
Detection of Aberration and Genetic Changes in Post-menopausal Women Presented with Bleeding Using Pap Smear, Immunocytochemical and Molecular Studies
*Corresponding Author(s):
Khalid Ahmed AlamaSenior Clinical Cytologist Dubai Hospital, Dubai, United Arab Emirates
Tel:+971 504182550,
Email:khalidalama@gmail.com
Abstract
Objective
Two of the most common malignancies of the female genital tract are endometrial and cervical cancers, these are highly prevalent cancers, and their further study has the potential for significant beneficial impact on women’s health on an international level. The p53 tumor suppressor gene has received considerable attention in endometrial cancer. Mutations in p53 are found in approximately 10%-20% of all endometrial carcinomas, the majority occurring in high-grade tumors. As many as 50% of all human tumors contain p53 mutants. In normal cells, the p53 protein level is low. DNA damage and other stress signals may trigger the increase of p53 proteins, which have three major functions: growth arrest, DNA repair and apoptosis (cell death). p53 also known as TP53 or tumor protein is a gene codes a protein that regulates the cell cycle and apoptosis, hence functions as tumor suppression. p53 has been described as "the guardian of the genome", referring to its role in conserving stability by preventing genome mutation, it is located on the seventeenth chromosome (17p13.1) and molecular weight 53 Kilodalton fraction of cell protein. Abnormalities of TP53 have been well described in various malignancies and germ-line mutations are associated with Le Fraumeni syndrome. The mutant p53 protein is non-functional but resists degradation and accumulates thereby acting as a dominant negative inhibitor of the wild-type p53. The accumulated mutant protein can be demonstrated immunohistochemically. The p53 signature, which (although morphologically unremarkable) displays diffuse and strong p53 nuclear staining, has been proposed to be a precursor of serous endometrial and/or endndocervicalintraepithelial neoplasia. We examined the overexpression of p53 in postmenopausal women with vaginal bleeding in exfoliated endometrial and endocervical cells collected by endo and ectocervical brushing.
Methods
Liquid Base (LBC) PAP smears of 70 women with postmenopausal bleeding with normal Cytomorphological findings ranging between Negative for Intraepithelial Lesion (NILM) and Reactive Cellular Changes (RCC), including 5 samples collected from postmenopausals without bleeding used as a control, were evaluated in this study. Menstruating women were excluded from this study. Expressions of p53 were immunohistochemically examined and the findings confirmed using molecular analysis (PCR). Overexpression of p53 was categorized as weak to strong in more than 18% of the samples.
Results
Strong overexpression of p53 was observed in 15 samples (10.5%) and 12 samples (8.4%) showed weak p53 overexpressionin Immunohistochemical studies. Molecular analysis (PCR) used to confirm the results and it showed 13 Positive using gel electrophoresis and read under Ultra Violet (UV) light. Twelve out of Fifteen samples which were strongly positive in immunomarker stain and One out of Twelve weak positive samples in immunomarker stain showed positive mutational changes in molecular study. Postmenopausals with bleeding exhibited significantly overexpression of P53 (P = 0.001).
Conclusion
Overexpression of p53 may be responsible for the high proliferative activity of postmenopausal endometrial intraepithelial neoplasia and /or endocervical intra epithelial neoplasia.
INTRODUCTION
General introduction
Post-menopausal bleeding accounts for 9-10% of gynecological complaints. 10% of patients presenting with post-menopausal bleeding are diagnosed to have carcinoma of endometrium. At the same time 90% of the patients with carcinoma of endometrium present with postmenopausal bleeding, therefore it is important to evaluate all patients with post-menopausal bleeding to rule out malignancies of the genital tract. Other causes of post-menopausal bleeding including atrophic endometrium (60%), endometrial hyperplasia (10%), carcinoma cervix (10%) and other rare conditions (10%) [7,8].
