Polyion Complex (PIC) Flower-shaped Nano-micelles formed from Anionic Triblock and Cationic Diblock Copolymers
*Corresponding Author(s):
Shin-ichi YusaDepartment Of Materials And Chemistry, University Of Hyogo, 2167 Shosha, Himeji, Hyogo, Japan
Tel:+81 792674954,
Fax:+81 792668868
Email:yusa@eng.u-hyogo.ac.jp
Abstract
Keywords
INTRODUCTION
Kadam et al., [2] have reported the synthesis of flower micelles composed of poly (2-methacryloyloxyethyl acrylate)-block-poly(ethylene oxide)-block-poly(2-meth- acryloyloxyethyl acrylate) triblock copolymer bearing polymerizable groups on the hydrophobic blocks. The transient flower micelles structures in water were permanently fixed by cross-linking the methacrylate moieties in the micelles cores under UV light. Graaf et al., [3] have reported that amphiphilic BAB triblock copolymers consisting of Poly (Ethylene Glycol) (PEG) as hydrophilic A block and thermo-responsive poly (N-isopropylacrylamide) (pNIPAm) B blocks from flower micelles above the Lower Critical Solution Temperature (LCST) for pNIPAm blocks in water. These reported examples of flower micelles are based on hydrophobic interactions.
In general, polymer-based nano-aggregates in water are formed due to various driving forces such as interpolymer hydrophobic interactions, hydrogen bonding, Van der Waals, and electrostatic interactions [4-8]. The driving forces of polymer micelle core formation are not only hydrophobic interactions but also electrostatic interactions, which have attracted attention. Kataoka et al., [9-11] reported preparation of oppositely charged double hydrophilic diblock copolymers, poly (ethylene glycol)-block-poly(L-lysine) (PEG-P(Lys)) and poly(ethylene glycol)-block-poly(α,ß-asparatic acid) (PEG-P(Asp)). When these oppositely charged diblock copolymers are neutralized in water, water-soluble Polyion Complex (PIC) micelles are formed due to the electrostatic interactions. The PIC micelles are composed of the segregated PIC core formed by charged blocks of cationic P (Lys) and anionic P (Asp), which are surrounded by electrically neutral hydrophilic PEG shells.
We prepared oppositely charged double hydrophilic diblock copolymers (PEG-PMAPTAC and PEG-PAMPS) via Reversible Addition-Fragmentation Chain Transfer (RAFT) controlled living radical polymerization of (3-(Methacryloylamino) Propyl) Trimethylammonium Chloride (MAPTAC) and sodium 2-(Acrylamido)-2-Methylpropanesulfonate (AMPS) using PEG-based monofunctional chain transfer agent [12-14]. When these oppositely charged PEG-PMAPTAC and PEG-PAMPS are mixed with stoichiometrically charge neutralization in water, water-soluble PIC micelles are formed, which composed of segregated PIC core composed of cationic PMAPTAC and anionic PAMPS blocks and the outer hydrophilic nonionic PEG shells [15].
In this study, we prepared PIC flower micelles in water (Figure 1). An anionic ABA triblock copolymer (PAMPS48-PEG227-PAMPS48, AEA) composed of PAMPS and PEG blocks was prepared via RAFT radical polymerization. Cationic diblock copolymers (PEG-PMAPTAC, EMm) with different chain lengths of the PMAPTAC block were also prepared via RAFT. When AEA and EMm were mixed in water, PIC flower micelles were formed through electrostatic interactions between the PAMPS and PMAPTAC blocks (Figure 1). The resulting structures , ζ-potential, and Transmission Electron Microscopy (TEM) measurement techniques.
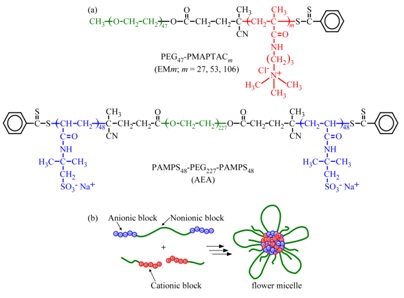
Figure 1: (a) Chemical structures of PAMPS48-PEG227-PAMPS48 (AEA) and PEG47-PMAPTACm (EMm, m = 27,53, and 106).
