
Toxicity Potential of Different Azadirachtin against Plutella Xylostella (Lepidoptera; Plutellidae ) and its Natural Enemy, Diadegma Species
*Corresponding Author(s):
Hayat ZadaAgriculture Services Academy, Peshawar, Pakistan
Tel:+92 03440920270,
Email:hayat82pk@yahoo.com
Abstract
The present study was conducted to assess different concentrations of azadirachtin against 3rd and 4thinstar larvae of P. xylostella and to determine their effects on natural enemy, Diadegma sp. Four different concentrations (0.31, 0.50, 0.60 and 1%) of azadirachtin were evaluated in the choice and non-choice tests methods. In the choice test, the LC50 value for the 3rd instar larvae were 0.66, 0.41 and 0.37 µg/ml on day 1st, 2nd and 3rd, respectively. Similarly the LC50 values for the 4th instar larvae were 0.55, 0.34 and 0.34 µg/ml after 24, 48 and 72 hours of treatment, respectively. Descending order of toxicity for azadirachtin was observed in no choice method. The LC50 values for the 3rd instar larvae in no choice test were 0.63, 0.32 and 0.29 µg/ml on 24, 48 and 72 hours of treatment, respectively. LC50 value for the 4thinstar larvae in no choice test were 0.52, 0.36 and 0.31 µg/ml on day 1st, 2nd and 3rd respectively. Maximum repellencies for 3rd (95%) and 4th instar larvae (90%) were noticed at 1% concentration. Highest mortalities of Diadegma sp were recorded for 1% concentration which started decreasing with decrease in tested concentrations. High LC50 values (4.91 and 1.63ppm) were recorded for Diadegma sp after 24 and 48h exposer time. Likewise, maximum adults emerged from pupae in lowest concentration 0.31% which increased with increase in concentration. Concluding, for better management of azadirachtin should be optimized with Diadegma sp.
Keywords
Biopesticide; Bio-rational; Dose-response; Eco-friendly; LC50
INTRODUCTION
Due to indiscriminant use of insecticides in developing countries, this pest has developed resistance to different groups of chemicals and adverse effects on natural enemies has also been noticed. Besides, negative impacts of these insecticides and strict government regulation have created interest in the preparation and use of botanical pesticides [3-6].
Azadirachtin is neem based insecticide derived from extracts of Azadirachta indica (Meliaceae). This product has played a vital role in crop protection in the last decade. This insecticide is very complex terpenoid, has been successfully used not only for the management of this pest, but against more than 400 species of insects. Azadirachtin has proved to be one of the most promising plant products for integrated pest management [7-11].
This compound can be used against insects as oviposition deterrent, anti-feedant, repellent, molting inhibitor, sterilant, growth retardant and preventing larvae from developing into adults [9,12,13].
Different azadirachtin concentrations have been used for the management of P. xylostella and other pest insects of cruciferous crops [14,15]. Neem based products can significantly reduce the pest damage on cauliflower and increase marketable head weight. Nonetheless, the efficacy of three new products of neem against P. xylostella has not been determined in USA [14].
Besides, this product has less toxic effect on natural enemies and environment [9,13]. Neem based insecticides are non-toxic to human and many beneficial insects, hence this product has regularly used many pest management programs [11,16,17].
Though there are many studies on crops regarding P. xylostella and Diadegma sp the main purpose of our study was to optimize the use of azadirachtin and Diadegma sp against P. xylostella. For these purpose different concentrations of azadirachtin were used to calculate percent mortality, larval repellency and its effect on its natural enemy in the laboratory.
MATERIALS AND METHODS
Host: Brassica oleracea italica
Fifteen days old broccoli Green seedlings were bought from a native nursery (Pohlmans Nursery) along with plant media. Media was transferred to 50 pots and a single broccoli seedling was then grown in each pot and were maintained at 25±2°C and 55±7% Relative Humidity (RH).