Mutations in mismatch repair genes
As many as 50% of all human tumors contain p53 mutants. In normal cells, the p53 protein level is low. DNA damage and other stress signals may trigger the increase of p53 proteins, which have three major functions: growth arrest, DNA repair and apoptosis (cell death) [15]. P53 also known as TP53 or tumor protein is a gene codes a protein that regulates the cell cycle and apoptosis, hence functions as tumor suppression. p53 has been described as "the guardian of the genome", referring to its role in conserving stability by preventing genome mutation, it is located on the seventeenth chromosome (17p13.1) and molecular weight 53 Kilodalton fraction of cell protein [16]. Abnormalities of TP53 have been well described in various malignancies and germ- line mutations are associated with Le Fraumeni syndrome. The mutant p53 protein is non-functional but resists degradation and accumulates thereby acting as a dominant negative inhibitor of the wild-type p53. The accumulated mutant protein can be demonstrated immunohistochemically. Wild type p53 protein has a short half-life; it is generally undetectable in normal cells, whereas mutant p53 protein is more stable and often reaches immunohistochemically detectable levels [17]. Mutations of the p53 gene are a frequent and characteristic finding in Type II serous tumour with positive immunohistochemistry reported in 71%-85% of tumour and are considered to be an early event in tumorigenesis [18]. This is supported by concordant p53 staining in multiple biopsies from different areas of individual tumour and by the finding of positive immunohistochemical results in Endometrial Intraepithelial Carcinoma (EIC), the putative precursor of serous carcinoma [19].
The aim of the study was to find out any possibility of gene mutational changes in postmenopausals with bleeding symptoms as an alert and early prediction for an occult endometrial or endocervical mutational genetic changes.
Problem identification and justification
The role of P53 mutation in cancer development
P53 plays a role in regulation or progression through the cell cycle, apoptosis, and genomic stability by means of several mechanisms:
- It can activate DNA repair proteins when DNA has sustained damage. Thus, it may be an important factor in aging [24]
- It can arrest growth by holding the cell cycle at the G1/S regulation point on DNA damage recognition if it holds the cell here for long enough, the DNA repair proteins will have time to fix the damage and the cell will be allowed to continue the cell cycle
- It can initiate apoptosis (i.e., programmed cell death) if DNA damage proves to be irreparable
- It is essential for the senescence response to short telomeres
Incidence and mortality
OBJECTIVES
General objectives
Specific objectives
• To determine the role of p53 overexpression in negative Pap smears of postmenopausals with bleeding as an early prediction of endometrial and endocervical cancer.
LITERATURE REVIEW
Endometrial cancer appears most frequently between the ages of 50 and 65 [26]. Overall, 75% of endometrial cancer occurs after menopause. Women younger than 40 make up 5% of endometrial cancer cases and 10-15% of cases occur in women under 50 years of age. This age group is at risk for developing ovarian cancer at the same time. [26]. The worldwide median age of diagnosis is 63 years of age; [27]. In the United States, the average age of diagnosis is 60 years of age. White American women are at higher risk for endometrial cancer than black American women, with a 2.88% and 1.69% lifetime risk respectively [28]. Japanese-American women and American Latina women have a lower rate and Native Hawaiian women have higher rates [29]. As of 2014, approximately 320,000 women and over 380,000 new cases in 2018 are diagnosed with endometrial cancer worldwide each year, and 76,000 die, making it the sixth most common cancer in women [6]. It is more common in developed countries, where the lifetime risk of endometrial cancer in people born with uteri is 1.6%, compared to 0.6% in developing countries [30]. It occurs in 12.9 out of 100,000 women annually in developed countries [31].
In the United States, endometrial cancer is the most frequently diagnosed gynecologic cancer and in women the fourth most common cancer overall [9] representing 6% of all cancer cases in women. In that country, as of 2014 it was estimated that 52,630 women were diagnosed yearly and 8,590 would die from the disease [28].