(b) Schematic representation of a flower micelle composed of AEA and EMm.
MATERIALS AND METHODS
Chemicals and materials
Synthesis of poly (ethylene glycol)-based bifunctional chain transfer agent (CpD-PEG-CpD)
Synthesis of PAMPS48-PEG227-PAMPS48 (AEA)
Preparation of cationic diblock copolymers (PEG47-PMAPTACm) [15]
Preparation of Polyion Complex (PIC) micelles
Measurements
1H NMR spectra were obtained using a Bruker DRX-500 spectrometer operating at 500 MHz using deuterium lock at a constant temperature of 20oC during the whole run. Sample solutions of the polymer for 1H NMR measurements were prepared in D2O containing 0.1 M NaCl at Cp = 1 g/L.
Light scattering measurements were performed using an Otsuka Electronics Photal DLS-7000 light scattering equipment with a multi-τ digital time correlator (ALV-5000/EPP). A He-Ne laser (10.0 Mw at 632.8 nm) was used as a light source. Sample solutions for light scattering measurements were filtered using a 0.2 µm pore size polytetrafluoroethylene filter. In Static Light Scattering (SLS) measurements, the weight-average molecular weight (Mw), z-average Radius of gyration (Rg), and second virial coefficient (A2) values were estimated from the relation,
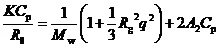
where Rθ is the difference between the Rayleigh ratio of the solution and that of the solvent, K = 4π2n2(dn/dCp)2/NAλ4 with dn/dCp representing the refractive index increment against Cp, NA is Avogadro's number, and q is the magnitude of scattering vector. The q value is calculated from q = (4πn/λ)(sin(θ/2)), where n is the refractive index of the solvent, λ is the wavelength of light source (= 632.8 nm), and θ is the scattering angle. The known Rayleigh ratio of toluene was used to calibrate the instrument. Values of dn/dCp at 633 nm were determined using an Otsuka Electronics Photal DRM-3000 differential refractometer. In the Dynamic Light Scattering (DLS) measurements, to obtain the relaxation time distribution τA (τ), an inverse Laplace Transform (ILT) analysis was performed using the REPES algorithm [18,19]. The relaxation rate (Γ = τ-1) is a function of θ [20]. The Diffusion coefficient (D) is calculated from D = (Γ/q2)q→0. The hydrodynamic radius (Rh) is given by the Stokes-Einstein equation, Rh = kBT/(6πηD), where kB is the Boltzmann constant, T is the absolute temperature, and η is the solvent viscosity. The details of DLS instrumentation and theory are described in the literature [21].
ζ-potential measurements were performed using a Malvern Zetasizer Nano-ZS equipped with a He-Ne laser light source (4 Mw at 632.8 nm) at 20oC. ζ-potential was calculated from the electrophoretic mobility (µ) using the Smoluchowski relationship, ζ = ηµ/ε (κa >> 1) where η is the viscosity, ε is the dielectric constant of the medium, and κ and a are the Debye-Huckel parameter and the particle radius, respectively [22].
Transmission Electron Microscopy (TEM) observations were carried out with a JEOL JEM-2100 microscope at an accelerating voltage of 200 kV. Samples for TEM observations were prepared by placing one drop of aqueous solution on a copper grid coated with thin films of Formvar. Excess water was blotted using a filter paper. The samples were stained with sodium phosphotungstate and dried under vacuum for one day.
RESULTS AND DISCUSSION
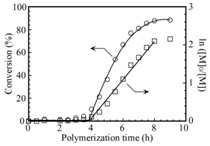
Figure 2: Time-conversion (?) and the first-order kinetic plots (?) for the polymerization of AMPS in the presence of CpD-PEG-CpD in water at 70oC. [M]0 and [M] represent the concentrations of the monomer at polymerization time = 0 and the corresponding time, respectively.
