. (2010) The nonpsychoactive cannabinoid cannabidiol inhibits 5-hydroxytryptamine3A receptor-mediated currents in Xenopus laevis oocytes. J Pharmacol Exp Ther 333: 547-554.
- Jarvis GE, Barbosa R, Thompson AJ (2016) Noncompetitive Inhibition of 5-HT3 Receptors by Citral, Linalool, and Eucalyptol Revealed by Nonlinear Mixed-Effects Modeling. J Pharmacol Exp Ther 356: 549-562.
- http://www.icrs.co/SYMPOSIUM.2014/ICRS2014.PROGRAMME.pdf
- Maione S, Piscitelli F, Gatta L, Vita D, de Petrocellis L, et al. (2011) Non-psychoactive cannabinoids modulate the descending pathway of antinociception in anaesthetized rats through several mechanisms of action. Br J Pharmacol 162: 584-596.
- Gonca E, Darici F (2015) The effect of cannabidiol on ischemia/reperfusion-induced ventricular arrhythmias: the role of adenosine A1 receptors. J Cardiovasc Pharmacol Ther 20: 76-83.
- Campos AC, Fogaca MV, Sonego AB, Guimaraes FS (2016) Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacological Res 112: 119-127.
- Park HM, Lee JH, Yaoyao J, Jun HJ, Lee SJ (2011) Limonene, a natural cyclic terpene, is an agonistic ligand for adenosine A(2A) receptors. Biochem Biophys Res Commun 404: 345-348.
- Liou GI, Auchampach JA, Hillard CJ, Zhu G, Youssufzai B, et al. Mediation of cannabidiol anti-inflammation in the retina by equilibrative nucleoside transporter and A2A adenosine receptor. Invest Ophthalmol Vis Sci 49: 5526-5531.
- Mecha M, Feliu A, Inigo PM, Mestre L, Carrillo-Salinas FJ, et al. Cannabidiol provides long-lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: a role for A2A receptors. Neurobiol Dis 59: 141-150.
- Ribeiro A, Ferraz-de-Paula V, Pinheiro ML, Vitoretti LB, Mariano-Souza DP, et al. (2012) Cannabidiol, a non-psychotropic plant-derived cannabinoid, decreases inflammation in a murine model of acute lung injury: role for the adenosine A(2A) receptor. Eur J Pharmacol 678: 78-85.
- Bakas T, van Nieuwenhuijzen PS, Devenish SO, McGregor IS, Arnold JC, et al. (2017) The direct actions of cannabidiol and 2-arachidonoyl glycerol at GABAA receptors. Pharmacol Res 119: 358-370.
- Fogaca MV, Duman RS (2019) Cortical GABAergic dysfunction in stress and depression: new insights for therapeutic interventions. Front Cell Neurosci 13: 87.
- Ruffolo G, Cifelli P, Roseti C, Thom M, van Vliet EA, et al. (2018) A novel GABAergic dysfunction in human Dravet syndrome. Epilepsia 59: 2106-2117.
- Gamlin CR, Yu WQ, Wong ROL Hoon M (2018) Assembly and maintenance of GABAergic and Glycinergic circuits in the mammalian nervous system. Neural Dev 13: 12.
- Ahrens J, Demir R, Leuwer M, de la Roche, Krampfl K, et al. (2009) The nonpsychotropic cannabinoid cannabidiol modulates and directly activates alpha-1 and alpha-1-Beta glycine receptor function. Pharmacology 83: 217-222.
- Xiong W, Cui T, Cheng K, Chen SR, Willenbring D, et al. (2012) Cannabinoids suppress inflammatory and neuropathic pain by targeting α3 glycine receptors. J Exp Med 209: 1121-1134.
- Zhang L (2016) Therapeutic Potential of Nonpsychoactive Cannabinoids by Targeting at Glycine Receptors. Open access peer-reviewed chapter.
- Nilius B, Szallasi A (2014) Transient receptor potential channels as drug targets: from the science of basic research to the art of medicine. Pharmacol Rev 66: 676-814.
- Perez de Vega MJ, Gomez-Monterrey I, Ferrer-Montiel A, Gonzalez-Muniz R (2016) Transient Receptor Potential Melastatin 8 Channel (TRPM8) modulation: cool entryway for treating pain and cancer. J Medicinal Chemistry 59: 10006-10029.
- Koivisto A, Jalava N, Bratty R, Pertovaara A (2018) TRPA1 antagonists for pain relief. Pharmaceuticals 11: 117.
- Wu Y-T, Yen S-L, Li C-F, Chan T-C, Chen T-J, et al. (2016) Overexpression of transient receptor protein cation channel subfamily A member 1, confers an independent prognostic indicator in nasopharyngeal carcinoma. J Cancer 7: 1181-1188.
- Jardin I, Lopez JJ, Diez R, Sanchez-Collado J, Cantonero C, et al. (2017) TRPs in pain sensation. Front Physiol. 8: 392.
