
Comparative Plasma Pharmacokinetics of Ziyuglycoside I from Sanguisorba Officinalis L. between Leukopenia and Normal Rats via UHPLC-MS/MS
*Corresponding Author(s):
Xu-Hua QinDepartment Of Chinese Materia Medica, School Of Pharmacy,Chengdu University Of Traditional Chinese Medicine, Chengdu, Sichuan 610075, China
Tel:+86 8303161506,
Email:jianmingwu@swmu.edu.cn
Jian-Ming Wu
Sichuan Key Laboratory Of New Drug Discovery And Drugability Evaluation, Luzhou Key Laboratory Of Bioactivity Screening In Traditional Chinese Medicine And Drugability Evalution, School Of Pharmacy, Southwest Medical University, Luzhou, Sichuan, China
Tel:+86 8303161506,
Email:qxhjsr@163.com
Abstract
Ziyuglycoside I (ZgI), one of the main active ingredients of Diyushengbai tablet made from Sanguisorba officinalis L., has been proved to relieve leukopenia. In our study, we compared the difference of pharmacokinetics of ZgI between normal and leukopenia rats.
Materials and methods
24 rats were divided into four groups, low and high dose (orally taken ZgI 5 or 20 mg/kg, respectively) control or leukopenia groups, induced by intraperitoneal injection of 70 mg/kg Cyclophosphamide (CY) twice. plasma samples were collected from orbital venous plexus at 0, 5, 10, 20, 40, 60 min, 1.5, 2, 4, 8, 12, 24, 48 h after oral administrated of ZgI, and concentrations of ZgI were analyzed by Ultra-High Performance Liquid Chromatography-tandem Mass-spectrometry (UHPLC-MS/MS).
Results
Compared with20 mg/kg normal group, peak time (Tmax) was significantly shortened (0.93 h to 0.33 h) and maximum Concentration (Cmax) was remarkably decreased (7.96 ng/L to 3.40 ng/L) in 20 mg/kg leukopenia group, on the contrary, elimination half-life (T1/2β) in it was obviously prolonged (5.02 h to 18.51 h). However, there were no clearly differences in distributed half-life (T1/2α) and area under the plasma concentration vs. time curve from zero to last sampling time (AUC0-t) between20 mg/kg leukopenia and control group (p>0.05). All above changes were similar between 5 mg/kg model and control group, except Cmax were nearly equal between them. Interestedly, there was also no evidently difference between the two leukopenia groups.
Conclusion
The pharmacokinetic process especially absorption and metabolism of ZgI were evidently influenced by leukopenia. Our study may provide guidance for clinical use of Diyushengbai tablet and development of ZgI as an agent for the treatment of leukopenia.
Keywords
INTRODUCTION
As we all known, pharmacokinetic study of a drug is an essential step for preclinical research and clinical trial and plays an important role in the process of innovative drug development and research [8]. However, there few literatures have been reported about the pharmacokinetic studies of ZgI during the past 20 years [9]. Recently years, a large number of studies have demonstrated that pharmacokinetics of drugs can be affected by many factors, including age, gender, altitude, drugs and variety of diseases, such as diabetes, Hypertension, estrogen level and so on [10-17]. Actually, drugs are used to treat diseases, and the patients are the ultimate consumers of drugs [18]. That is to say, study on drug pharmacokinetics in a disease state is more important than the normal condition and is more clinically relevant [19]. Therefore, we consider that the previous pharmacokinetic study of ZgI was not suitable for clinic as the animal model they used.
In the present study, we established an UHPLC-MS/MS method to investigate the pharmacokinetics of ZgI in a rat model of leukopenia and further explored the difference to that of the normal for the first time. This study may provide a rationale for clinical use of Diyushengbai tablet and development of ZgI as an agent for the treatment of leukopenia.