Figure 3 compares GPC elution curves (RI response) for HO-PEG-OH and PAMPS48-PEG227-PAMPS48. Values of Mn and Mw/Mn for all the block copolymers are listed in Table 1. PAMPS48-PEG227-PAMPS48 and PEG47-PMAPTACm, are further abbreviated as AEA and EMm, A, E, M, and m representing PAMPS, PEG, PMAPTAC, and DP of PMAPTAC, respectively.
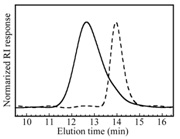
Figure 3: GPC elution curves for a sample of HO-PEG-OH (Mn = 9.40 × 103; Mw/Mn = 1.06) (----) and triblock copolymer of PAMPS48-PEG227-PAMPS48 (AEA, Mn = 2.32 × 104; Mw/Mn = 1.42) (--).
Figure 4 shows 1H NMR spectra of EM53, AEA, and AEA/ EM53 micelle in D2O containing 0.1 M NaCl. DP (= m) and Mn(NMR) of the PMAPTAC block in EMm were determined from the integral intensity ratio of the resonance bands due to the pendant methyl and methylene protons in the PMAPTAC block around 3.1 to 3.4 ppm and the PEG main chain protons at 3.8 ppm. DP and Mn (NMR) of the PAMPS block in AEA were calculated from the integral intensity ratio of the resonance bands due to the pendent methylene protons in the PAMPS block at 3.4 ppm and PEG main chain protons at 3.8 ppm. Figure 4c shows the 1H NMR spectrum of a stoichiometrically charge neutralized mixture of EM53 and AEA in D2O containing 0.1 M NaCl. The intensities of the resonance bands associated with the PMAPTAC pendent methyl protons at 3.1 ppm and PAMPS pendent methyl protons at 1.5 ppm were extremely weak compared with those associated with the PEG main chain protons at 3.8 ppm. This observation suggested that motions of the PMAPTAC and PAMPS blocks were restricted as a result of the formation of PIC by these oppositely charged block chains. On the other hand, the motion of the PEG blocks was not restricted due to the formation of PEG shells.
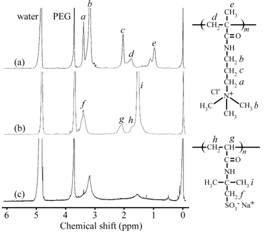
Figure 4: 1H NMR spectra for (a) EM53, (b) AEA, and (c) AEA/EM53 micelle in D2O containing 0.1 M NaCl at 20°C. Assignments are indicated for the resonance peaks.
If the polymerization is assumed to be ideally living in nature, then the theoretical Mn (Mn(theo)) can be calculated as
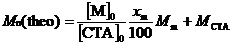
where [M]0 is the initial molar concentration of monomer, [CTA]0 is the initial molar concentration of Chain Transfer Agent (CTA), xm is the percentage conversion of the monomer, Mm is the molecular weight of the monomer, and MCTA is the molecular weight of CTA. The Mn(NMR) values for EMm and AEA were calculated from 1H NMR data. As shown in Table 1, the Mn(NMR) values for EMm and AEA were in reasonable agreement with Mn(theo). However, the Mn(theo) and Mn(GPC) values for EMm and AEA were slightly different, because poly(2-vinylpyridine) or poly(sodium styrenesulfonate) were used as a standard polymer to calibrate Mn(GPC), respectively, and its volume-to-mass ratio may be different from that of EMm and AEA [24].
| Samples | Mn(theo)a × 10-4 | Mn(NMR)b ×10-4 | Mn(GPC)c ×10-4 | Mw/Mnc | Rhd (nm) | ?-potential (mV) |
| EM27 | 0.78 | 0.83 | 0.82 | 1.03 | 4.5 | 18.2 |
| EM53 | 1.36 | 1.41 | 1.11 | 1.02 | 4.3 | 24.2 |
| EM106 | 2.52 | 2.58 | 1.51 | 1.02 | 6.1 | 25.4 |
| AEA | 3.21 | 3.26 | 2.32 | 1.42 | 6.1 | -14.4 |
Table 1: Number-average Molecular weight (Mn), Molecular weight distribution (Mw/Mn), hydrodynamic radius (Rh), and ζ-potential for the polymers.
aCalculated from Equation (2), bEstimated from 1H NMR, cEstimated from GPC, dEstimated from DLS.
