
Journal of Cytology & Tissue Biology Category: Clinical
Type: Research Article
3D Cell Culture on VitroGel System
*Corresponding Author(s):
John HuangDepartment Of Biotechnology, TheWell Bioscience, New Jersey, United States
Tel:+1 9738554955,
Email:info@thewellbio.com, hz_huang@hotmail.com
Received Date: May 29, 2019
Accepted Date: Jun 24, 2019
Published Date: Jul 01, 2019
Abstract
Standard Two-Dimensional (2D) cell culture systems have facilitated research of cellular and molecular biology for decades; however, they have also engendered significant limitations in replicating in vivoconditions including inaccurate duplication of physiological cell-cell interaction, changes in gene expression between in vivo and in vitro conditions, and unbalanced nutrient supply. Three-Dimensional (3D) cell culture models have been shown to be more accurate; they can overcome many of the limitations of two-dimensional culture and can and allow for dynamic culture environment. As we have learned more about how cells interact with scaffolds, we have come to understand that, similar to the 2D environment, many variables of the 3D scaffold including stiffness, density, porosity, and composition can alter cellular response. Moreover, while some animal-derived hydrogel system have been successful at mimicking an in vivo environment, comparative in vivo/in vitro studies or clinical applications are very difficult on such undefined, batch-to-batch different hydrogel system. Here we discuss some of the different applications of the versatile VitroGelsystem. VitroGelis a ready-to-use, xeno-free tunable hydrogel system that closely mimics the natural Extracellular Matrix (ECM) by creating a functional and optimized environment to make cells feel like at home. This hydrogel system can bridge the in vitro and in vivo studies and allows for comparable 2D and 3D studies platforming the same platform system.
Keywords
3D cell culture; Cancer; Drug screen; Hydrogel; VitroGel
INTRODUCTION
Since its inception nearly a century ago, 2D cell culture has been used to study cellular responses to intra - and extracellular stimuli [1]. Although 2D approaches have made a substantial contribution to our understanding of cell behavior, the repeated publication of research identifying and discussing the limitations of 2D cell culture have underlain the ambition to develop novel 3D culture models. Conventional 2D cell culture relies adherence to a hard, flat surface as a means of providing mechanical support for the cells. Cells grow in monolayers allowing for access to a constant amount of nutrients, growth factors and any other small molecules, which result in homogenous growth and proliferation [2]. Although this can seem ideal for the purpose of study, this is not an accurate representation of the in vivoenvironment, a necessary aspect for understanding tissue regeneration and studying tumor microenvironments, which studies have demonstrated to have direct and indirect effects on cellular response.
The advent of three-dimensional cell culture began in the 1980s after researchers began to understand the various physiological differences that occur when cells are cultured ex vivo. One of the earliest 3D scaffolds was developed in 1988 by Eric Simon, who was conducting an NIH sponsored feasibility study for fibrous scaffold inserts to facilitate 3D tissue culture [3]. Although he concluded that the electrospun polycarbonate scaffolds were inferior to standard 2D tissue culture, this initial study identified several of improvement including lack of mechanical strength, low-level adhesion of the cells to the scaffold that resulted in unintentional dissociation, and inability to image cells within the scaffold. Since that time, researchers have developed various scaffolds that have successfully addressed these issues and promoted cell growth. For example, although the initial polymer scaffold developed by Simon was unsuccessful, scientists have since found other ways to develop polymer scaffolds that very closely mimic the extracellular matrix and allow cells to settle in and incorporate into pre-fabricated geometric scaffolds with consistent pore sizes [4]. Some of the more common materials to make these scaffolds include polystyrene and polycaprolactone, which have been popular for use in bone regeneration studies because to generate scaffolds that closely mimic the structure of the bone matrix [5]. There are also scaffolds known as biological scaffolds which are built using components that are found naturally in the in vivoextracellular matrix. For example, collagen matrices, which are primarily intended to mimic cartilage for the purposes of joint regeneration [6,7], can be altered for use in other applications such as ligament or skin regeneration. However, they have limitations related to their rigid mechanical properties [8,9].
Cells cultured in 3D environments have many advantages over 2D culture in terms of mimicking the in vivo environment. They exhibit more realistic distribution and cellular access to oxygen and nutrients/media components, they experience increased cell-to-cell and cell-to-ECM interactions [10], and they can develop district zones of different cell proliferation or differentiation ability [11]. However, research has demonstrated that a single cell line cultured in different 3D systems can exhibit different cellular behavior [12-14]. Conversely, within a single 3D system, some cells may have a positive response, while others may fail to thrive, making it different to study multicellular tissues within a single 3D model [15]. Moreover, many 3D scaffolds cannot be used in both in vitro and in vivo settings, making it difficult to conduct crossover studies or to culture cells in vitro for the purposes of transplantation [16].
One type of scaffold that may be able to overcome some of the limitations of previous scaffolds is hydrogel. A hydrogel is a versatile macromolecular polymer gel comprised of crosslinked polymer chains synthesized from hydrophilic monomers. Hydrogels can be used to generate multi-layer tissue-like structures during cell culture: different cell types can be embedded into separate hydrogel suspensions and layered on top of each other. Permeable supports can also be incorporated to simulate various air-liquid interfaces in an in vitro manner [17]. The hydrogel system can be composited with growth factors and biological functional ligands, which is able to better promote cellular function, leading to increased viability and recapitulating a more accurate in vivo like cellular environment. Despite these many advantages, hydrogels also have several limitations. In research, hydrogels are often too opaque to allow for live imaging, instead requiring dissociation of cells for successful imaging [16,18]. With regard to clinical applications, hydrogels tend to have low tensile strength which can limit their use in load-bearing applications like bone engineering. The tensile strength can also impede drug discovery applications; it can cause premature dissolution of the hydrogel [19]. Moreover, hydrogels have not been ideal in applications involving drug delivery, particularly if the drugs are hydrophobic. Their water content and large pore size can impede drugs that need to be released over longer periods of time, releasing the drug too quickly. Each of these issues significantly restricts the practical use of hydrogel-based drug delivery therapies in the clinic [20]. Additionally, most current hydrogel systems are limited by such conditions as poor scaffold structure, unknown growth factors, and undesirable pH or temperature of the pre-gel solution, which lead to the challenges of complex operation, difficulties in downstream analysis, and product reproducibility.
VitroGel is a ready-to-use, xeno-free tunable hydrogel system which closely mimics the natural extracellular matrix environment. The hydrogel system is room stable at room temperature, has a neutral pH and is transparent, permeable and compatible with different imaging systems. The solution transforms into a tunable hydrogel matrix simply by mixing with cell culture medium, which shorten the operation time from hours to 20 min Cells cultured in this system can also be easily harvested for downstream analysis. The hydrogel can also be tuned to be injectable for in vivo studies. From 2D coating and 3D culture to animal injection, VitroGel makes it possible to bridge the in vitro and in vivo studies with the same platform system. VitroGel offers solutions to many of the existing problems presented by hydrogels. Mechanical properties and rate of hydrogel can be adjusted and the biological functional of the hydrogel system can be modified to mimic the endogenous physiological microenvironment. Since different cell types prefer different tissue-specific microenvironments, the hydrogel conditions can be optimized for different cell types and culture media, allowing for the ideal environment to be created for each cell type in multicellular applications. In addition to offering these solutions, VitroGel can be used in 2D applications and 3D in vitro and in vivo applications, offering a single platform by which to bridge the gap between traditional 2D system and in vivo study.