MATERIALS AND METHODS
Chemicals and reagents
Animals
Establishment of leukopenia model in rats
Instrumentation and analytical conditions
The mass spectrometer was handled in the negative pattern. Quantification was obtained using Multiple Reaction Monitoring (MRM) acquisition mode by monitoring the precursor ion to product ion transitions of m/z 765.45→m/z 603.50 for ZgI and m/z 469.15→355.10 for GA (IS). The ion spray voltage and source temperature were maintained at 3.5 kV and 400°C, respectively. DL temperature was 200°C. The collision energy for ZgI and IS were set at 51 eV and 52 eV, respectively. The atomizing gas, heating gas, and drying gas were 2.5 L/min, 8.0 L/min and 10.0 L/min, respectively.
Preparation of samples
Nine calibration samples and four Quality Control (QC) samples were prepared with appropriate amounts of the Intermediate Stock solutions of ZgI and GA (IS) in blank plasma. The concentrations of the calibration samples of ZgI in plasma were 5, 10, 20, 50, 100, 200, 500, 1000 and 2000 ng/mL. QC samples were prepared at four concentrations: 5 ng/mL (lower limit of quantification quality control, LLQC), 50 ng/mL (low quality control, LQC), 500 ng/mL (middle quality control, MQC), and 1000 ng/mL (high quality control, HQC), respectively. The IS solution (1000 ng/mL) was prepared from the intermediate solution.
All plasma samples were thawed and equilibrate at room temperature before analysis, and then centrifuged at 10000 ×g for 10 min at 4°C. 5 μL ZgI standard solution and 10 μL IS solution were added to 45 μL of plasma sample and vortexed for 30 s. Next, 200 μL acetonitrile was added to each sample followed by vortexing for 1 min. Finally, samples were centrifuged at 10000 ×g for 10 min at 4°C. 150 μL of supernatant was transferred and 10 μL sample for LC-MS analysis.
Method validation
The linearity of the method was analyzed with calibration standards at nine different concentrations. The calibration curves were constructed by plotting the peak area ratios of analyte/ARE concentrations. Curve fitting was performed by Lab Solutions LC-MS Ver.5.85 analysis. The calibration curve was accepted if the residuals had at least 75% of the calibration standards as previous described.
The intra-batch and the inter-batch accuracy and precision were calculated by analyzing four levels of QC samples (5, 50, 500 and 1000 ng/mL, respectively). Intra-batch accuracy and precision were analyzed five times with each QC level on the same day. The inter-batch accuracy and precision were determined on three different days of each QC level. Precision was expressed as percentage (%) of Relative Standard Deviation (RSD), while the accuracy was calculated by % of Relative Error (RE).
The matrix effect was assessed by comparing the mean area response of ZgI in plasma with the mean area of standard solutions in the absence of matrix at four QC levels. Three replicates for each QC level were performed.
The stability of method was calculated by triplicate assay at four conditions (stored at 25°C for 2 h, 10°C for 24 h, -20°C for three weeks, and after three freeze-thaw cycles). The samples were evaluated by comparing the measured concentrations to freshly prepared QC samples, respectively.
Pharmacokinetics study
Statistical analysis
RESULTS
Establishment of leukopenia model in rats
|
Group |
Numbers |
WBC (×107) before CY treatment |
WBC (×107) after CY treatment (12 h) |
|
Control |
6 |
7.92 ± 1.68 |
8.02 ± 1.57 |
|
CY 40 mg/kg |
6 |
8.03 ± 1.47 |
3.97 ± 1.93** |
|
CY 70 mg/kg |
6 |
8.19 ± 1.51 |
1.89 ± 0.51** |
Determination of chromatographic and mass spectrometric conditions
 Figure 1: The chemical structures of ZgI (A) and GA (B).
Figure 1: The chemical structures of ZgI (A) and GA (B). Figure 2: The MS/MS spectrums of ZgI (A) and GA (B), and the main corresponding product ion of ZgI (C) and GA (D).