Northern Europe, Eastern Europe, and North America have the highest rates of endometrial cancer, whereas Africa and West Asia have the lowest rates. In Asia 41% of the world's endometrial cancer diagnoses in 2012, whereas Northern Europe, Eastern Europe, and North America together comprised 48% of diagnoses [6]. Unlike most cancers, the number of new cases has risen in recent years, including an increase of over 40% in the United Kingdom between 1993 and 2013 [30]. Some of this rise may be due to the increase in obesity rates in developed countries [31]. Increasing life expectancies, and lower birth rates [9]. In the UK, approximately 7,400 cases are diagnosed annually, and in the EU, approximately 88,000 [27]. There were over 500,000 new cervical cancer cases in 2018, representing 6.6% of all female cancers. Approximately 90% of deaths from cervical cancer occurred in low- and middle-income countries. The high mortality rate from cervical cancer globally could be reduced through a comprehensive approach that includes prevention, early diagnosis, and effective screening and treatment programmers. There are currently vaccines that protect against common cancer-causing types of human papilloma virus and can significantly reduce the risk of cervical cancer. 90% of CIN lesions and cervical cancer are associated with HPV.
MATERIALS AND METHODS
Study area
Study Design
Study area and period
Study population and sample size
Ethical consideration
Data collection
Inclusion criteria
Exclusion criteria
Cytological examination
1. 95% Ethanol 1minute
2. Rinse in tap water
3. Harris or Gill Hematoxylin 1-3 minutes (Time vary with selection of hematoxylin solution)
4. Rinse in tap water or Scott's tap water
5. 95% Ethanol 1 minute
6. OG-6 stain for 1.5 minutes.
7. 95% Ethanol 1 minute
8. EA-50 for 2.5 minutes.
9. 95% Ethanol 1 minute, 2 changes
10. 100% Ethanol 1 minute
11. Clear in 2 changes of xylene, 2 minutes each
12. Mount with DPX
using Bethesda system for reporting cervical cytopathology as follows:
NILM: In case of Negative for cellular Lesion
RCC: Reactive Cellular Changes
ASCUS: Atypia of Undetermined Significance
LSIL: Low grade Squamous Intraepithelial Lesion
HSIL: High grade Squamous Intraepithelial Lesion
AGC: Atypical Glandular Cells
AIS: Adenocarcinoma Insitu either endocervical or endometrial
SCC: Squamous Cell Carcinoma
Molecular analysis
DNA extraction: The samples were centrifuged for 10 min and the pellet resuspended in 30 to 50 p.1 of 50 mmol/L Tris (pH 9.0) with 200 ng/,ul proteinase K, and covered by 20 p.l of mineral oil. After overnight digestion at 600C, the samples were heated at 1 000C for 10 minutes to inactivate proteinase K. Negative controls were included with each extraction and processed similarly. Internal controls included 3 USCs (SE4, SE8 and SE10) that underwent separate extractions of DNA known positive samples. Repeat DNA extractions of the cases were performed for confirmation of sequence results.
Polymerase Chain Reaction (PCR): PCR amplification was performed using Techne 512, UK thermal cycler to optimize the procedure and the product was analyzed by gel electrophoresis in 1.5% Agarose, and stained with 0.15 % Ethidium bromide and visualized by using UV gel documentation system. (DNA products expected size was 141 bp. The reaction volumes was 25ul containing 5 ul master mix, 2 ul of primer, 5 ul of DNA and 13 ul of distilled water. The genomic DNA 2 to 500 ng , 10 mmol/L Tris/HCI (pH 9.2), 75 mmol/L KCI, 1.5 mmol/L MgCl2, 160 p.mol/L each of dGTP, dATP, dTTP, and dCTP, 0.5 p.mol/L of each primer, and 0.5 U of Taq polymerase (Perkin-Elmer, Norwalk, CT, or Gibco BRL, Gaithersburg, MD). dinucleotides were added. Amplification was performed with 40 cycles consisting of 1 minute at 95 ºC, 1 minute at 61 ºC, and 1 minute at 72 ºC followed by a single 5-minute extension at 72 ºC.