Rearing of P. xylostella in the laboratory
After oviposition, seedlings with eggs were removed from the cages and kept caged until the eggs hatched. Seedlings with newly-hatched larvae were transferred to large cages (120×60×60 cm) in the insect rearing room where they were kept until the larvae pupated. Pupae were then harvested and transferred to the laboratory, some for mating immediately, the rest kept at 4°C until needed to maintain the population
Plant insecticides
Repellency effect and developmental disruption (Larval Stage)
Laboratory conditions for the experiments were maintained at 25ºC±2 (temperature) and 54±10% (relative humidity). During the course of experiment, recordings were made for the following categories of effects:
• Repellency test (Choice and No choice method)
• Dose-response mortality
Extract efficacy and repellence effect against parasitoids of Diadegma sp Parasitoids of P. xylostella
Data analysis
RESULTS
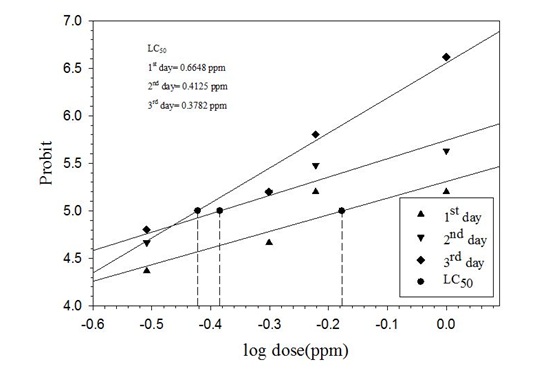
Percent mortality of P. xylostella larvae (Choice Method)
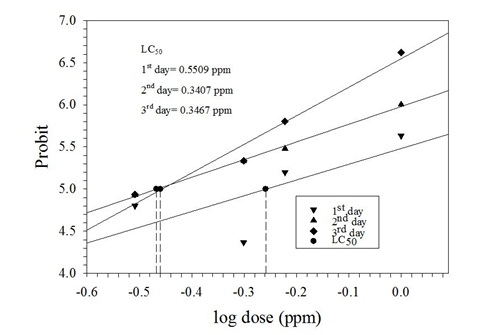
Figure 1: Graph showing the LC50 values of azadirachtin concentrations against 4th instar larvae of P. xylostella (L.) in choice test.
|
Concentration (%) |
Percent Mortality of 3rd instar |
Percent Mortality of 4th instar |
||||
|
24 h |
48 h |
72 h |
24 h |
48 h |
72 h |
|
|
0.31 |
30 b |
40 b |
45 c |
45 b |
50 b |
55 b |
|
0.5 |
40 ab |
60 ab |
60 bc |
40 b |
60 b |
65 b |
|
0.6 |
60 a |
70 a |
80 ab |
55 ab |
65 b |
80 a |
|
1 |
60 a |
75 a |
95 a |
75 a |
85 a |
95 a |
|
LSD value (0.05) |
29.5 |
21.32 |
20.86 |
24.91 |
16.51 |
15.07 |
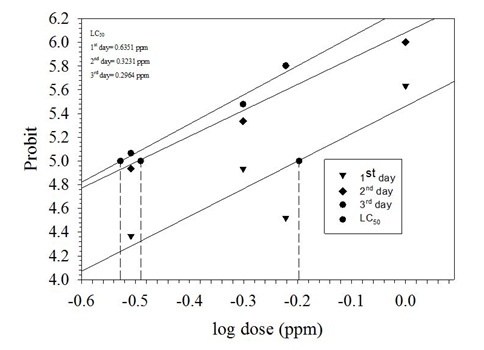
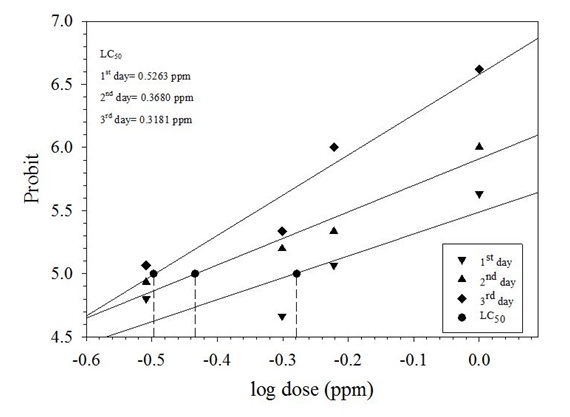
Mortality of P. xylostella (No Choice Method)
|
Concentration (%) |
Percent Mortality of 3rd instar |
Percent Mortality of 4th instar |
||||
|
24 h |
48 h |
72 h |
24 h |
48 h |
72 h |
|
|
0 |
0 c |
5 d |
10 d |
0 c |
0 c |
5 c |
|
0.31 |
30 b |
50 c |
55 c |
45 b |
50 b |
55 b |
|
0.5 |
50 b |
65 bc |
70 bc |
40 b |
60 b |
65 b |
|
0.6 |
35 b |
80 ab |
80 b |
55 ab |
65 b |
85 a |
|
1 |
75 a |
85 a |
100 a |
75 a |
85 a |
95 a |
|
LSD value (0.05 ) |
21.31 |
17.83 |
16.96 |
24.91 |
16.51 |
15.07 |
Repellency test
| Concentration (%) | Repellency effect against 3rd instar larvae (%) | Repellency effect against 4th instar larvae (%) |
| 0 | 0 c | 0 d |
| 0.31 | 60 b | 40 c |
| 0.5 | 65 b | 75 b |
| 0.6 | 90 a | 85 ab |
| 1 | 95 a | 100 a |