Figure 2: The MS/MS spectrums of ZgI (A) and GA (B), and the main corresponding product ion of ZgI (C) and GA (D). Figure 3: The MRM chromatograms of blank plasma (A), standard solution with 10 ng/mL ZgI (B), blank plasma with ZgI at LLOQ (C), and plasma of the rat with leukopenia at 20 min after a single oral administration of 5 mg/kg ZgI (D).
Figure 3: The MRM chromatograms of blank plasma (A), standard solution with 10 ng/mL ZgI (B), blank plasma with ZgI at LLOQ (C), and plasma of the rat with leukopenia at 20 min after a single oral administration of 5 mg/kg ZgI (D).Method validation
The calibration curves were linear in peak area rations over the concentration ranges of 0.5-200.0 ng/mL for ZgI. The results of linearity are shown in figure 4. After process of the calibration data and fitting the standard curve, the regression curve presented high linearity in the ranges of 0.5-200 ng/mL ZgI (R=0.9990). The linear equation was y=0.00418679x-0.000701950, where the y represents the peak area, and the x indicates the concentration ratio of ZgI and IS.
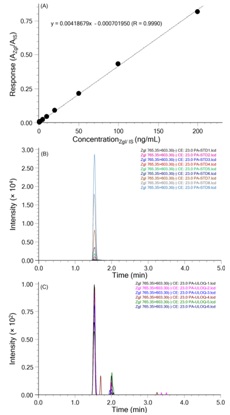
The intra-day and inter-day precision and accuracy were performed at four QC levels and the results are shown in table 2. The data suggest that precision values at each QC level were lower than 15% and accuracy values were within 102.6%–110.8%, indicating that the assay in precision and accuracy for ZgI is in accordance with the standard of the China Food and Drug Administration (CFDA).
|
QC level |
Intra-batch (% ) |
|
Inter-batch (% ) |
||
|
RSD |
Accuracy |
|
RSD |
Accuracy |
|
|
LLQC |
13.8 |
104.0 ± 14.4 |
|
10.7 |
108.7 ± 11.6 |
|
LQC |
5.7 |
110.8 ± 6.3 |
|
6.0 |
105.0 ± 6.3 |
|
MQC |
1.9 |
105.3 ± 2.0 |
|
5.0 |
105.2 ± 5.3 |
|
ULQC |
1.0 |
102.6 ± 1.0 |
|
2.2 |
97.0 ± 2.1 |
The matrix effect of measured compounds in plasma was defined as the overall effect of all components in the samples other than only the analytes of interest. As shown in table 3, the values of matrix effects for all levels were over 85%, indicating that the matrix effect could be ignored and free of endogenous interference in this method.
|
QC level |
Concentration (ng/mL) |
Matrix effect (%) |
|
LLQC |
0.5 |
98.1 ± 9.0 |
|
LQC |
5 |
106.1 ± 6.9 |
|
MQC |
50 |
100.3 ± 3.3 |
|
HQC |
100 |
98.7 ± 1.8 |
The data of stability of ZgI in rat plasma under the various conditions described above are listed in table 4. The results show that the levels of RSD% of two concentrations were within 15% under the different conditions, which is within the standard of the CFDA.
|
Storage condition |
RSD (%) |
|
|
1 ng/mL |
200 ng/mL |
|
|
Short-term (25°C, 2 h) |
11.9 |
4.3 |
|
Post-preparative (10°C, 24 h) |
6.9 |
3.9 |
|
Long-term (-20°C, 21 days) |
8.1 |
2.7 |
|
After three freeze-thaw cycles |
6.2 |
3.6 |
Pharmacokinetics study
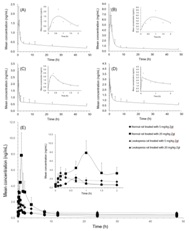 Figure 5: Pharmacokinetic profiles of normal rat with single oral administration of 5 mg/kg ZgI (A), and 20 mg/kg ZgI (B), and leukopenia rat with single oral administration of 5 mg/kg ZgI (C), and 20 mg/kg ZgI (D), and the integrated plasma concentration versus time curves of two above dosages in each group (E). Six samples were tested for each group.