The PCR amplification technique was performed and the test carried out following primers:
F
3'TCCCCCTTGCCGTCCCAA'5
R
3'CTGGTGCAGGGGCCACGC'5
The primers sequences were cited from previous studies and obtained from Macrogen Korea (10F, 254 Beotkkot-ro Geumcheon-gu, Seoul. 08511, Rep of Korea). Each reaction was performed in total volume of 25 ul.
STATISTICAL ANALYSIS
All information about the study uploaded in a spread sheet as well as obtained results. The data analyzed using 11.5 statistical packages for Social Science programme (SPSS). Frequencies mean chi-square test value then calculated.
RESULTS
Seventy liquid base samples were collected from ecto and endocervices of postmenopausal women with bleeding and examined cytologically for any cellular changes, Five samples collected from postmenopausals without vaginal bleeding used as a control, they were normal cytologically (NILM) in PAP smear, Negative for IHC stain, and used as control in PCR while the Seventy samples were ranging between reactive cellular changes (Twelve samples) and Normal in cytological examination. While in IHC Twenty seven samples were IHC positive for p53 overexpression. Figures 1-3, ranging between strong positive (Fifteen samples) and weak positive (Twelve samples) (Table 1). Molecular confirmation using Polymerase Chain Reaction (PCR) product was analyzed by gel electrophoresis in 1.5% Agarose, and stained with 0.15 % Ethidium bromide and the product was visualized by using UV gel documentation system. Thirteen samples were p53 expressed with mutational changes. Figures 4 and 5 Data of endometrial and endocervical assessment reports was collected from the HIS, thirteen patients had endometrial thickness ranging between 3 to 12 mm and the endocervices was ranging between normal and inflamed. All samples were HPV negative except one case was Positive for HPV 16 and showed intraepithelial neoplasia in Pap smear and positive IHC and PCR. Thirteen samples out of Twenty seven samples showed strong p53 overexpression in PCR and the Fourteen IHC weak positive in addition to one case strong positive showed negative p53 overexpression in PCR.
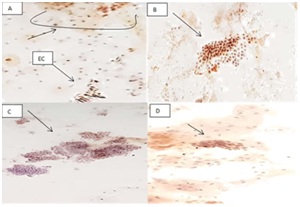 Figure 1: (A) Glandular endocervical cells showed positive p53 Immunostain. (B) Showed positive p53Immunostain while (C) was negative for p53 Immunostain (D).
Figure 1: (A) Glandular endocervical cells showed positive p53 Immunostain. (B) Showed positive p53Immunostain while (C) was negative for p53 Immunostain (D).
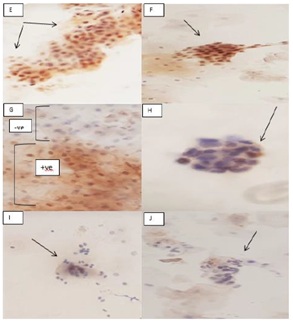 Figure 2: (E,F) Showed squamous epithelial cells p53 overexpression in IHC immunostain. (G) Showed diphasic picture of positive and negative p53 Immunostain in the same field. (H) Endometrial cells showed positive p53 IHC Immunostain. (I,J) Endocervical cells showed negative p53 Immunostain.
Figure 2: (E,F) Showed squamous epithelial cells p53 overexpression in IHC immunostain. (G) Showed diphasic picture of positive and negative p53 Immunostain in the same field. (H) Endometrial cells showed positive p53 IHC Immunostain. (I,J) Endocervical cells showed negative p53 Immunostain.
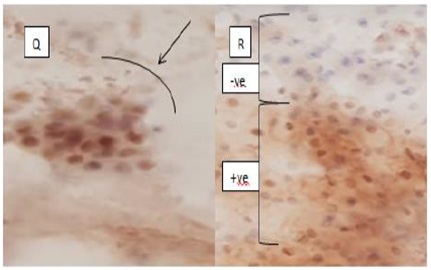 Figure 3: (Q) Showed Strong p53 overexpression in endocervical cells, while (R) showed positive and negative picture of p53 expression.