The advent of three-dimensional cell culture began in the 1980s after researchers began to understand the various physiological differences that occur when cells are cultured ex vivo. One of the earliest 3D scaffolds was developed in 1988 by Eric Simon, who was conducting an NIH sponsored feasibility study for fibrous scaffold inserts to facilitate 3D tissue culture [3]. Although he concluded that the electrospun polycarbonate scaffolds were inferior to standard 2D tissue culture, this initial study identified several of improvement including lack of mechanical strength, low-level adhesion of the cells to the scaffold that resulted in unintentional dissociation, and inability to image cells within the scaffold. Since that time, researchers have developed various scaffolds that have successfully addressed these issues and promoted cell growth. For example, although the initial polymer scaffold developed by Simon was unsuccessful, scientists have since found other ways to develop polymer scaffolds that very closely mimic the extracellular matrix and allow cells to settle in and incorporate into pre-fabricated geometric scaffolds with consistent pore sizes [4]. Some of the more common materials to make these scaffolds include polystyrene and polycaprolactone, which have been popular for use in bone regeneration studies because to generate scaffolds that closely mimic the structure of the bone matrix [5]. There are also scaffolds known as biological scaffolds which are built using components that are found naturally in the in vivoextracellular matrix. For example, collagen matrices, which are primarily intended to mimic cartilage for the purposes of joint regeneration [6,7], can be altered for use in other applications such as ligament or skin regeneration. However, they have limitations related to their rigid mechanical properties [8,9].
Cells cultured in 3D environments have many advantages over 2D culture in terms of mimicking the in vivo environment. They exhibit more realistic distribution and cellular access to oxygen and nutrients/media components, they experience increased cell-to-cell and cell-to-ECM interactions [10], and they can develop district zones of different cell proliferation or differentiation ability [11]. However, research has demonstrated that a single cell line cultured in different 3D systems can exhibit different cellular behavior [12-14]. Conversely, within a single 3D system, some cells may have a positive response, while others may fail to thrive, making it different to study multicellular tissues within a single 3D model [15]. Moreover, many 3D scaffolds cannot be used in both in vitro and in vivo settings, making it difficult to conduct crossover studies or to culture cells in vitro for the purposes of transplantation [16].
One type of scaffold that may be able to overcome some of the limitations of previous scaffolds is hydrogel. A hydrogel is a versatile macromolecular polymer gel comprised of crosslinked polymer chains synthesized from hydrophilic monomers. Hydrogels can be used to generate multi-layer tissue-like structures during cell culture: different cell types can be embedded into separate hydrogel suspensions and layered on top of each other. Permeable supports can also be incorporated to simulate various air-liquid interfaces in an in vitro manner [17]. The hydrogel system can be composited with growth factors and biological functional ligands, which is able to better promote cellular function, leading to increased viability and recapitulating a more accurate in vivo like cellular environment. Despite these many advantages, hydrogels also have several limitations. In research, hydrogels are often too opaque to allow for live imaging, instead requiring dissociation of cells for successful imaging [16,18]. With regard to clinical applications, hydrogels tend to have low tensile strength which can limit their use in load-bearing applications like bone engineering. The tensile strength can also impede drug discovery applications; it can cause premature dissolution of the hydrogel [19]. Moreover, hydrogels have not been ideal in applications involving drug delivery, particularly if the drugs are hydrophobic. Their water content and large pore size can impede drugs that need to be released over longer periods of time, releasing the drug too quickly. Each of these issues significantly restricts the practical use of hydrogel-based drug delivery therapies in the clinic [20]. Additionally, most current hydrogel systems are limited by such conditions as poor scaffold structure, unknown growth factors, and undesirable pH or temperature of the pre-gel solution, which lead to the challenges of complex operation, difficulties in downstream analysis, and product reproducibility.
VitroGel is a ready-to-use, xeno-free tunable hydrogel system which closely mimics the natural extracellular matrix environment. The hydrogel system is room stable at room temperature, has a neutral pH and is transparent, permeable and compatible with different imaging systems. The solution transforms into a tunable hydrogel matrix simply by mixing with cell culture medium, which shorten the operation time from hours to 20 min Cells cultured in this system can also be easily harvested for downstream analysis. The hydrogel can also be tuned to be injectable for in vivo studies. From 2D coating and 3D culture to animal injection, VitroGel makes it possible to bridge the in vitro and in vivo studies with the same platform system. VitroGel offers solutions to many of the existing problems presented by hydrogels. Mechanical properties and rate of hydrogel can be adjusted and the biological functional of the hydrogel system can be modified to mimic the endogenous physiological microenvironment. Since different cell types prefer different tissue-specific microenvironments, the hydrogel conditions can be optimized for different cell types and culture media, allowing for the ideal environment to be created for each cell type in multicellular applications. In addition to offering these solutions, VitroGel can be used in 2D applications and 3D in vitro and in vivo applications, offering a single platform by which to bridge the gap between traditional 2D system and in vivo study.
MATERIALS AND METHODS
Cell culture
Cells were maintained prior to 3D culture according to their respective distributor protocols. PANC-1 (ATCC) cells were maintained in a T-25 flask with DMEM containing 10%FBS + 1x pen-strep and passaged at ~80% confluency. U-87 cells (ATCC) were maintained in Eagle’s Minimum Essential Medium supplemented with 10% FBS and 1x pen-strep, as indicated by the distributor. Cells were passaged when the cultures reached ~80% confluency. OP9 cells (ATCC) were maintained in Alpha Minimum Essential Medium supplemented with 10% FBS, 2.2g/L sodium bicarbonate, and 1x pen-strep, as indicated by the distributor. Cells were passaged when the cultures reached ~80% confluence. HCT116 (ATCC) cells were maintained in T-25 flask with McCoy's 5a with 10% FBS and 1x pen-strep. Cells were passaged when the cultures reached ~80% confluence.
2D coating culture method
2D coating hydrogel was prepared at 1:0, 1:1 and 1:3 dilutions (v/v) with VitroGel Dilution Solution (MS01-100, TheWell Bioscience, NJ) and then mixed with the respective cell culture medium without cells at 4:1 ratio; 300µl added to each 24-well plate. The hydrogel was stabilized at room temperature for 20 minutes, then cells were added on the top of the hydrogel suspended in 300µl of cover medium at a seeding density of 2-5 x 105 cells/mL. Following cell incorporation, culture medium on top of the hydrogel was changed every other day.
3D culture method
Hydrogel solution was diluted with VitroGel Dilution Solution at 1:0, 1:1, 1:2 or 1:3 ratio (v/v). Cell suspension was mixed in at a 4:1 or 2:1 ratio (4mL diluted hydrogel solution with 1mL cell suspension, final cell concentration: 150K to 300K cells/mL). Hydrogel/cell mixture was added to a 24-well plate at 3000µl per well. Hydrogel was then allowed to stabilize at room temperature for 15 min. After stabilization, 300µl complete cell culture medium was added on the top of the hydrogel. On day 2, additional 200µl complete cell culture medium was added to the top medium. After that, the medium was changed according to TheWell Bioscience user handbook (8), 60-70% of top medium changed every other day. In applications where confocal imaging was necessary, cells were cultured 8 well chambers with cover glass on the bottom, using 200µl hydrogel per well. Medium was changed every other day.
PANC-1 and HCT116 cells were cultured in both VitroGel 3D (TWG001, TheWell Bioscience, NJ) and VitroGel 3D-RGD (TWG002, TheWell Bioscience, NJ), while OP9 and U-87 cells were cultured in both VitroGel 3D-RGD, and the newer VitroGel RGD-PLUS (TWG003, TheWell Bioscience, NJ).
PANC-1 and HCT116 cells were cultured in both VitroGel 3D (TWG001, TheWell Bioscience, NJ) and VitroGel 3D-RGD (TWG002, TheWell Bioscience, NJ), while OP9 and U-87 cells were cultured in both VitroGel 3D-RGD, and the newer VitroGel RGD-PLUS (TWG003, TheWell Bioscience, NJ).
Live/Dead assay
8-chambered cover glass slides containing 200µL of hydrogel and 200µL cover medium cover media was removed, and the hydrogel was washed with 3 times with PBS prior to proceeding with the manufacturer’s protocol for Live/Dead assessment using the LIVE/DEADTM Cell Imaging Kit (488/570) (ThermoFisher). Images were then taken using confocal microscopy.
Immunofluorescence
HCT116, PANC-1, OP9 and U-87 cells were grown in chambered cover glass slides in lieu of 24-well plates. Each chamber holds 200µL hydrogel/cell mixture. Cells were fixed and stained by using the Image-iT™ Fixation/Permeabilization Kit, ActinGreen™ 488 ReadyProbes™ Reagent and NucBlue™ Fixed Cell ReadyProbes™ Reagent (ThermoFisher) according to manufacturer protocols. The images were taken using a Leica TCS SP8 scanning confocal microscope with z-stack function at 10X and 20X magnification and processed by LAS X software and ImageJ.