Figure 5: Pharmacokinetic profiles of normal rat with single oral administration of 5 mg/kg ZgI (A), and 20 mg/kg ZgI (B), and leukopenia rat with single oral administration of 5 mg/kg ZgI (C), and 20 mg/kg ZgI (D), and the integrated plasma concentration versus time curves of two above dosages in each group (E). Six samples were tested for each group.|
Parameters |
Normal rats |
|
Leukopenia rats |
||
|
5 mg/kg |
20 mg/kg |
|
5 mg/kg |
20 mg/kg |
|
|
Cmax (ng/L) |
2.26 ± 0.74 |
7.96 ± 2.68 |
2.70 ± 0.87 |
3.40 ±1.26 ** |
|
|
Tmax (h) |
0.67 ± 0.00 |
0.93 ± 0.15 |
0.29 ± 0.08 ## |
0.33 ± 0.37 ** |
|
|
AUC0-t (ng/h/L) |
29.16 ± 15.88 |
69.86 ±34.12 |
39.76 ± 17.30 |
57.47 ± 26.39 |
|
|
AUC0-∞ (ng/h/L) |
32.80 ± 16.54 |
72.02 ±33.01 |
47.96 ± 13.39 |
65.34 ± 29.51 |
|
|
t1/2α (h) |
0.31 ± 0.04 |
1.22 ±0.91 |
0.25 ± 0.13 |
1.01 ± 0.85 |
|
|
t1/2β (h) |
8.64 ± 3.65 |
5.02 ± 6.74 |
16.31 ± 6.96 ## |
18.51 ± 8.41 ** |
|
##p<0.01 vs normal rats treated with 5 mg/kg ZgI.
DISCUSSION
Chromatographic separation conditions, in particular the composition of mobile phase, play a crucial role for achieving good chromatographic behavior and proper ionization [21]. Through the investigation of the mobile phase system, the addition of 0.05% ammonia to the aqueous phase was able to significantly increase in the sensitivity and peak symmetry. In addition, acetonitrile as organic phase can provide better peak shape and lower background noise compared to methanol. Finally, we got an ideal separation with acetonitrile and H2O both containing 0.05% ammonia as the mobile phase within 5 min. additionally, compared to methanol, protein precipitation with acetonitrile in the current experiment was more complete and less inference.
It is also necessary to have a proper IS to obtain desirable assay for mass spectrometer detection. Next, we selected optimal IS for ZgI. ZgI belongs to triterpenoid saponins and has similar structures with many other saponins including ginsenosides, ursolic acid, glycyrrhizic acid, and glycyrrhetinic acid [22]. Initially, we have tested ursolic acid, glycyrrhizic acid, and glycyrrhetinic acid as the candidates of IS. Finally, glycyrrhetinic acid was selected as the IS of ZgI because it not only belongs to the ursane type pentacyclic triterpenoid saponins but also has minimal endogenous interferences for mass spectrometer assay.
Leukopenia caused by myelosuppression, induced by Chemotherapy, is the most common side reaction in clinical treatment of tumors [23]. Leukopenia not only affects the continuation of chemotherapy, but also causes serious medical infections that lead to additional medical care or even early death [24]. Effective and efficient improvement of white blood cell levels in chemotherapy patients is a major problem which has long plagued the clinicians [25]. In this situation, Study on drug pharmacokinetics in a disease state is more important than the normal condition and more able to guide clinical medication plan [16]. The previous pharmacokinetic studies of ZgI were limited to normal animal models [9]. In present study, we revealed that the Cmax of ZgI didn’t rise with its increasing dosage by one-time administration in the state of leukopenia, which meant you may need to divide the previous dose into multiple doses to maintain its effective concentration in vivo, and this would be a big impact on the way of its administered, or even other relative drugs, to treat leucopenia. Contrarily, our results also showed that the metabolism time of ZgI in normal rats were almost 2 to 3 times to that of leukopenia rats, indicating that it is necessary to reduce the frequency of drug administration to prevent other side effects caused by excessive drug dose in the adjuvant chemotherapy treatment. Our study may provide a useful reference for the administration of ZGI and other related drugs in the treatment of chemotherapy-induced leukopenia.