- Bang S, Yoo S, Oh U, Hwang SW (2010) Endogenous lipid-derived ligands for sensory TRP ion channels and their pain modulation. Arch Pharm Res 33: 1509-1520.
- Nakamura T, Miyoshi N, Ishii T, Nishikawa M, Ikushiro S, et al. (2016) Activation of transient receptor potential ankyrin 1 by quercetin and its analogs. Biosci Biotechnol Biochem 80: 949-954.
- De Petrocellis L, Ligresti A, Moriello AS, Allara M, Bisogno t, et al. (2011) Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Brit J Pharmacol 163: 1479-1494.
- Kaneko Y, Szallasi A (2014) Transient receptor potential (TRP) channels: a clinical perspective. Br J Pharmacol 171: 2474-2507.
- Meotti FC, de Andrade EL, Calixto JB (2014) TRP modulation by natural compounds. Handb Exp Pharmacol 223: 1177-1238.
- Premkumar LS (2014) Transient receptor potential channels as targets for phytochemicals. ACS Chem Neurosci 5: 1117-1130.
- Lesch A, Rubil S, Thiel G (2014) Activation and inhibition of transient receptor potential TRPM3-induced gene transcription. Br J Pharmacol 171: 2645-2658.
- Rodrigues T, Sieglitz F, Bernardes GJ (2016) Natural product modulators of transient receptor potential (TRP) channels as potential anti-cancer agents. Chem Soc Rev 45: 6130-6137.
- Dussor G, Cao YQ (2016) TRPM8 and migraine. Headache 56: 1406-1417.
- Philpott HT, O’Brien M, McDougall JJ (2017) Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. Pain 158: 2442-2451.
- Mickle AD, Shepherd AJ, Mohapatra DP (2016) Nociceptive TRP channels: Sensory detectors and transducers in multiple pain pathologies. Pharmaceuticals (Basel) 9: 72.
- Panchal SK, Bliss E, Brown L (2018) Capsaicin in metabolic syndrome. Nutrients 10: 630.
- Burstein S (2015) Cannabidiol (CBD) and its analogs: a review of their effects on inflammation. Bioorg Med Chem 23: 1377-1385.
- De Petrocellis L, Orlando P, Schiano Moriello A, Aviello G, Stott C, et al. Cannabinoid actions at TRPV channels: effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol (Oxf) 204: 255-266.
- Vogt-Eisele AK, Weber K, Sherkheli MA, Vielhaber G, Panten J, et al. Monoterpenoid agonists of TRPV3. Br J Pharmacol 151: 530-540.
- Huang SM, Chung MK (2013) Targeting TRPV3 for the development of novel analgesics. Open Pain J 6: 119-126.
- Zhan L, Li J (2018) The role of TRPV4 in fibrosis. Gene 642: 1-8.
- Adapala RK, Thoppil RJ, Ghosh K, Cappelli HC, Dudley AC, et al. Activation of mechanosensitive ion channel TRPV4 normalizes tumor vasculature and improves cancer therapy. Oncogene 35: 314-322.
- Alpizar YA, Boonen B, Sanchez A, Jung C, Lopez-Requena A, et al. (2018) TRPV4 activation triggers protective responses to bacterial lipopolysaccharides in airway epithelial cells. Nat Commun 8: 1059.
- Cai W, Yang T, Liu H, Han L, Zhang K, et al. (2018) Peroxisome proliferator-activated receptor γ (PPARγ): A master gatekeeper in CNS injury and repair. Prog Neurobiol 164: 27-58.
- Pucci M, Rapino C, Di Francesco A, Dainese E, D’Addario C, et al. (2013) Epigenetic control of skin differentiation genes by phytocannabinoids. Br. J. Pharmacol 170: 581-591.
- Kozela E, Juknat A, Vogel Z (2017) Modulation of astrocyte activity by cannabidiol, a nonpsychoactive cannabinoid. Int J Mol Sci 18: 1669.
- Lafuente H, Pazos MR, Alvarez A, Mohammed N, Santos M. et al. (2016) Effects of Cannabidiol and Hypothermia on Short-Term Brain Damage in New-Born Piglets after Acute Hypoxia-Ischemia. Front Neurosci 10: 323.
- Juknat A, Rimmerman N, Levy R, Vogel Z, Kozela E (2012) Cannabidiol affects the expression of genes involved in zinc homeostasis in BV-2 microglial cells. Neurochemistry International 61: 923-930.
- Hartung JE, Eskew O, Wong T, Tchivileva IE, Oladosu FA, et al. (2015) Nuclear factor-kappa B regulates pain and COMT expression in a rodent model of inflammation. Brain Behav Immun 50: 196-202.
- Kunnumakkara AB, Sailo BL, Banik K, Harsha C, Prasad S, et al. (2018) Chronic diseases, inflammation, and spices: how are they linked? J Transl Med 16: 14.
- Nadal X, Del Rio C, Palomares B, Ferreiro-Vera C, Navarrete C, et al. (2017) Tetrahydrocannabinolic acid is a potent PPARγ agonist with neuroprotective activity. Br J Pharmacol 174: 4263-4276.