| LSD value (0.05 ) | 16.51 | 21.31 |
Percent adult mortality of Diadegma sp
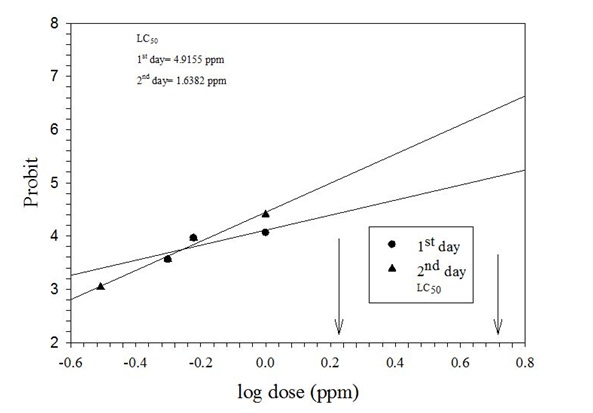
| Concentration (%)r | Percent Mortality after 24 h (%) | Percent Mortality after 48 h (%) | Mean (%) |
| 0.31 | 0.00 c | 2.50 cd | 1.25 |
| 0.5 | 7.50 b | 7.50 c | 7.5 |
| 0.6 | 15.00 a | 15.00 b | 15 |
| 1 | 17.50 a | 27.50 a | 22.5 |
| Control | 0.00 c | 0.00 d | 0 |
| LSD value (0.05 ) | 6.15 | 7.01 | ---- |
Percent adult emergence of Diadegma sp
| Concentrations (%) | Emergence after 24 h (%) | Emergence after 48 h (%) | Emergence after 72 h (%) | Mean (%) |
| 0.31 | 35.00 b | 77.50 a | 90.00 a | 67.5 |
| 0.5 | 30.00 b | 40.00 b | 60.00 b | 43.33 |
| 0.6 | 27.50 b | 35.00 b | 57.50 b | 40 |
| 1 | 22.50 b | 27.50 b | 52.50 b | 34.17 |
| Control | 60.00 a | 82.50 a | 97.50 a | 80 |
| LSD value (0.05 ) | 12.61 | 16.16 | 13.62 | --- |
Means in columns followed by different letters are significantly different at α = 0.05 level of significance by using Fischer's Protected LSD test.
DISCUSSION
Order of toxicity of different azadirachtin concentrations was found to be 1% > 0.6% > 0.5% > 0.31%. Additionally, time of exposure also increased the lethal and repellent activity of azadirachtin. The results are in accordance with the findings of as they reported insecticidal and repellent activities of plant parts increased with increase in dose and time. In the present study, it was further concluded that a 0.6-1% concentration would be the highest and most appropriate dosage for the management of P. xylostellabecause no phytotoxic effects were found in the treated plants after 24 hour application of this extract. Similarly, these compounds had lethal effects on diamondback moth larvae and neem oil reduced egg hatching and survival larvae of H. armigera [30,31]. Likewise, repellency increased with increase in concentration for 3rd instar larvae. Similarly, for 4th instar larvae, maximum repellency was observed at 1% concentration whilst all other concentrations including control attracted maximum number of 4thinstar larvae.
It is usually assumed that parasitoids life history (e.g., growth, survival, and adult emergence) respond directly to host properties such as body size [33,34]. A variety of studies has shown that host size influences parasitoid size and survival, and that parasitoid size is positively correlated with other fitness factors such as fecundity, mating capacity and longevity [35].