Since OP9 cells have a GFP reporter, we conducted live cell imaging. The images were taken using a Leica TCS SP8 scanning confocal microscope with z-stack function at 10X and 20X magnification and processed by LAS X software and ImageJ.
Since OP9 cells have a GFP reporter, we conducted live cell imaging. The images were taken using a Leica TCS SP8 scanning confocal microscope with z-stack function at 10X and 20X magnification and processed by LAS X software and ImageJ.
Drug testing
HCT116 cells were seeded at 500K cells/mL on top of chambered coverglass slides in VitroGel 3D-RGD (1:1 dilution, 2:1 mixing) for 3 days respectively. On Day 4, the anti-cancer drug (5-fluorouracil, Sigma) was added on the top of both 2D and 3D cultured cells at the concentrations of 0µM, 10µM, 25µM, 50µM. Cells were treated with the drug for 3 days. On Day 7, the top medium was removed, and the live/dead assay was performed according to manufacturer instruction (Thermo Fisher R37601, Live/Dead cell imaging kit). Quantification of Live/Dead Staining was processed using ImageJ.
STATISTICAL ANALYSIS
Statistics were done using an unpaired t-test comparing the drug concentrations to 0µM as a control. P-values less than 0.05 are considered to be significant (*), p-values less than 0.005 are very statistically significant (**) and values less than 0.001 are extremely statistically significant (***).
RESULTS AND DISCUSSION
VitroGel has been used to successfully culture a large series of cells and in many independent studies. Here we discuss only a few of the possible applications of this new adjustable hydrogel.
VitroGel 3D can mimic the tumor microenvironment
Three-dimensional culture is becoming the standard for studying the tumor microenvironment in an in vitro setting. These systems allow for insight into the cell-cell interactions and communications that occur in vivo. Traditionally, 3D culture systems have been established in Matrigel, which changed the face of in vitro study of cancer allowing for proper cell differentiation and organization into 3D spheroids. Despite the innovation, this method introduces some difficulties into experimentation including difficulty in reproducibility from batch to batch and difficulty in controlling the temperature of the experiment leading to poor reproducibility. The VitroGel 3D system has been used for sustained culturing of multiple cell lines one of which is PANC-1, an immortalized line of cells derived from a human pancreatic carcinoma. One of the largest challenges in developing novel treatments for pancreatic cancer has been the high rate of response to treatments in 2D culture but ultimate failure in the clinic. Recent work revealed an in vivo like tumor microenvironment represented in 3D sphere formation with the PANC-1 model [21]. In this study, they elucidated significant alterations in multiple matrix proteins, cell metabolism, and responsiveness to drug treatment when comparing 2D to 3D models. A similar study conducted with the VitroGel system allowed for homogeneous distribution and sphere formation without any of the caveats of Matrigel or traditional hydrogel systems. VitroGel-3D provides a faster, easier and more versatile solution for 3D cell culture and beyond.
PANC-1 cells were cultured in dilutions of hydrogel solution. At day 2 (Figure 1A) in the 3D cell culture, cells were able to distribute homogeneously within the hydrogel (Figure 1), showing no difference between dilutions (not shown). Cells maintained through day 7 showed significant concentration-based differences were seen based on the concentration of the hydrogel (Figures 1B-1D). Individual cells formed colonies in each condition, however, the 1:1 and 1:3 dilutions of hydrogel (Figures 1C,1D) allowed for more rapid division and expansion of the single cells, developing larger 3D colonies. Another critical component of 3D culture comes from the development of an in vivo like extracellular matrix which can be recapitulated in vitro. Act in and nuclear staining (Figures 2A-2C) reveal increasing complexity and dispersion of the ECM generated by the cell-cell contact of PANC-1 cells when maintained in VitroGel 3D-RGD hydrogel. Although different cells may exhibit a distinct response, our studies demonstrated that PANC-1 cells grow into larger spheres with higher dilutions of the hydrogel (Figures 2B,2C). A concern of growing 3D spheres comes from the notion that the center of the sphere may become necrotic [22]. To verify that cells are viable in our 3D system, we conducted a live/dead assay. This assessment revealed no significant death in each of the three hydrogel dilutions (Figures 2D-2F), indicating that the growth rate and complexity of PANC-1 3D spheres cultured in VitroGel more accurately mimic the in vivo environment, thus allowing for better studies for pancreatic cancer.
PANC-1 cells were cultured in dilutions of hydrogel solution. At day 2 (Figure 1A) in the 3D cell culture, cells were able to distribute homogeneously within the hydrogel (Figure 1), showing no difference between dilutions (not shown). Cells maintained through day 7 showed significant concentration-based differences were seen based on the concentration of the hydrogel (Figures 1B-1D). Individual cells formed colonies in each condition, however, the 1:1 and 1:3 dilutions of hydrogel (Figures 1C,1D) allowed for more rapid division and expansion of the single cells, developing larger 3D colonies. Another critical component of 3D culture comes from the development of an in vivo like extracellular matrix which can be recapitulated in vitro. Act in and nuclear staining (Figures 2A-2C) reveal increasing complexity and dispersion of the ECM generated by the cell-cell contact of PANC-1 cells when maintained in VitroGel 3D-RGD hydrogel. Although different cells may exhibit a distinct response, our studies demonstrated that PANC-1 cells grow into larger spheres with higher dilutions of the hydrogel (Figures 2B,2C). A concern of growing 3D spheres comes from the notion that the center of the sphere may become necrotic [22]. To verify that cells are viable in our 3D system, we conducted a live/dead assay. This assessment revealed no significant death in each of the three hydrogel dilutions (Figures 2D-2F), indicating that the growth rate and complexity of PANC-1 3D spheres cultured in VitroGel more accurately mimic the in vivo environment, thus allowing for better studies for pancreatic cancer.
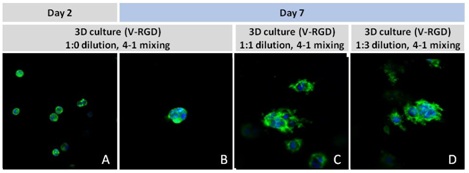
Figure 1: Day 2 and Day 7 representative fluorescent micrographs of PANC-1 cells at three different dilutions of VitroGel 3D-RGD with corresponding antibody staining: NucBlue (nucleus) and ActinGreen (actin).
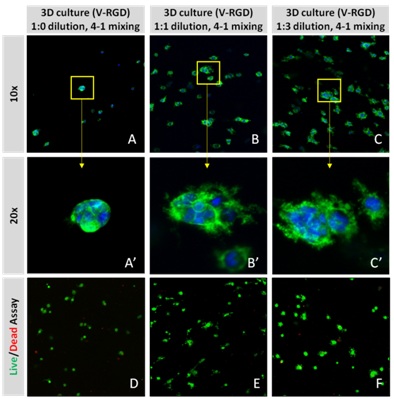 Figure 2: Day 7 representative and enlarged (bottom) fluorescent micrographs of PANC-1 cells at three different dilutions of VitroGel 3D-RGD with corresponding antibody staining: NucBlue (nucleus) and ActinGreen (actin). Live/Dead assay in varying concentrations of hydrogel strength at day 7 showing live cells in green and dead cells in red.