- Meng LQ, Yang FY, Wang MS, Shi BK, Chen DX, et al. (2018) Quercetin protects against chronic prostatitis in rat model through NF-κB and MAPK signaling pathways. Prostate 78: 790-800.
- Sabzichi M, Hamishehkar H, Ramezani F, Sharifi S, Tabasinezhad M, et al. (2014) Luteolin-loaded phytosomes sensitize human breast carcinoma MDA-MB 231 cells to doxorubicin by suppressing Nrf2 mediated signalling. Asian Pac J Cancer Prev 15: 5311-5316.
- Sznarkowska A, Kostecka A, Meller K, Bielawski KP (2017) Inhibition of cancer antioxidant defense by natural compounds. Oncotarget 8: 15996-16016.
- Shehzad A, Anwar MN, Zahid H, Ravinayagam V, Al-Rumaih HS, et al. (2016) Multifactorial role of flavonoids in prevention and treatment of various cancers. An Real Acad Farm 82: 297-302.
- Porter AC, Sauer JM, Knierman MD, Becker GW, Berna MJ, et al. (2002) Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J Pharmacol Exp Ther 301: 1020-1024.
- Fanelli F, Mezzullo M, Repaci A, Belluomo I, Ibarra-Gasparini D, et al. (2018) Profiling plasma N-acylethanolamine levels and their ratios as a biomarker of obesity and dysmetabolism. Mol Metab 14: 82-94.
- Fezza F, Bari M, Florio R, Talamonti E, Feole M, et al. (2014) Endocannabinoids, related compounds and their metabolic routes. Molecules 19: 17078-17106.
- Barrie N, Manolios N (2017) The endocannabinoid system in pain and inflammation: Its relevance to rheumatic disease. Eur J Rheumatol 4: 210-218.
- Tuo W, Leleu-Chavain N, Spencer J, Sansook S, Millet R, et al. (2016) Therapeutic potential of fatty acid amide hydrolase, monoacylglycerol lipase, and N-acylethanolamine acid amidase inhibitors. J Med Chem 60: 4-46.
- Björklund E, Blomqvist A, Hedlin J, Persson E, Fowler CJ (2014) Involvement of fatty acid amide hydrolase and fatty acid binding protein 5 in the uptake of anandamide by cell lines with different levels of fatty acid amide hydrolase expression: a pharmacological study. PLoS One 9: 103479.
- Cable JC, Tan GD, Alexander SP, O’Sullivan SE (2014) The effects of obesity, diabetes and metabolic syndrome on the hydrolytic enzymes of the endocannabinoid system in animal and human adipocytes. Lipids Health Dis 13:43.
- Maccarone M (2017) Metabolism of the endocannabinoid anandamide: open questions after 25 years. Front Mol Neurosci 10:166.
- Thors L, Belghiti M, Fowler CJ (2008) Inhibition of fatty acid amide hydrolase by kaempferol and related naturally occurring flavonoids. Br J Pharmacol 155: 244-252.
- Brose SA, Golpovko Sa, Golovko MY (2016) Brain 2-arachidonoylglycerol levels are dramatically and rapidly increased under acute ischemia-injury which is prevented by microwave irradiation. Lipids 51: 487-495.
- Fanelli F, Mezzullo M, Belluomo I, Di Lallo VD, Baccini M, et al. (2017) Plasma 2-arachidonoylglycerol is a biomarker of age and menopause related insulin resistance and dyslipidemia in lean but not in obese men and women. Mol Metab 6: 406-415.
- Lau BK, Vaughan CW (2014) Targeting the endogenous cannabinoid system to treat neuropathic pain. Front Pharmacol 5:28.
- Moody JS, Kozak KR, Ji C, Marnett LJ (2001) Selective oxygenation of the endocannabinoid 2-arachidonylglycerol by leukocyte-type 12-lipoxygenase. Biochemistry 40: 861-866.
- Turcotte C, Chouinard F, Lefebvre JS, Flamand N (2015) Regulation of inflammation by cannabinoids, the endocannabinoids 2-arachidonoyl-glycerol and arachidonoyl-ethanolamide, and their metabolites. J Leukoc Biol 97: 1049-1070.
- Van Zadelhoff G, van der Stelt M (2016) Oxygenation of anandamide by lipoxygenases. Methods Mol Biol 1412: 217-225.
- Di Marzo V, Stella N, Zimmer A (2015) Endocannabinoid signalling and the deteriorating brain. Nat Rev Neurosci 16: 30-42.
- Krustev E, Reid A, McDougall JJ (2014) Tapping into the endocannabinoid system to ameliorate acute inflammatory flares and associated pain in mouse knee joints. Arthritis Research & Therapy 16: 437.
Citation: Nahler G, Jones TM, Russo EB (2019) Cannabidiol and Contributions of Major Hemp Phytocompounds to the “Entourage Effect”; Possible Mechanisms. J Altern Complement Integr Med 5: 070.
Copyright: © 2019 Gerhard Nahler, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