Figure 5 shows light scattering intensities and hydrodynamic radius (Rh) for a mixture of AEA and EMm in 0.1 M NaCl as a function of fAMPS (= [AMPS]/([AMPS] + [MAPTAC])). The total polymer concentration (Cp) was kept constant at 1 g/L. At Cp = 1 g/L of AEA/EMm, an increase in viscosity of the solution cannot be observed, which indicates that network formation due to open association of interpolymer electrostatic interaction cannot be occurred. If the Cp value increases, the solution viscosity may increase. However, in this study we focused on PIC flower micelles at diluted state (Cp = 1 g/L). An increase in the scattering intensity indicates an increase in the size of the micelle. Maximum Rh and scattering intensity were observed at close to stoichiometric charge neutralization of PAMPS and PMAPTAC segments. The PIC micelles with maximum Rh and scattering intensity, i.e., AEA/EM106, AEA/EM53, and AEA/EM27 with fAMPS = 45, 50, and 45 %, respectively, were used in this study unless otherwise stated.
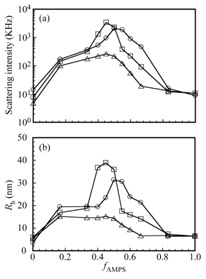
Figure 5: (a) Light scattering intensities and (b) Rh for PIC micelles of AEA/EM106 (?), AEA/EM53 (?), and AEA/M27 (?) as a function of fAMPS (= [AMPS]/([AMPS] + [MAPTAC])) in 0.1 M NaCl aqueous solutions. [AMPS] and [MAPTAC] represent the concentrations of the AMPS and MAPTAC units, respectively. The total polymer concentration was kept constant at 1 g/L.
ζ-potential of EMm and AEA are presented in Table 1. The ζ-potential values for EMm were positive values, which increased with increasing DP of the cationic PMAPTAC block. The ζ-potential value for AEA was negative due to pendant sulfonate anions in the PAMPS blocks. When EMm and AEA were mixed to prepare PIC micelles, the ζ-potential values for PIC micelle of AEA/EMm were close to zero (Table 2). This observation suggested that AEA and EMm were almost stoichiometric charge neutralization of PAMPS and PMAPTAC segments.
| PIC micelles | Mwa × 10-5 | Rga | Rhb | Rg/Rh | Naggc | dPICd |
?-potential (mV) |
| (nm) | (nm) | ||||||
| AEA/EM27 | 8.48 | 15.1 | 15.2 | 0.99 | 50 | 0.096 | -0.88 |
| AEA/EM53 | 189 | 36.6 | 41.0 | 0.89 | 735 | 0.109 | -0.53 |
| AEA/EM106 | 111 | 28.6 | 32.4 | 0.88 | 302 | 0.129 | -0.20 |
Table 2: Dynamic and static light scattering data for PIC micelles in 0.1 M NaCl.
aEstimated by SLS in 0.1 M NaCl, bEstimated by DLS in 0.1 M NaCl, cAggregation number of PIC micelles calculated from Mw(SLS) of PIC micelles determined by SLS and Mw of the corresponding unimers determined by 1H NR and GPC, dDensity calculated from Equation (3).
The Rh and scattering intensity values of AEA/EM53 micelle were the largest compared to those of AEA/EM106 and AEA/EM27 micelles. Kataoka et al., reported that when the anionic and cationic chain length of block copolymers are approximately the same, the aggregation number (Nagg), defined as the total number of polymer chains forming one micelle, is the largest of the PIC micelles [11]. The values of DP for anionic PAMPS in AEA and cationic PMAPTAC in EM53 are 48 and 53, respectively. AEA and EM53may form complex easily, because these DP values of charged blocks in AEA/EM53 are closer than those of AEA/EM106 and AEA/M27. Therefore, a pair of AEA/EM53 micelles may have the largest Nagg.