Figure 2: Day 7 representative and enlarged (bottom) fluorescent micrographs of PANC-1 cells at three different dilutions of VitroGel 3D-RGD with corresponding antibody staining: NucBlue (nucleus) and ActinGreen (actin). Live/Dead assay in varying concentrations of hydrogel strength at day 7 showing live cells in green and dead cells in red.Treatment for glioblastoma has also been difficult to develop because two-dimensional culture systems fail to mimic the tumor microenvironment. Tumor progression depends critically upon the interactions between the tumor cells and their microenvironment [23]. The tumor microenvironment is heterogeneous and dynamic; it consists of extracellular matrix, stromal cells, immune cells, progenitor cells, and blood and lymphatic vessels, which is impossible to recreate in a 2D environment. Various studies have shown that tumor cells cultured two-dimensional environments exhibit distinct differences in cell proliferation ability, resistance to apoptosis, differentiation ability, invasive ability and even biomarker expression [24,25]. Moreover, it isn’t enough to simply study tumor-derived cells; tumors are inherently three-dimensional, and that morphology plays a role in tumor progression, namely angiogenesis, a critical factor in malignancy [26]. Additionally, cancer cells with properties similar to stem cells have been found in glioblastoma and are thought to contribute the high resistance to conventional treatments, and the high recurrence rate [27]. Therefore, accurate and effective development of treatments for glioblastoma will require 3D models that closely mimic the cell-cell interactions exhibited in 3D tumors.
U-87 cells are a line of human primary glioblastoma cells [28] that are commonly used in studies aimed at understanding causality and progression of glioblastoma. To investigate whether our 2D VitroGel system is conducive to the growth of U-87 cells in a two-dimensional system, we cultured them in various dilutions of two of our VitroGel products. U-87 cells were harvested from a human glioblastoma tumor, and although it is in their inherent nature to cluster, when they are cultured in standard 2D culture, they adopt fibroblast-like morphology and only make small aggregates (Figure 3A). In the VitroGel hydrogels, the cells exhibited substantially more spheroid clustering, even at the lower dilutions (Figures 3B-3E). In the 1:3 dilutions of both hydrogels (Figures 3C,3E), the colonies formed are smaller, but in the new softer VitroGel RGD-PLUS, the cell clusters exhibit an interesting morphological characteristic: they form bridges between the clusters (Figure 3E). Culture of U-87 cells on 2D VitroGel demonstrates that this system promotes spheroid morphology even in a 2D environment.
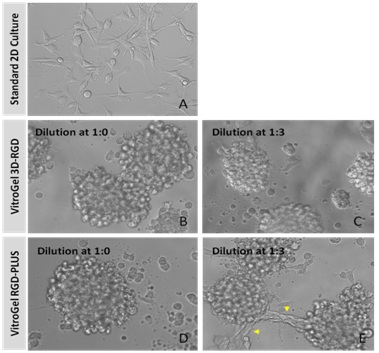 Figure 3: U-87 MG cells grown on the top of 2D hydrogel thick coating surface of VitroGel 3D-RGD and VitroGel RGD-PLUS for 2 days. At 1:0 dilution, cells aggregate to form the spheroids on the surface of both hydrogels (A,B). At 1:3 dilution (C-E), the soft substance help cells making connection between different spheroids on the surface of VitroGel RGD-PLUS (yellow arrows).
Figure 3: U-87 MG cells grown on the top of 2D hydrogel thick coating surface of VitroGel 3D-RGD and VitroGel RGD-PLUS for 2 days. At 1:0 dilution, cells aggregate to form the spheroids on the surface of both hydrogels (A,B). At 1:3 dilution (C-E), the soft substance help cells making connection between different spheroids on the surface of VitroGel RGD-PLUS (yellow arrows).A tunable hydrogel suitable for drug screening applications
in vitro assays have become increasingly popular in drug discovery applications because of their high throughput functionality and cost-effectiveness in conducting optimization and preclinical validation. Three-dimensional culture assays have become especially popular because compared to traditional 2D cell culture, 3D culture provides a more physiologically relevant environment [8,14,29,30]. Comparative studies have shown that 2D cultures lose tissue-specific architecture, causing changes in mechanical and biochemical cues, and interrupting cell-cell or cell-matrix interactions [18]. These differences become exceedingly important in drug development. While many drugs exhibit success in 2D cultures, this may not be translatable in vivo because the 3D environment of the great majority of cells in the body may make it difficult for the drugs to attack all cells [31].
We evaluated the viability and survival of HCT116 cells in the VitroGel system for 2D coating, 3D culture, and drug testing. Both VitroGel 3D and VitroGel 3D-RGD (the RGD peptide modified hydrogel system for better cell adhesion) were used in our studies. HCT116 cells are a line of human colorectal carcinoma cells commonly used in colon cancer studies and therapeutic drug development. These studies established the VitroGel system as a viable means by which to study drug development using HCT116 cells as a model for colorectal cancer.
We evaluated HCT116 cells using both versions of the VitroGel system (Figure 4A): the standard VitroGel-3D system, which is ideal for suspension cells, and the RGD-modified version, which is contains the RGD peptide modification, a peptide present in all major extracellular matrix proteins, and which can promote cell adhesion to biomaterials such as Hydrogels [32]. Cells were evaluated 24 hours after seeding cells and again 7 days later. When using the 2D coating method (Figures 4B,4C,4E,4F), in which the VitroGel hydrogel is allowed to set before cells are seeded, cells adopted a different morphology depending on which version of the VitroGel system was used. In the VitroGel 3D setup (Figures 4A,4D), cells acquired a spheroid shape, albeit small, after 24 hours, and continued to increase in size out to 7 days. Like the standard VitroGel 3D, cells on VitroGel 3D-RGD also began to form spheroid cell clusters after 24 hours. However, unlike the standard hydrogel, cells spread out after 7 days, adopting morphology reminiscent of the 2D culture on standard treated culture plastic. When using the 3D cell culture method in the VitroGel 3D-RGD system, in which cells are seeded into the hydrogel prior to the setting of the gel, HCT116 cells are found to distribute homogenously within the hydrogel. Strength of the hydrogel can affect its mechanical properties, and thus cell viability and response within the hydrogel. We analyzed growth potential (Figure 5) and viability (Figure 6) of HCT116 cells in three different hydrogel strengths (concentrations): 1:0 dilution (mostly hydrogel), and 1:1 and 1:2 dilutions of hydrogel. After 7 days, the 1:1 hydrogel dilution allowed for big colonies (Figure 5B), while other dilutions only allowed for small cell clusters/colonies with abnormal morphology (Figures 5A,5C). Analysis of cell viability by live/dead assay showed that there was no significant cell death in any of the hydrogel dilutions (Figures 6A-6C), despite reduced colony size. Although the cells in the 1:0 and 1:2 dilutions (Figures 6B,6C) aren't exhibiting many dead markers, their morphology indicates they may be dying if not already dead. In the 1:1 dilution, there are more cells, and although they have a flatter morphology commonly associated with poor cell health, it is thought that the increased cell-matrix interaction allowed in scaffolds prevents the spheroid morphology seen in ultra-low adhesion plates used in 2D culture. This morphology might indicate a good cell-matrix interaction which could be good to mimic in vivo situation.
We evaluated the viability and survival of HCT116 cells in the VitroGel system for 2D coating, 3D culture, and drug testing. Both VitroGel 3D and VitroGel 3D-RGD (the RGD peptide modified hydrogel system for better cell adhesion) were used in our studies. HCT116 cells are a line of human colorectal carcinoma cells commonly used in colon cancer studies and therapeutic drug development. These studies established the VitroGel system as a viable means by which to study drug development using HCT116 cells as a model for colorectal cancer.