Figure 3: (Q) Showed Strong p53 overexpression in endocervical cells, while (R) showed positive and negative picture of p53 expression.
|
Procedure |
Positive |
|
RCC (Cytomorphology) |
12 |
Table 1: Showing the frequency of positive reactions.
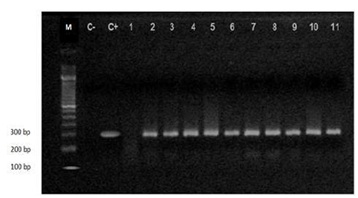 Figure 4: Electrophoresis using Agarose gel of p53products. Lane M: 1.5 Kb DNA Ladder. C- Is control negative, C+ is control positive Lanes 2 to 11: p53 PCR products (296 bp). Lane 1: Negative sample.
Figure 4: Electrophoresis using Agarose gel of p53products. Lane M: 1.5 Kb DNA Ladder. C- Is control negative, C+ is control positive Lanes 2 to 11: p53 PCR products (296 bp). Lane 1: Negative sample.
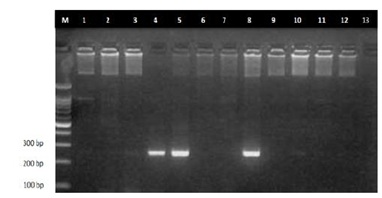 Figure 5: Electrophoresis using Agarose gel of p53 PCR product. Lane M: 1.5 kb DNA ladder. Lanes4, 5 and 8 p53 PCR products (296 bp). Lanes 1, 2, 3, 6, 7, 9, 10, 11, 12 and 13) are negative samples.
Figure 5: Electrophoresis using Agarose gel of p53 PCR product. Lane M: 1.5 kb DNA ladder. Lanes4, 5 and 8 p53 PCR products (296 bp). Lanes 1, 2, 3, 6, 7, 9, 10, 11, 12 and 13) are negative samples.
DISCUSSION
Knowledge of the oncogenetic pattern of a tumor can explain its natural history and behavior in single cases. Thus, a number of oncogenetic studies of cervical and endometrial cancer have been performed to identify prognostic and predictive markers the p53 tumor suppressor gene is correlated with cervical and endometrial tumor induction and progression, and its significant role as a prognostic marker was found in previous studies in advanced cervical and endometrial tumors. The aim of the present study was to detect the genetic changes that can lead to uterine cancer. It is important to note that the cohort of patients considered was not consecutive due to the inclusion criteria used. Patients were selected on the basis of Clinical history. This allowed more significance to be assigned to acquire data, as even more emphasis could be placed, in the single case and the association with the risk of mortality. Malignant tumors result from an accumulation of genetic alterations that lead to overexpression of oncogenes and inactivation of tumor suppressor genes. The consequences of these changes in cells progressing to malignancy are the loss of negative regulation of cellular proliferation or programmed cell death. Evidence thus far accumulated suggests that normal p53 protein is a negative regulator of cell growth, possibly playing a role in maintaining genomic integrity by arresting cells with DNA damage at the GL/S boundary of the cell cycle. Mutations of the p53 gene are the most common genetic alterations associated with cancers in humans. An important consequence of these various mutations is post translational stabilization of the altered protein, leading to elevated p53 levels in tumor cells. Because of its short half-life, wild type p53 protein is almost undetectable at IHC, and its positive immunoreactivity is considered synonymous with altered protein structure or function due to mutation or viral oncogene involvement. The presence of point mutations in the p53 gene is not the only cause of protein accumulation. Change in p53 half-life may also be caused by exposing normal cells to DNA-damaging agents, such as ultraviolet light. This critical observation has generated renewed interest and led to important new models of p53 protein function. A cell in distress usually reacts by activation of biochemical pathways controlled by the p53 gene. The increase in p53 protein level is associated with an increase in transcription of p53-responsive genes and induction of growth arrest and apoptosis. The precise mechanism by which DNA damage and other stimuli result in p53 stabilization is not yet elucidated, but may involve the action of other gene products. IHC positive and PCR negative results can be explained by the presence of either mutations outside the exons