Values of Rh for the block copolymers were determined by DLS at Cp = 1 g/L in 0.1 M NaCl, as listed in Table 1. The Rh values ranging from 4.5 to 6.1 nm appear to be reasonable for unimers of these block copolymers. Figure 6a shows Rh distributions for AEA/EMm micelles. The values of Rh estimated from the distributions were summarized in Table 2. The Rh values of AEA/EM106, AEA/EM53, and AEA/EM27micelles were 32.4, 41.0, and 15.2 nm, respectively. When 1.5 M NaCl was added to the AEA/EM53aqueous solution, the Rh value decreased. This observation suggests that the micelle is dissociated by adding NaCl.
The relaxation rates (Γ) measured at different scattering angles (θ) were plotted as a function of the square of the magnitude of the scattering vector (q2) in Figure 6b. A linear relation passing through the origin indicates that the relaxation modes are virtually diffusive [25]. The Rh value estimated from slope of the Γ versus q2 plot, was found to be in good agreement with the Rh value calculated from the peak of the Rh distribution obtained at θ = 90o (Figure 6a). Because the angular dependence was negligible, Rhvalues were estimated at a fixed θ of 90o. In Figure 6c, the Rh values are plotted against Cp. The Rh values of AEA/EM106, AEA/EM53, and AEA/EM27 micelles were approximately 32, 41, and 15 nm, respectively, which were practically constant independent of Cp in the range of 0.2 to 1 g/L. From DLS results, the stoichiometrically charge neutralized mixture of AEA and EMm may form flower micelles without intermicellar aggregates because of the unimodal Rh distributions and independence of Cp in the range of 0.2 to 1 g/L.
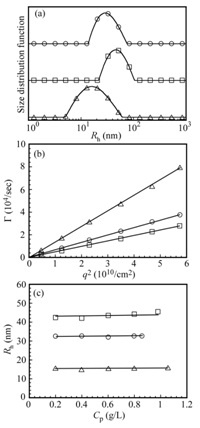
Figure 6: (a) Distributions of Rh for the PIC micelles of AEA/EM106 (?), AEA/EM53 (?), and AEA/EM27 (?) in 0.1 M NaCl aqueous solutions. (b) Relationship between relaxation rate (Γ) and square of the magnitude of the scattering vector (q2). (c) Plots of Rh as a function of Cp.
Figure 7 shows a typical example of Zimm plots for AEA/EM106 micelle. Apparent values of Mw and Rg, determined from Zimm plots, were listed in Table 2. Nagg can be calculated from the ratio of Mw values for PIC micelle and unimer. Nagg for AEA/EM27, AEA/EM53, and AEA/EM106 micelles were 50,735 and 302, respectively. Nagg for AEA/EM53 micelle shows maximum number compared with those of AEA/EM27 and AEA/EM106 micelles.
The Rg/Rh value is useful for characterizing the shape of molecular assemblies. The theoretical value of Rg/Rh for a homogeneous hard sphere is 0.778, however the ratio increases substantially for less dense structures and polydisperse mixtures; for example, Rg/Rh = 1.5 to 1.7 for flexible linear chains in good solvents, whereas Rg/Rh >= 2 for a rigid rod [26-28]. As shown in Table 2, the Rg/Rh ratios for the micelle were found to be 0.88-0.99, which suggested that the shape of PIC micelles was fairly close to spherical shape.
The density of PIC micelles (dPIC) can be calculated by
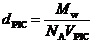
where NA is Avogadro's number and VPIC is the volume of a PIC micelle. VPIC can be calculated to be VPIC= 4πRh3/3. Values of dPIC for AEA/EM27, AEA/EM53 and AEA/EM106 micelles were calculated to be 0.096, 0.109 and 0.129 g/cm3, respectively. These values are close to the density (dPIC = 0.050 - 0.148 g/cm3) of PIC micelles formed from the mixture of PEG-P (Lys) with PEG-P(Asp) [9]. The dPIC value for AEA/EM106micelle with long cationic PMAPTAC block was larger than that for AEA/EM27 micelle with short cationic PMAPTAC block. This observation suggested that PIC micelle of AEA/EMm with short cationic block length may be more hydrated, i.e., the content of water molecules in AEA/EM27 micelle may be larger than that in AEA/EM106 micelle, because the volume of PEG chains in one AEA/EM27 micelle was larger than those in AEA/EM53 and AEA/EM106 micelles.