We evaluated HCT116 cells using both versions of the VitroGel system (Figure 4A): the standard VitroGel-3D system, which is ideal for suspension cells, and the RGD-modified version, which is contains the RGD peptide modification, a peptide present in all major extracellular matrix proteins, and which can promote cell adhesion to biomaterials such as Hydrogels [32]. Cells were evaluated 24 hours after seeding cells and again 7 days later. When using the 2D coating method (Figures 4B,4C,4E,4F), in which the VitroGel hydrogel is allowed to set before cells are seeded, cells adopted a different morphology depending on which version of the VitroGel system was used. In the VitroGel 3D setup (Figures 4A,4D), cells acquired a spheroid shape, albeit small, after 24 hours, and continued to increase in size out to 7 days. Like the standard VitroGel 3D, cells on VitroGel 3D-RGD also began to form spheroid cell clusters after 24 hours. However, unlike the standard hydrogel, cells spread out after 7 days, adopting morphology reminiscent of the 2D culture on standard treated culture plastic. When using the 3D cell culture method in the VitroGel 3D-RGD system, in which cells are seeded into the hydrogel prior to the setting of the gel, HCT116 cells are found to distribute homogenously within the hydrogel. Strength of the hydrogel can affect its mechanical properties, and thus cell viability and response within the hydrogel. We analyzed growth potential (Figure 5) and viability (Figure 6) of HCT116 cells in three different hydrogel strengths (concentrations): 1:0 dilution (mostly hydrogel), and 1:1 and 1:2 dilutions of hydrogel. After 7 days, the 1:1 hydrogel dilution allowed for big colonies (Figure 5B), while other dilutions only allowed for small cell clusters/colonies with abnormal morphology (Figures 5A,5C). Analysis of cell viability by live/dead assay showed that there was no significant cell death in any of the hydrogel dilutions (Figures 6A-6C), despite reduced colony size. Although the cells in the 1:0 and 1:2 dilutions (Figures 6B,6C) aren't exhibiting many dead markers, their morphology indicates they may be dying if not already dead. In the 1:1 dilution, there are more cells, and although they have a flatter morphology commonly associated with poor cell health, it is thought that the increased cell-matrix interaction allowed in scaffolds prevents the spheroid morphology seen in ultra-low adhesion plates used in 2D culture. This morphology might indicate a good cell-matrix interaction which could be good to mimic in vivo situation.
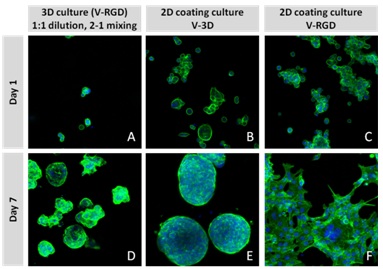
Figure 4: HCT116 cell in VitroGel 3D and VitroGel 3D-RGD system for both 2D coating and 3D culture.
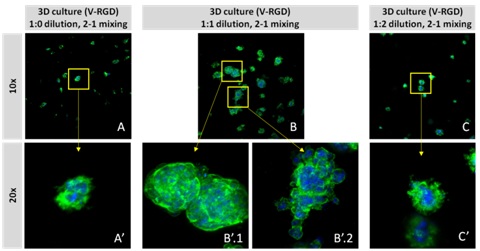
Figure 5: 3D cell culture of HCT116 Cell in different hydrogel concentrations (enlarged cells showing at the bottom.
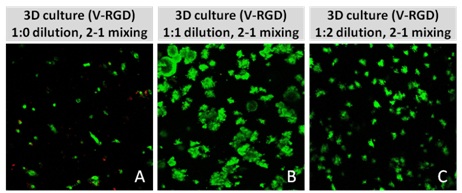
Figure 6: Cell viability of HCT116 cells in different hydrogel strengths.
5-Fluorouracil (5-FU) is a common anti-cancer drug used to treat several cancers including colorectal cancer. While it has some demonstrated some success in colon cancer, more recent studies have pointed out that many patients develop a resistance to 5-FU [33], and other studies have found that 5-FU only acts on a small subset of colon cancers that have a mismatch repair gene mutation [34]. Testing 5-FU on HCT116 cells using traditional 2D cell culture system juxtaposed against the 3D culture system demonstrated that the effect of the 5-FU is insignificant (Figure 7). We found that in 2D cultures, drug efficiency increased as the drug concentration increased from 10µM to 50µM (Figures 7A-7D). However, in the 3D cell culture system, there was no significant drug effect at any stage (Figures 7E-7H). Only in the case of 50µM (Figure 7H) is there any noticeable effect, reduced size of cell colonies clearly observable. Quantification of cell death with a live/dead assay (Figure 7I) showed increasing levels of dead cells in the 2D cultures, suggesting an effectiveness of 5-FU in killing cancer cells. However, in 3D culture, there was not a significant level of dead cells at any stage; nearly non-existent until 50µM. Quantification of live cells using ImageJ showed a steady decline with increased drug concentration. However, in 3D cultures, this decline was not present, demonstrating the importance of 3D culture for accurate interpretation of drug effect.
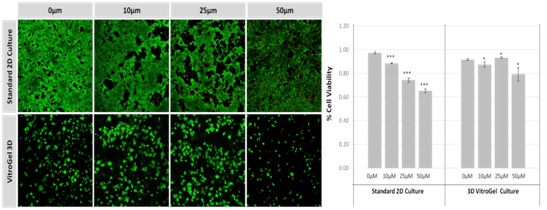
Figure 7: Cell viability of HCT116 cells following treatment with 5-FU at different concentrations. P-values less than 0.05 are considered to be significant (*), p-values less than 0.005 are very statistically significant (**) and values less than 0.001 are extremely statistically significant (***).
In 3D cell culture, we studied the cell morphology, growth, and survival in response to different hydrogel strength (hydrogel dilution). The results of live/dead assay showed that there was not an increase in cell death, suggesting that the reduction in total number of cells in the lower and higher hydrogel dilutions resulted from the mechanical interactions of the cells with the hydrogel. Previous studies have found that the number of live cells is dependent upon substrate stiffness, while the number of dead cells is unrelated, suggesting that the stiffness of the hydrogel can affect proliferation [11,35]. In fact, studies conducted on hepatocellular carcinoma cells demonstrated that the mechanical properties of a matrix can not only promote proliferation, but also can moderate chemotherapeutic response [12].
In figure 5, we observed varying levels of Actin in different parts of the clusters and even between clusters in different 3D scaffold stiffness. Some clusters have more Actin than others, and Actin localization in scaffolds of different stiffness varies. Although we used Actin simply to observe cluster morphology (i.e., flat vs. spherical), it should be noted that there are studies that show that actin levels can fluctuate in cancer cells at different stages [36-38]. In research, Actin is useful as a morphology marker because it is a homeostatic gene, but its actual function relates to multiple cellular processes from contraction to cell division and motility. One study found that actin filaments located at the leading edge of cancer cells are motile in nature and have a higher rate of turnover [37]. Another study found that actin plays a major role in both pre-malignant and malignant breast tumor cells by first controlling cell stiffness to promote proliferation and then cell softness to allow for invasion [36]. The variation in Actin localization and expression levels between different scaffold rigidities concomitant with studies that show that Actin expression varies in cancer cells suggests that the 3D scaffold can affect the motility of the cell. Because cells respond uniquely to matrix rigidity, it is likely that each cell line will have to be optimized prior to experimentation. However, the wide array of flexibility this system offers with regard to culture medium compatibility and its potential to be used in vitro and in vivo make VitroGel a great option for drug testing, especially for tumor applications.
Three-dimensional hydrogels are more suited to promoted intercellular networking
Mesenchymal stem cells are a type of adult multipotent stem cell that gives rise to bone, cartilage, adipose and connective tissue. Over the last two decades, they have become a wildly popular potential alternative to embryonic stem cells for regenerative medicine because of their broad plasticity, immunomodulatory, and anti-inflammatory properties, and the relative ease in which they can be isolated [39]. in vitro, they have been repeatedly demonstrated to be capable of generating various non-mesodermal and non-mesenchymal tissues including hepatocytes [40,41], cardiomyocytes [42], pancreatic cells [43], neuronal cells [44], and melanocytes [45]. They can be isolated with reasonable abundance and in a patient-specific fashion from adipose tissue, bone marrow, cord blood and synovial fluid [46-48]. Their immunomodulatory abilities make them popular not only because they hold promise for possible treatments for previously difficult-to-treat diseases such as graft-versus-host disease and for organ transplantation, but also because any tissues derived from MSCs will themselves engender little to no immune response compared to iPSC- and ESC-derived tissues [49].
However, for all of their therapeutic potential, they have one major setback: in vitro, their proliferation rate tends to slow drastically, and they begin to senesce readily, making them difficult to expand enough for therapeutic use [50]. Some studies have suggested that adjusting the glucose concentration in the culture media can mitigate some of the senescence [51], but this means culturing the cells in an environment that is not clinically relevant and which could ultimately alter their genetic stability. Studies have been conducted on the effect of 2D cell culture on the expression patterns and they have found that 2D culture tends to result in the overall flattening of the cytoskeleton and nucleus which in turn results in a change in gene and gene expression and rearrangement of the extracellular matrix. Three-dimensional cultures have proven to be much more successful in maintain the ECM microenvironment and produce more uniform, reproducible results and have demonstrated more success with clinical applications including transplantation.