screened or mutant conformers migrating like wild-type strands. However, a non-mutational stabilization of p53 may well be operative. Distressed cells express high levels of wild-type protein, perhaps through interruption of the p53 degradative pathway. Moreover, some p53- dependent genes involved in cell cycle regulation may be altered and may indirectly cause p53 overexpression in cell nuclei. On the other hand, IHC-negative, and positive PCR may result from nonsense point mutations or from deletions, because such alterations lead either to premature cessation of protein synthesis or generation of quite different protein products, making IHC detection impossible or may be related to the presence of point mutations that do not cause p53 protein stabilization
CONCLUSION
In the present study, Liquid Base (LBC) PAP smears of 70 women with postmenopausal bleeding with normal Cytomorphological findings ranging between Negative for Intraepithelial Lesion (NILM) and Reactive Cellular Changes (RCC), including 5 samples collected from postmenopausals without bleeding used as a control, were evaluated in this study. Menstruating women or woman in reproductive age were excluded from this study. Expressions of p53 were immunohistochemically examined and the findings confirmed using molecular analysis (PCR). Overexpression of p53 was categorized as weak to strong in more than 18% of the samples. Strong overexpression of p53 was observed in 15 samples (10.5%) and 12 samples (8.4%) showed weak p53 overexpression in Immunohistochemical studies. Molecular analysis (PCR) used to confirm the results and it showed 13 Positive using gel electrophoresis and read under Ultra Violet (UV) light. Twelve out of Fifteen samples which were strongly positive in immunomarker stain and One out of Twelve weak positive samples in immunomarker stain showed positive mutational changes in molecular study. Postmenopausals with bleeding exhibited significantly overexpression of p53.
Overexpression of p53 may be responsible for the high proliferative activity of postmenopausal endometrial intraepithelial neoplasia and /or endocervical intra epithelial neoplasia.
RECOMMENDATIONS
In this study we found that p53 plays an important role to predict the endometrial or endocervical cancer in postmenopausals with bleeding. Any woman has a vaginal bleeding especially after menopause which may be endometrial or endocervical origin. 10%-20% have genetic aberration and they have the liability to develop endometrial or endocervical cancer has to be under medical care.
REFERENCES
- Ellenson LH, Wu T-C (2004) Focus on endometrial and cervical cancer. J Cancer cell 5: 533-538.
- Kong A, Johnson N, Kitchener HC, Lawrie TA (2012) Adjuvant radiotherapy for stage I endometrial cancer. Cochrane Database Syst Rev 18: CD003916.
- National Cancer Institute NCI (2014) National Cancer Institute NCI. US Department of Health and Human Services. Washington, USA.
- Purdie DM, Green AC (2001) Epidemiology of endometrial cancer. Best Practice & Research Clinical Obstetrics & Gynaecology 15: 341-354.
- Saso S, Chatterjee J, Georgiou E, Ditri AM, Smith JR, et al. (2011) Endometrial cancer. BMJ Pg no: 343.
- (IARC &WHO) International Agency for Research on Cancer (2003) World Cancer Report. World Health Organization, IARC Press, Lyon, France.
- A.V U, G I. POST MENOPAUSAL BLEEDING (PMB) -. Ibom Medical Journal, 6(1). Available from: http://www.ibommedicaljournal.com/m. 2013.
- Newell S, Overton C (2012) Postmenopausal bleeding should be referred urgently. Practitioner 256: 13-15.
- Miller DS, Schorge JO (2012) Endometrial cancer. In: Hoffman BL, Schorge JO, Schaffer JI, et al (eds.). Williams Gynecology. McGraw-Hill, New York, USA.
- McGraw-Hill (2014) Williams Gynecology Endometrial Cancer (2nd edn.) pg no: 818.
- Ma J, Ledbetter N, Glenn L (2013) Testing women with endometrial cancer for lynch syndrome: should we test all? J Adv Pract Oncol 4: 322-330.