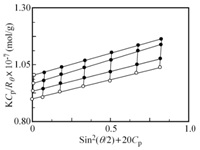
Figure 7: A typical example of Zimm plots for AEA/EM106 micelle in 0.1 M NaCl aqueous solution.
Figure 8 shows TEM images for PIC micelles composed of AEA/EM27, AEA/EM53, and AEA/EM106. The average diameters of PIC flower micelles composed of AEA/EM27, AEA/EM53 and AEA/EM106 estimated from TEM were 14 &plusMn; 4, 53 &plusMn; 2 and 49 &plusMn; 5 nm, respectively, which were smaller than the 2Rh values, estimated from DLS (Table 2). This implied that the PIC micelles shrank after the removal of water in dry state to measure TEM [29]. Spherical shape for PIC micelles can be observed, suggesting that the aggregates are individual flower micelles without intermicellar aggregation.

Figure 8: TEM images for (a) AEA/EM27, (b) AEA/EM53, and (c) AEA/EM106 micelles.
CONCLUSION
ACKNOWLEDGEMENTS
REFERENCES
- Nuopponen M, Kalliomäki K, Aseyev V, Tenhu H (2008) Spontaneous and thermally induced self-oRganization of A-B-A stereoblock polymers of N-isopropylacrylamide in aqueous solutions. Macromolecules 41: 4881-4886.
- Kadam VS, Nicol E, Gaillard C (2012) Synthesis of flower-like poly (ethylene oxide) based macromolecular architectures by photo-cross-linking of block copolymers self-assemblies. Macromolecules 45: 410-419.
- de Graaf AJ, Boere KWM, KEMmink J, Fokkink Rg, van Nostrum CF, et al., (2011) Looped structure of flowerlike micelles revealed by 1H NMR relaxometry and light scattering. Langmuir 27: 9843-9848.
- Lee W, Chang J, Ju S (2010) Hydrogen-bond structure at the interfaces between water/poly (methyl methacrylate), water/poly(methacrylic acid), and water/poly(2-aminoethylmethacrylamide). Langmuir 26: 12640-12647.
- Foreman MB, Coffman JP, Murcia MJ, Cesana S, Jordan R, et al., (2003) Gelation of amphiphilic lipopolymers at the air-water interface: 2D analogue to 3D gelation of colloidal systems with grafted polymer chains. Langmuir 19: 326-332.
- Xu R, Winnik MA, Riess G, Chu B, Croucher MD (1992) Micellization of polystyrene-poly(ethylene oxide) block copolymers in water. 5. A test of the star and mean-field models. Macromolecules 25: 644-652.
- Kabanov VA, Zezin AB, Kasaikin VA, Zakharova JA, Litmanovich EA, et al., (2003) Self-assembly of ionic amphiphiles on polyelectrolyte chains. Polymer International 52: 1566-1572.
- Santis SDA, Ladogana RD, Diociaiuti M, Masci G (2010) Pegylated and thermosensitive polyion complex micelles by self-assembly of two oppositely and permanently charged diblock copolymers. Macromolecules 43: 1992-2001.
- Harada A, Kataoka K (1995) Formation of polyion complex micelles in an aqueous milieu from a pair of oppositely-charged block copolymers with poly (ethylene glycol) segments. Macromolecules 28: 5294-5299.
- Harada A, Kataoka K (1999) Novel polyion complex micelles entrapping enzyme molecules in the core. 2. Characterization of the micelles prepare at nonstoichiometric mixing ratios. Langmuir 15: 4208-4212.
- Harada A, Kataoka K (2003) Effect of charged segment length on physicochemical properties of core-shell type polyion complex micelles from block ionomers. Macromolecules 36: 4995-5001.
- Lokitz BS, Convertine AJ, Ezell Rg, Heidenreich A, Li Y, et al., (2006) Responsive nanoassemblies via interpolyelectrolyte complexation of amphiphilic block copolymer micelles. Macromolecules 39: 8594-8602.
- Li Y, Lokitz BS, McComick CL (2006) Thermally responsive vesicles and their structural "locking" through polyelectrolyte complex formation. Angew Chem Int Ed 118: 5924-5927.