OP9 cells are a characterized line of murine bone marrow stromal cells that do not produce functional macrophage colony-stimulating factor (M-CSF) due to an osteopetrotic mutation in the gene encoding M-CSF [52]. OP9 cells were initially used to coculture mouse embryonic stem cells to induce their differentiation into blood cells, but they have been characterized as mesenchymal stem cells [53]. Here we discuss culturing of OP9 mesenchymal stem cells in the VitroGel tunable hydrogel system including the new VitroGel RGD-PLUS. OP9 cells in VitroGel 2D culture exhibit cell clustering and healthy cell morphology. We evaluated OP9 cells in 2D culture in both the original VitroGel 3D-RGD and the new VitroGel RGD-PLUS. In standard 2D culture, OP9 cells, like many mesenchymal cell types, cells adopt a fibroblast like shape and fail to cluster (Figure 8A). Mesenchymal stem cells in this environment don’t proliferate well and can lead to senescence. Moreover, they exhibit the characteristic bright spotting in the cell culture commonly associated with stages of apoptosis. To understand optimal conditions for OP9 culture within our tunable system, we cultured these cells various dilutions of VitroGel. In the VitroGel 3D-RGD (Figures 8B-8D), cells form clusters in all dilutions after just 2 days of cell culture. With the newer VitroGel-3D-PLUS (Figures 8E-8G), cells are even more robust, forming large colonies and exhibiting a healthy MSC morphology at the lowest dilution. A major factor of the in vivo 3D microenvironment is the ability of cells to form an intercellular network. This characteristic is typically not possible in two-dimensional culture and can vary with three-dimensional culture. To understand whether our hydrogel system can facilitate this, we cultured OP9 cells in three-dimensional cultures with two of our hydrogels. With VitroGel 3D-RGD, after 7 days of culture, cells have settled into the 3D hydrogel (Figures 9A-9E,9G), but cells cultured in VitroGel RGD-PLUS have already begun to make intercellular connections (Figures 9F,9H).
However, for all of their therapeutic potential, they have one major setback: in vitro, their proliferation rate tends to slow drastically, and they begin to senesce readily, making them difficult to expand enough for therapeutic use [50]. Some studies have suggested that adjusting the glucose concentration in the culture media can mitigate some of the senescence [51], but this means culturing the cells in an environment that is not clinically relevant and which could ultimately alter their genetic stability. Studies have been conducted on the effect of 2D cell culture on the expression patterns and they have found that 2D culture tends to result in the overall flattening of the cytoskeleton and nucleus which in turn results in a change in gene and gene expression and rearrangement of the extracellular matrix. Three-dimensional cultures have proven to be much more successful in maintain the ECM microenvironment and produce more uniform, reproducible results and have demonstrated more success with clinical applications including transplantation.
OP9 cells are a characterized line of murine bone marrow stromal cells that do not produce functional macrophage colony-stimulating factor (M-CSF) due to an osteopetrotic mutation in the gene encoding M-CSF [52]. OP9 cells were initially used to coculture mouse embryonic stem cells to induce their differentiation into blood cells, but they have been characterized as mesenchymal stem cells [53]. Here we discuss culturing of OP9 mesenchymal stem cells in the VitroGel tunable hydrogel system including the new VitroGel RGD-PLUS. OP9 cells in VitroGel 2D culture exhibit cell clustering and healthy cell morphology. We evaluated OP9 cells in 2D culture in both the original VitroGel 3D-RGD and the new VitroGel RGD-PLUS. In standard 2D culture, OP9 cells, like many mesenchymal cell types, cells adopt a fibroblast like shape and fail to cluster (Figure 8A). Mesenchymal stem cells in this environment don’t proliferate well and can lead to senescence. Moreover, they exhibit the characteristic bright spotting in the cell culture commonly associated with stages of apoptosis. To understand optimal conditions for OP9 culture within our tunable system, we cultured these cells various dilutions of VitroGel. In the VitroGel 3D-RGD (Figures 8B-8D), cells form clusters in all dilutions after just 2 days of cell culture. With the newer VitroGel-3D-PLUS (Figures 8E-8G), cells are even more robust, forming large colonies and exhibiting a healthy MSC morphology at the lowest dilution. A major factor of the in vivo 3D microenvironment is the ability of cells to form an intercellular network. This characteristic is typically not possible in two-dimensional culture and can vary with three-dimensional culture. To understand whether our hydrogel system can facilitate this, we cultured OP9 cells in three-dimensional cultures with two of our hydrogels. With VitroGel 3D-RGD, after 7 days of culture, cells have settled into the 3D hydrogel (Figures 9A-9E,9G), but cells cultured in VitroGel RGD-PLUS have already begun to make intercellular connections (Figures 9F,9H).
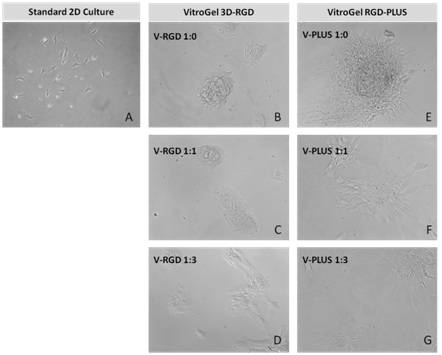 Figure 8: OP9-GFP cells grown on the top of 2D hydrogel coating surface of VitroGel 3D-RGD and VitroGel RGD-PLUS. Compared to standard 2D culture (A), OP9 cells attach and cluster much better in the 3D VitroGel environment (B-G) after only 2 days in culture Cells show aggregation at 1:0 dilution for both VitroGel products (B, E), but the cells spread and attach better on V-PLUS at lower gel strength substance.
Figure 8: OP9-GFP cells grown on the top of 2D hydrogel coating surface of VitroGel 3D-RGD and VitroGel RGD-PLUS. Compared to standard 2D culture (A), OP9 cells attach and cluster much better in the 3D VitroGel environment (B-G) after only 2 days in culture Cells show aggregation at 1:0 dilution for both VitroGel products (B, E), but the cells spread and attach better on V-PLUS at lower gel strength substance.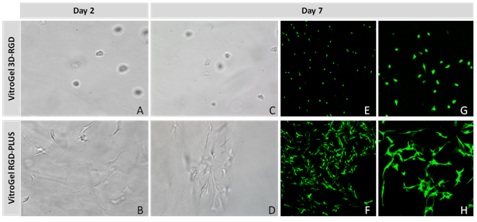 Figure 9: Three-dimensional culture of OP9-GFP cells in both VitroGel 3D-RGD and VitroGel RGD-PLUS. While VitroGel 3D-RGD promotes cell expansion (A-D), the new VitroGel RGD-PLUS has better support for OP9 cell proliferation and cell-cell communication. Stronger cell-matrix interactions help the cells to form the cell-networking structures (E-H). (images A,B,C,D,G,H at 20X; images E&F at 10X).
Figure 9: Three-dimensional culture of OP9-GFP cells in both VitroGel 3D-RGD and VitroGel RGD-PLUS. While VitroGel 3D-RGD promotes cell expansion (A-D), the new VitroGel RGD-PLUS has better support for OP9 cell proliferation and cell-cell communication. Stronger cell-matrix interactions help the cells to form the cell-networking structures (E-H). (images A,B,C,D,G,H at 20X; images E&F at 10X).Our studies indicate that our new VitroGel 3D RGD-PLUS can be an ideal three-dimensional scaffold for cellular applications that rely on intercellular networks. Cellular differentiation and identity are regulated by complex cell-cell communication which can only be achieved at a short range by diffusible ligands; a behavior which can be difficult to model in vitro. It can lead to variability between in vitro and in vivostudies which can hinder translational studies [54]. For tissue regeneration, especially mesenchymal differentiation, the ability to communicate with surrounding cells is absolutely necessary. Cell communication commonly happens at relatively short-range via diffusible factors between cells, facilitated by the close contact provided by a three-dimensional environment. However, longer range signals require a cell communications network. Multiple studies have demonstrated that different kinds of scaffolds and different elasticities can drive differentiation down different pathways. VitroGel 3D provides a platform that is adjustable enough to fit any of the applications, reducing variability and streamlining the process.