- O'Mara TA, Glubb DM, Amant F, Annibali D, Ashton K, et al. (2018) Identification of nine new susceptibility loci for endometrial cancer. Nat Commun 9: 3166.
- Enomoto T, Inoue M, Perantoni AO, Buzard GS, Miki H, et al. (1991) K-ras activation in premalignant and malignant epithelial lesions of the human uterus. Cancer Res 51: 5308-5314.
- Takata M, Matsui Y (1994) Proliferating Cell Nuclear Antigen (PCNA) and p53 Protein Expression in Bowen's Disease. J Dermatol 21: 947-952.
- Blagosklonny MV (2002) P53: an ubiquitous target of anticancer drugs. Int J Cancer 98: 161-166.
- Strachan T, Read A (2018) Human Molecular Genetics (5th edn.), CRC Pres, Pg no: 770.
- Sherman ME, Bur ME, Kurman RJ (1995) P53 in endometrial cancer and its putative precursors: evidence for diverse pathways of tumorigenesis. Human Pathol 26: 1268-1274.
- Kounelis S, Kapranos N, Kouri E, Coppola D, Papadaki H, et al. (2000) Immunohistochemical profile of endometrial adenocarcinoma: a study of 61 cases and review of the literature. Mod Path 13: 379-388.
- Lecce G, Meduri G, Ancelin M, Bergeron C, Perrot-Applanat M (2001) Presence of Estrogen Receptor β in the Human Endometrium through the Cycle: Expression in Glandular, Stromal, and Vascular Cells. Journal of Clinical Endocrinology and Metabolism 86: 1379-1386.
- American Cancer Society (2017) Breast Cancer Facts & Figures. American Cancer Society, Atlanta, Georgia, USA.
- Lane D, Levine A (2010) P53 Research: The Past Thirty Years and the Next Thirty Years. Cold Spring Harb Perspect Biol 2: 000893.
- Ambros RA, Sherman ME, Zahn CM, Bitterman P, Kurman RJ (1995) Endometrial intraepithelial carcinoma: a distinctive lesion specifically associated with tumors displaying serous differentiation. Hum Pathol. 26: 1260-1267.
- Baergen RN, Warren CD, Isaacson C, Ellenson LH (2001) Early uterine serous carcinoma: clonal origin of extrauterine disease. Int J Gynecol Pathol 20: 214-219.
- Gilbert SF (2000) Developmental Biology (6th edn). Sinauer Associates, Sunderland, Massachusetts, USA.
- Lawrence L (2018) Postmenopausal Bleeding Common in Women With Endometrial Cancer, Cancer Net Work, journal of oncology.
- Soliman PT, Lu KH (2014) Endometrial Hyperplasia, Endometrial Carcinoma, and Sarcoma: Diagnosis and Management. Neoplastic Diseases of the Uterus Pg no: 1-23.
- Colombo N, Preti E, Landoni F, Carinelli S, Colombo A, et al. (2013) Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology 24: 33-38.
- SGO Clinical Practice Endometrial Cancer Working Group, Burke WM, Orr J, Leitao M, Salom E, et al. (2014) Endometrial cancer: a review and current management strategies: part I. Gynecol Oncol 134: 385-392.
- PDQ NIH (2015) Archived from the original.
- Galaal K, Al Moundhri M, Bryant A, Lopes AD, Lawrie TA (2014) Adjuvant chemotherapy for advanced endometrial cancer. Cochrane Database Syst Rev 15: CD010681.
- Vale CL, Tierney J, Bull SJ, Symonds PR (2012) Chemotherapy for advanced, recurrent or metastatic endometrial carcinoma. Cochrane Database Syst Rev 8 : 003915.
Citation: Alama KA, Abkar AD (2019) Detection of Aberration and Genetic Changes in Post-menopausal Women Presented with Bleeding Using Pap Smear, Immunocytochemical and Molecular Studies. J Cytol Tissue Biol 6: 021.
Copyright: © 2019 Khalid Ahmed Alama, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