- Xu X, Smith AE, Kirkland SE, McComick CL (2008) Aqueous RAFT synthesis of pH-responsive triblock copolymer mPEO-PAPMA-PDPAEMA and formation of shell cross-linked micelles. Macromolecules 41: 8429-8435.
- Yusa S, Yokoyama Y, Morishima Y (2009) Synthesis of oppositely charged block copolymers of poly (ethylene glycol) via reversible addition-fragmentation chain transfer radical polymerization and characterization of their polyion complex micelles in water. Macromolecules 42: 376-383.
- Mitsukami Y, Donovan MS, Lowe AB, McCormick CL (2001) Water-soluble polymers. 81. Direct synthesis of hydrophilic styrenic-based homopolymers and block copolymers in aqueous solution via RAFT. Macromolecules 34: 2248-2256.
- Achilleos M, Legge TM, Perrier S, Patrickios CS (2008) Poly (ethylene glycol)-based amphiphilic model conetworks: Synthesis by RAFT polymerization and characterization. J Polym Sci Part A: Polym Chem 46: 7556-7565.
- Jakes J (1995) Regularized Positive Exponential Sum (REPES) program - A way of inverting Laplace transform data obtained by dynamic light scattering. Collect Czech Chem Commun 60: 1781-1797.
- Brown W, Nicolai T, Hvidt S, Stepanek P (1990) Relaxation time distributions of entangled polymer solutions from dynamic light scattering and dynamic mechanical measurements. Macromolecules 23: 357-359.19. Brown W, Nicolai T, Hvidt S, Stepanek P (1990) Relaxation time distributions of entangled polymer solutions from dynamic light scattering and dynamic mechanical measurements. Macromolecules 23: 357-359.
- Stockmayer WH, Schmidt M (1982) Effects of polydispersity, branching and chain stiffness on quasielastic light scattering. Pure Appl Chem 54: 407-414.
- Phillies GDJ (1990) Quasielastic light scattering. Anal Chem 62: 1049-1057.
- Ali SI, Heuts JPA, van Herk AM (2010) Controlled synthesis of polymeric nanocapsules by RAFT-based vesicle templating. Langmuir 26: 7848-7858.
- Donovan MS, Lowe AB, Sumerlin BS, McCormik CL (2002) RAFT polymerization of N,N-dimethylacrylamide utilizing novel chain transfer agents tailored for high reinitiation efficiency and structural control. Macromolecules 35: 4123-4132.
- Yusa S, Fukuda K, Yamamoto T, Ishihara K, Morishima Y (2005) Synthesis of well-defined amphiphilic block copolymers having phospholipid polymer sequences as a novel biocompatible polymer micelle reagent. Biomacromolecules 6: 663-670.
- Xu R, Winnik MA, Hallett FR, Riess G, Croucher MD (1991) Light-scattering study of the association behavior of styrene-ethylene oxide block copolymers in aqueous solution. Macromolecules 24: 87-93.
- Huber K, Bantle S, Lutz P, Burchard W (1985) Hydrodynamic and thermodynamic behavior of short-chain polystyrene in toluene and cyclohexane at 34.5°C. Macromolecules 18: 1461-1467.
- Akcasu AZ, Han CC (1979) Molecular weight and temperature dependence of polymer dimensions in solution. Macromolecules 12: 276-280.
- Konishi T, Yoshizaki T, Yamakawa H (1991) On the "universal constants" ? and F. of flexible polymers. Macromolecules 24: 5614-5622.
- Zhang M, Xue YN, Liu M, Zhuo RX, Huang SW (2010) Biocleavable polycationic micelles as highly efficient gene delivery vectors. Nanoscale Res Lett 5: 1804-1811.
Citation: Yokoyama Y, Enomoto R, Yusa SI (2014) Polyion Complex (PIC) Flower-shaped Nano-micelles formed from Anionic Triblock and Cationic Diblock Copolymers. J Nanotechnol Nanomed Nanobiotechnol 1: 001.
Copyright: © 2014 Shin-ichi Yusa, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.