To further investigate the ability of VitroGel RGD-PLUS in promoting inter cellular networking, we culture U-87 MG glioblastoma cells in three-dimensional culture at different dilutions. We found that in both concentrations of the original VitroGel 3D-RGD, cells settle into the hydrogel after only two days (Figures 10A-10C), but after seven days (Figures 10D,10E), their overall spread has not changed, and they do not exhibit any intercellular network connections (Figure 10F,10G). In the VitroGel RGD-PLUS, not only do cells settle into the hydrogel by day two, but they also exhibit markedly different morphology from the cells in the VitroGel 3D-RGD: they are more elongated. After 7 days, they exhibit broad cell-cell connections within the 3D hydrogel (Figures 10H,10I) in clusters much larger than the VitroGel 3D-RGD (Figure 10J).
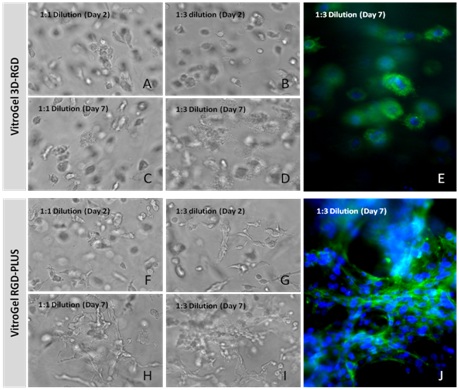 Figure 10: U-87 MG cells cultured in both VitroGel 3D-RGD and VitroGel RGD-PLUS. Cells can grow in 3D hydrogel at 1:1 and 1:3 dilutions of both hydrogels. Cells incorporate into the hydrogel in VitroGel 3D-RGD (A-D), but are not able to form network connections (E). However, in the new VitroGel RGD-PLUS, cells incorporate after 2 days (F-G), but they are able to form intercellular actin connections by day 7 (H-J).
Figure 10: U-87 MG cells cultured in both VitroGel 3D-RGD and VitroGel RGD-PLUS. Cells can grow in 3D hydrogel at 1:1 and 1:3 dilutions of both hydrogels. Cells incorporate into the hydrogel in VitroGel 3D-RGD (A-D), but are not able to form network connections (E). However, in the new VitroGel RGD-PLUS, cells incorporate after 2 days (F-G), but they are able to form intercellular actin connections by day 7 (H-J).Unlike the spheroid structure in VitroGel 3D-RGD, U-87 MG cells create better cell networking structures in VitroGel RGD-PLUS, indicating better cell-cell and cell-matrix interaction. These interactions are a crucial part of properly recapitulating in vivo tissue organization and regeneration and cancer in vitro. Although this is acknowledged, researchers have found it difficult to address, since there isn’t merely one solution to this problem. For example, in a standard in vivo cell niche, there are various types of cell interactions, shapes, junctions, etc., [55]. Studies on currently existing 3D scaffolds have shown that the cohesive forces between cells, which drive the surface tension of the tissue and thus cell fate, can vary greatly between different parts of a tissue [56,57]. This can become problematic considering that some 3D matrices are more suitable to certain cell types and not others, introducing a certain level of variability into studies trying to use 3D methods of cell culture. This is evident even with our own studies. We see that different concentrations of the same matrix can lead to different cell behavior, even if cells are capable of growing in all concentrations.
The ability generates proper extracellular matrix interactions will also be a crucial part to properly understand the tumor microenvironment. A great deal of cancer progression is due to the ability of tumor cells to interact with their surroundings. For example, during tumor growth, the metastatic process begins with oxygen and nutrient deprivation, which triggers the release of angiogenic growth factors and cytokines that will cause blood vessel generation [58,59]. This nutrient and oxygen deprivation only happens after a certain threshold of growth that cannot be achieved in 2D culture, and which will require appropriate cell-cell and cell-matrix interaction in order to achieve in vivo modeling [60].
CONCLUSION
TheWell Biosciences VitroGel System is a tunable hydrogel system that is ready to use. It can be used for both 2D and 3D systems, and in both in vitro and in vivo applications. The hydrogel is adjustable based on the concentration of the VitroGel and the density of cells and can be adjusted to fit different cell type and mixed with various culture medium to generate the extracellular matrix. The VitroGel system has been used to create 3D structures of many different cell types for various applications. For example, a recent study analyzing the efficacy of a novel anti-cancer peptide found that breast cancer cells form spheroid groups with other cells in culture [61]. Addition of their novel peptide resulted in disruption of the spheroid, suggesting it could inhibit adhesion independent cell growth, a conclusion that may not have been elucidated in a two-dimensional environment. Our studies and others suggest that a more tunable system can ultimately have the most promise to truly model in vivo systems in vitro. The future of organ regeneration will require the development of platforms that can capture the complexity of the in vivoextracellular matrix properties while also being able to tune matrix properties to provide the in vivoenvironment for the variety of cells within a tissue. Future cancer studies will rely on a 3D tunable system that will give a realistic representation of solid tumor growth and will also give a realistic recapitulation of how the tumor will respond to putative treatments. Previous studies with VitroGel tunable hydrogels have demonstrated that they are a viable option for drug testing, and various studies have already demonstrated success with this hydrogel in cancer studies [61-64]. The enhancement of cell networking capabilities in the new VitroGel RGD-PLUS indicates that this new matrix may provide a viable solution to cancer and regenerative medicine studies.
REFERENCES
- Benien P, Swami A (2014) 3D tumor models: history, advances and future perspectives. Future Oncol 10: 1311-1327.
- Duval K, Grover H, Han LH, Mou Y, Pegoraro AF, et al. (2017) Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology (Bethesda) 32: 266-277.
- Simon E (2017) Simon Fibrous Substrates For Cell Culture-NIH. Final Rept 1-23.
- Worthington P, Pochan DJ, Langhans SA (2015) Peptide Hydrogels - Versatile Matrices for 3D Cell Culture in Cancer Medicine. Front Oncol 5: 92.
- Patel BB, Sharifi F, Stroud DP, Montazami R, Hashemi NN, et al. (2019) 3D Microfibrous Scaffolds Selectively Promotes Proliferation and Glial Differentiation of Adult Neural Stem Cells: A Platform to Tune Cellular Behavior in Neural Tissue Engineering. Macromol Biosci 19: 1800236.
- Irawan V, Sung TC, Higuchi A, Ikoma T (2018) Collagen Scaffolds in Cartilage Tissue Engineering and Relevant Approaches for Future Development. Tissue Eng Regen Med 15: 673-697.
- Sun X, Wang J, Wang Y, Zhang Q (2018) Collagen-based porous scaffolds containing PLGA microspheres for controlled kartogenin release in cartilage tissue engineering. Artif Cells Nanomed Biotechnol 46: 1957-1966.
- Langhans SA (2018) Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front Pharmacol 9: 6.
- Ratcliffe A, Butler DL, Dyment NA, Cagle PJ, Proctor CS, et al. (2015) Scaffolds for tendon and ligament repair and regeneration. Ann Biomed Eng 43: 819-831.
- Kapa?czy?ska M, Kolenda T, Przyby?a W, Zaj?czkowska M, Teresiak A, et al. (2014) 2D and 3D cell cultures - a comparison of different types of cancer cell cultures. Arch Med Sci 14: 910-919.
- Cavo M, Fato M, Peñuela L, Beltrame F, Raiteri R, et al. (2016) Microenvironment complexity and matrix stiffness regulate breast cancer cell activity in a 3D in vitro model. Sci Rep 6: 35367.
- Schrader J, Gordon-Walker TT, Aucott RL, van Deemter M, Quaas A, et al. (2011) Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology 53: 1192-1205.
- Wingate K, Floren M, Tan Y, Tseng PON, Tan W (2014) Synergism of matrix stiffness and vascular endothelial growth factor on mesenchymal stem cells for vascular endothelial regeneration. Tissue Eng Part A 20: 2503-2512.