It is no doubt that chemotherapy leads to damage of liver and change its related metabolic enzymes, as well as it induces leucopenia [26]. We should have explored the mechanism of ZgI absorption reduction caused by leucopenia, limited by the experimental conditions and other uncontrollable factors. Our current study just regrettably illuminated the pharmacokinetic features of the speed and grade of absorption of ZgI could be changed in leucopenia state. Next, we would further investigate the mechanism of ZgI absorption reduction caused by leucopenia and the distinction in pharmacokinetics of ZgI among several of species or relative diseases, and then explore the relationship between its pharmacokinetics and pharmacodynamics. Thus, providing a better and more rigorous guidance on clinical of drugs which use ZgI as the main ingredient.
CONCLUSION
ACKNOWLEDGMENT
CONFLICTS OF INTEREST
REFERENCES
- Zhao ZF, He XR, Zhang Q, et al. (2017) Meta-analysis of Diyushengbai Tablets in the treatment of leukopenia induced by tumor chemotherapy. Northwest Pharmaceut J 32: 648-652.
- Chen XK (2015) Clinical observation of Diyushengbai tablets combined with granulocyte colony -stimulating factor in the treatment of leukopenia after tumor chemotherapy. Chin Mod Med 22: 66-67.
- Chen Z (2015) The clinical study on burnet root tablets for the treatment of acquired immunodeficiency syndrome with leukocytopenia. Proc Clin Med 24: 259-260.
- Gui-Yan Y, Liang-Min D, Yong-Ai X, Yi-Hang H, Ming Y, et al. (2016) Determination of the content of ziyuglycoside I and ziyuglycoside II in Sanguisorba officinalis L. by HPLC-ELSD. Chin J Trad Chin Med Pharm 31: 3340-3343.
- Yong-Ai X, Jun-Bo Z, Min Y, Pharmaceutical College, Chengdu University of Traditional Chinese Medicine, et al. (2014) Protective Effect of Tannins from Sanguisorba officinalis on Cyclophosphamide Induced Myelosuppression in Mice. Nat Prod Res Dev 26: 499-503.
- Xuewen Z, Wei W, Yue C, Haowen L, Jianping C, et al. (2013) Determination of Total saponins and Ziyuglycoside I in Sanguisorba officinalis L.. Trad Chin Drug Res Clin Pharm 24: 186-191.
- Wang G, Fu H, Ye W, Zheng X, Xiao J, et al. (2016) Comprehensive characterization of the in vitroand in vivo metabolites of ziyuglycoside I in rat microsome, intestinal flora, excretion specimen and fresh tissues based on LC-Q-TOF/MS, J Pharm Biomed Anal 128: 191-200.
- Jiang LX, Yan HY, Yang SH et al. (2006) Application of pharmacokinetics in new drug research and development. J Pharm Prac 24: 260-263.
- Ye W, Fu H, Xie L, Zhou L, Rao T, et al. (2015) Development and validation of a quantification method for ziyuglycoside I and II in rat plasma: Application to their pharmacokinetic studies. J Sep Sci 38: 2340-2347.
- Liu W, Wang X, Chen R, Zhang K, Li Y, et al. (2016) Yang, Effect of age on the pharmacokinetics of polymorphic nimodipine in rats after oral administration. Acta Pharmaceutica Sinica B 6: 468-474.
- Gong H, Tang W, Wang H, Xia Q, Huang X (2011) Effects of food and gender on the pharmacokinetics of rhein and emodin in rats after oral dosing with Da-Cheng-Qi decoction. Phytother Res 25:74-80.
- Wenbin L, Rong W, Hua X, Juanhong Z, Xiaoyu W, et al. (2015) Effects on Pharmacokinetics of Propranolol and Other Factors in Rats After Acute Exposure to High Altitude at 4,010 m. Cell Biochem Biophy 72: 27-36.