The P. xylostella feeding on broccoli plants treated with azadirachtin are smaller than those that have been feeding on control plants [36]. The development time of Diadegma sp would therefore be significantly prolonged if it were to wait for the host to develop a larger size. Results from this study confirmed that the time until cocoon formation of Diadegma sp is significantly longer if they have been feeding on P. xylostella that have been exposed to botanical compounds. This may indicate that there is some delay in development time of Diadegma sp feeding on larvae that have been exposed to the botanical pesticides, which would ensure that P. xylostella had increased sufficiently in size.
With further demonstration of their efficacies, these concentrations should enhance the management of lepidopteran pests in the vegetable agroecosystems because they do not persist in the environment, have unique modes of action, low mammalian toxicity, and may be potentially compatible with natural enemies. Azadirachtin extracts seem to be suitable for combination with biological control, as these extracts did not have a detrimental effect on longevity or behaviour of Diadegma sp, one of the most abundant parasitoid species found in the field in Australia.
CONCLUSION
ACKNOWLEDGEMENT
REFERENCES
- Ninsin KD (2015) Cross-resistance assessment in cartap- and esfenvalerateselected strains of the diamondback moth, Plutella Xylostella (L.) (Lepidoptera: Plutellidae). West African Journal of Applied Ecology 23.
- Neto JEL, Amaral MHP, Siqueira HAA, Barros R, Silva PA (2016) Resistance monitoring of Plutella Xylostella (L.) (Lepidoptera: Plutellidae) to risk-reduced insecticides and cross resistance to spinetoram. Phytoparasitica 44: 631-640.
- Magaro JJ, Edelson JV (1990) Diamondback Moth (Lepidoptera: Plutellidae) in South Texas: A Technique for Resistance Monitoring in the Field. J Econ Entomol 83: 1201-1206.
- Ascher KRS (1993) Nonconventional Nonconventional insecticidal effects of pesticides available from the Neem tree, Azadirachta indica. Arch Insect Biochem Physiol 22: 433-449.
- Saljoqi AUR, Salim M, Rehman S, Khalil SK, Khurshid I (2015) Field Application of Trichogramma chilonis (Ishii) for the Management of Sugarcane Borers. Pakistan J Zool 47: 783-791.
- Naqqash MN, Gökçe A, Bakhsh A, Salim M (2016) Insecticide resistance and its molecular basis in urban insect pests. Parasitol Res 115: 1363-1373.
- Leskovar DI, Boales AK (1996) Azadirachtin: Potential Use for Controlling Lepidopterous Insects and Increasing Marketability of Cabbage. HortScience 31: 405-409.
- Rembold H (1989) Isomeric azadirachtins and their mode of action. In: Jacobson M (ed.). Focus on Biochemical Pesticides, I. The Neem Tree. CRC Press, Boca Raton, USA. Pg no: 47-67.
- Schmutterer H (1990) Properties and potential of natural pesticides from the neem tree, Azadirachta indica. Annu Rev Entomol 35: 271-297.
- Isman MB (1999) Neem and Related Natural Products. In: Hall FR, Menn JJ (eds.). Biopesticides: Use and Delivery. Methods in Biotechnology, Humana Press, New York, USA. 5: 139-153.
- Walter JF (1999) Commercial experience with neem products. In: Hall FR, Menn JJ (Eds.). Biopesticides: Use and Delivery. Methods in Biotechnology. Humana Press, New York, USA. 5: 155-170.
- Mordue AJ, Blackwell A (1993) Azadirachtin: an update. J Insect Physiol 39: 903-924.
- Schmutterer H, Ascher KRS (1995) The neem tree: Azadirachta indica A. Juss. and other meliaceous plants : sources of unique natural products for integrated pest management, medicine, industry and other purposes. VCH, Weinheim, Germany. Pg no: 696.
- Leskovar DI, Boales AK (1996) Azadirachtin: Potential Use for Controlling Lepidopterous Insects and Increasing Marketability of Cabbage. Hortic Sci 31: 405-409.
- Perera DR, Armstrong G, Senanayake N (2000) Effect of antifeedants on the diamondback moth (Plutella xylostella) and its parasitoid Cotesia plutellae. Pest Manage Sci 56: 486-490.