- Hwang YH, Yang HJ, Kim DG, Ma JY (2017) Inhibitory effects of multiple-dose treatment with baicalein on the pharmacokinetics of ciprofloxacin in rats. Phytother Res 31: 69-74.
- Deng Y, Mo YF, Chen XM, Zhang LZ, Liao CF, et al. (2016) Effect of ginkgo biloba extract on the pharmacokinetics and metabolism of clopidogrel in rats. Phytother Res 30: 1886-1892.
- He LH, Li J, Deng YX, Zhang XJ, Chen R, et al. (2016) Comparative investigation on the pharmacokinetics of geniposide in type 2 diabetic and normal rats after oral administration of Fructus Gradeniae extract. J Chromatogr B Analyt Technol Biomed Life Sci 1033: 180-186.
- van Schaik BA, Geyskes GG, van der Wouw PA, van Rooij HH, Porsius AJ (1988) Pharmacokinetics of Lisinopril in Hypertensive Patients with Normal and Impaired Renal Function. Eur J Clin Pharmacol 34:61-65.
- An J, Hu F, Wang C, Zhang Z, Yang L, et al. (2016) Pharmacokinetics and tissue distribution of five active ingredients of Eucommiae cortex in normal and ovariectomized mice by UHPLC-MS/MS. Xenobiotica 46: 1-12.
- Gong ZP, Chen Y, Zhang RJ, Yang Q, Zhu XX (2015) Advances on pharmacokinetics of traditional Chinese medicine under disease states. Zhongguo Zhong Yao Za Zhi 40: 169-173.
- Yang T, Liu S, Zheng TH, Tao YY, Liu CH (2015) Comparative pharmacokinetics and tissue distribution profiles of lignan components in normal and hepatic fibrosis rats after oral administration of Fuzheng Huayu recipe. J Ethnopharmacol 166: 305-312.
- Lohrmann HP (1984) The problem of permanent bone marrow damage after cytotoxic drug treatment. Oncology 41: 180-184.
- Dainiak MB, Galaev IY, Mattiasson B (2006) Affinity cryogel monoliths for screening for optimal separation conditions and chromatographic separation of cells. J Chromatogr A 2:145-150.
- Xie JB, Bi ZM, Li P (2003) HPLC-ELSD determination of triterpenoids and triterpenoid saponins in Ilex pupurea leaves. Acta Pharm Sin 7: 534-536.
- Lyman GH, Allcott K, Garcia J, Stryker S, Li Y, et al. (2017) The effectiveness and safety of same-day versus next-day administration of long-acting granulocyte colony-stimulating factors for the prophylaxis of chemotherapy-induced neutropenia: a systematic review. Support Care Cancer 8: 2619-2629.
- Alenzi EO, Kelley GA (2017) The association of hyperglycemia and diabetes mellitus and the risk of chemotherapy-induced neutropenia among cancer patients: A systematic review with meta-analysis. J Diabetes Complications 1: 267-272.
- Choi TY, Lee MS, Ernst E (2015) Moxibustion for the treatment of chemotherapy-induced leukopenia: a systematic review of randomized clinical trials. Support Care Cancer 6:1819-1826.
- Di Re F, Bohm S, Oriana S, Spatti GB, Zunino F (1990) Efficacy and safety of high-dose cisplatin and cyclophosphamide with glutathione protection in the treatment of bulky advanced epithelial ovarian cancer. Cancer Chemother Phaimacol 5: 355-360.
Citation: Zhu L-J, Chen L, Bai C-F, Wu A-G, Huang F-H, et al. (2019) Comparative Plasma Pharmacokinetics of Ziyuglycoside I from Sanguisorba Officinalis L. between Leukopenia and Normal Rats via UHPLC-MS/MS. J Altern Complement Integr Med: S1005.
Copyright: © 2019 Lin-Jie Zhu, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