- Feng R, Isman MB (1995) Selection for resistance to azadirachtin in the green peach aphid, Myzus persicae. Experientia 51: 831-833.
- Immaraju JA (1998) The commercial use of azadirachtin and its integration into viable pest control programmes. Pest Manag Sci 54: 285-289.
- Talekar NS, Ying Lin M (1998) Training Manual on IPM of Diamondback Moth. AVRDC, Shanhua, Taiwan.
- Park B-S, Lee S-E, Choi W-S, Jeong C-Y, Song C, et al. (2002) Insecticidal and acaricidal activity of pipernonaline and piperoctadecalidine derived from dried fruits of Piper longum L. Crop Protection 21: 249-251.
- Steel RGD (1997) Principles and Procedures of Statistics: A Biometrical Approach (3rdedn). McGraw-Hill, New York, USA. Pg no: 666.
- Finney D (2009) Probit analysis. Cambridge University Press, Cambridge, UK.
- Chi H (1997) Computer program for the probit analysis. National Chung Hsing University, Taiwan.
- Reddy PP (2016) Biorational Pest Management. In: Reddy PP (ed.). Sustainable Crop Protection under Protected Cultivation. Springer, Berlin, Germany. Pg no: 99-108.
- Bezzar-Bendjazia R, Kilani-Morakchi S, Aribi N (2016) Larval exposure to azadirachtin affects fitness and oviposition site preference of Drosophila melanogaster. Pestic Biochem Physiol 133: 85-90.
- Greenberg SM, Showler AT, Liu TX (2005) Effects of neem?based insecticides on beet armyworm (Lepidoptera: Noctuidae). Insect Sci 12: 17-23.
- Arnason JT, Philogere BJR, Donskov N, Hudon M, McDougall C, et al. (1985) Antifeedant and insecticidal properties of azadirachtin to the European Corn Borer, Ostrinia nubilalis. Entomol Exp Appl 38: 29-34.
- Tanzubi PB (1995) Effects of neem Azadirachta indica (A. Juss) extracts on food intake and utilization in the African armyworm, Spodoptera exempta (Walker). Insect Sci Appl 16: 167-170.
- Mehta PK, Vaida DN, Kashyap NP (1995) Antifeedant properties of some plant extracts against brinjal hadda beetle Henosepilachna vigintioctopunctata. J Entomol Res 19: 147-150.
- Schmutterer H (1988) Potential of azadirachtin-containing pesticides for integrated pest control in developing and industrialized countries. J Insect Physiol 34: 713-719.
- Pugazhvendan SR, Narendiran NJ, Kumaran R, Kumaran S, Alagappan K (2009) Effect of Malathion Toxicity in the Freshwater Fish Ophiocephalus punctatus-A Histological and Histochemical Study. World Journal of Fish and Marine Sciences 1: 218-224.
- Ma D-L, Gordh G, Zalucki MP (2000) Biological effects of azadirachtin on Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) fed on cotton and artificial diet. Aust J Entomol 39: 301-304.
- Schneider A, Madel G (1992) Fekunditat und Vitalitat adulter Schlupfwespen nach Exposition auf Niem (Azadirachta indica) behandelten Flachen. Mitteilungen der Deutschen Gesellschaft fuer Allgemeine und Angewandte Entomologie Pg no: 273-278.
- Godfray HCJ (1994) Parasitoids: Behavioural and Evolutionary Ecology. Princeton University Press, New Jersey, USA.
- Visser ME (1994) The importance of being large: the relationship between size and fitness in females of the parasitoid Aphaereta minuta (Hymenoptera: Braconidae). J Anim Ecol 63: 963-978.
- Harvey JA, Kadash K, Strand MR (2000) Differences in larval feeding behavior correlate with altered developmental strategies in two parasitic wasps: implications for the size?fitness hypothesis. Oikos 88: 621-629.
- Charleston DS (2004) Integrating biological control and botanical pesticides for management of Plutella xylostella. Wageningen University, Netherlands.
Citation: Zada H, Ahmad B, Hassan E, Ur Rehman Saljoqi A, Naheed H, et al. (2018) Toxicity Potential of Different Azadirachtin against Plutella Xylostella (Lepidoptera; Plutellidae) and its Natural Enemy, DiadegmaSpecies. J Agron Agri Sci 1: 003.
Copyright: © 2018 Hayat Zada, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