- Edmondson R, Broglie JJ, Adcock AF, Yang L (2014) Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol 12: 207-218.
- Hoarau-Véchot J, Rafii A, Touboul C, Pasquier J (2018) Halfway between 2D and Animal Models: Are 3D Cultures the Ideal Tool to Study Cancer-Microenvironment Interactions? Int J Mol Sci 19: 181.
- Prestwich GD (2007) Simplifying the extracellular matrix for 3-D cell culture and tissue engineering: A pragmatic approach. J Cell Biochem 101: 1370-1383.
- Andersen T, Auk-Emblem P, Dornish M (2015) 3D Cell Culture in Alginate Hydrogels. Microarrays (Basel) 4: 133-161.
- Zanoni M, Piccinini F, Arienti C, Zamagni A, Santi S, et al. (2016) 3D tumor spheroid models for in vitro therapeutic screening: a systematic approach to enhance the biological relevance of data obtained. Sci Rep 6: 19103.
- Castilho M, Hochleitner G, Wilson W, van Rietbergen B, Dalton PD, et al. (2018) Mechanical behavior of a soft hydrogel reinforced with three-dimensional printed microfibre scaffolds. Sci Rep 8: 1245.
- Hoare TR, Kohane DS (2008) Hydrogels in drug delivery: Progress and challenges. Polymer 49: 1993-2007.
- Longati P, Jia X, Eimer J, Wagman A, Witt M-R, et al. (2013) 3D pancreatic carcinoma spheroids induce a matrix-rich, chemoresistant phenotype offering a better model for drug testing. BMC Cancer 13: 95.
- Chen Y-C, Lou X, Zhang Z, Ingram P, Yoon E (2015) High-Throughput Cancer Cell Sphere Formation for Characterizing the Efficacy of Photo Dynamic Therapy in 3D Cell Cultures. Sci Rep 5: 12175.
- Wu M, Swartz MA (2014) Modeling tumor microenvironments in vitro. J Biomech Eng 136: 021011.
- Stock K, Estrada MF, Vidic S, Gjerde K, Rudisch A, et al. (2016) Capturing tumor complexity in vitro: Comparative analysis of 2D and 3D tumor models for drug discovery. Sci Rep 6: 28951.
- Riedl A, Schlederer M, Pudelko K, Stadler M, Walter S, et al. (2017) Comparison of cancer cells in 2D vs 3D culture reveals differences in AKT-mTOR-S6K signaling and drug responses. J Cell Sci 130: 203-218.
- Amann A, Zwierzina M, Koeck S, Gamerith G, Pechriggl E, et al. (2017) Development of a 3D angiogenesis model to study tumour - endothelial cell interactions and the effects of anti-angiogenic drugs. Sci Rep 7: 2963.
- Murat A, Migliavacca E, Gorlia T, Lambiv WL, Shay T, et al. (2008) Stem cell-related “self-renewal” signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. J Clin Oncol 26: 3015-3024.
- Oh SJ, Yang JI, Kim O, Ahn EJ, Kang WD, et al. (2017) Human U87 glioblastoma cells with stemness features display enhanced sensitivity to natural killer cell cytotoxicity through altered expression of NKG2D ligand. Cancer Cell Int 17: 22.
- Fang Y, Eglen RM (2017) Three-Dimensional Cell Cultures in Drug Discovery and Development. SLAS Discov 22: 456-472.
- Kloxin AM, Kloxin CJ, Bowman CN, Anseth KS (2010) Mechanical properties of cellularly responsive hydrogels and their experimental determination. Adv Mater Weinheim 22: 3484-3494.
- Powell K (2017) Adding depth to cell culture. Science. American Association for the Advancement of Science 356: 96-98.
- Bellis SL (2011) Advantages of RGD peptides for directing cell association with biomaterials. Biomaterials 32: 4205-4210.
- 33. Bracht K, Nicholls AM, Liu Y, Bodmer WF (2010) 5-Fluorouracil response in a large panel of colorectal cancer cell lines is associated with mismatch repair deficiency. British Journal of Cancer. 103: 340-346.
- He J, Pei L, Jiang H, Yang W, Chen J, et al. (2017) Chemoresistance of colorectal cancer to 5-fluorouracil is associated with silencing of the BNIP3 gene through aberrant methylation. J Cancer 8: 1187-1196.
- Cook JL, Fox DB, Kuroki K, Jayo M, De Deyne PG (2008) In vitro and in vivo comparison of five biomaterials used for orthopedic soft tissue augmentation. Am J Vet Re 69: 148-156.
- Lorente G, Syriani E, Morales M (2014) Actin filaments at the leading edge of cancer cells are characterized by a high mobile fraction and turnover regulation by profilin PLoS ONE 9: 85817.
- Tavares S, Vieira AF, Taubenberger AV, Araújo M, Martins NP, et al. (2017) Actin stress fiber organization promotes cell stiffening and proliferation of pre-invasive breast cancer cells. Nat Commun. 8: 15237.
- Yamaguchi H, Condeelis J (2007) Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta 1773: 642-652.
- Ullah I, Subbarao RB, Rho GJ (2015) Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 35: 1-18.
- Stock P, Brückner S, Ebensing S, Hempel M, Dollinger MM, et al. (2010) The generation of hepatocytes from mesenchymal stem cells and engraftment into murine liver. Nat Protoc 5: 617-627.
- Itaba N, Noda I, Oka H, Kono Y, Okinaka K, et al. (2018) Hepatic cell sheets engineered from human mesenchymal stem cells with a single small molecule compound IC-2 ameliorate acute liver injury in mice. Regen Ther 9: 45-57.
- Arslan YE, Galata YF, Sezgin Arslan T, Derkus B (2018) Trans-differentiation of human adipose-derived mesenchymal stem cells into cardiomyocyte-like cells on decellularized bovine myocardial extracellular matrix-based films. J Mater Sci Mater Med 29: 127.
- Shivakumar SB, Bharti D, Subbarao RB, Park JM, Son YB, et al. (2019) Pancreatic endocrine-like cells differentiated from human umbilical cords Wharton's jelly mesenchymal stem cells using small molecules. J Cell Physiol 234: 3933-3947.
- Srivastava A, Singh S, Pandey A, Kumar D, Rajpurohit CS, et al. (2108) Secretome of Differentiated PC12 Cells Enhances Neuronal Differentiation in Human Mesenchymal Stem Cells Via NGF-Like Mechanism. Mol Neurobiol. 55: 8293-8305.
- Paino F, Ricci G, De Rosa A, D'Aquino R, Laino L, et al. (2010) Ecto-mesenchymal stem cells from dental pulp are committed to differentiate into active melanocytes. Eur Cell Mater 20: 295-305.
- Jia Z, Liang Y, Xu X, Li X, Liu Q, et al. (2018) Isolation and characterization of human mesenchymal stem cells derived from synovial fluid by Magnetic-Activated Cell Sorting (MACS). Cell Biol Int 42: 262-271.
- Amati E, Sella S, Perbellini O, Alghisi A, Bernardi M, et al. (2017) Generation of mesenchymal stromal cells from cord blood: Evaluation of in vitro quality parameters prior to clinical use. Stem Cell Research & Therapy 8: 14.
- Baer PC (2104) Adipose-derived mesenchymal stromal/stem cells: An update on their phenotype in vivo and in vitro. World J Stem Cells 6: 256-265.
- Gao F, Chiu SM, Motan DAL, Zhang Z, Chen L, et al. (2016) Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death & Disease. 7: 2062.
- McKee C, Chaudhry GR (2017) Advances and challenges in stem cell culture. Colloids Surf B Biointerfaces 159: 62-77.
- Li YM, Schilling T, Benisch P, Zeck S, Meissner-Weigl J, et al. (2007) Effects of high glucose on mesenchymal stem cell proliferation and differentiation. Biochem Biophys Res Commun 363: 209-215.
- Nakano T, Kodama H, Honjo T (1994) Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science 265: 1098-1101.
- Gao J, Yan XL, Li R, Liu Y, He W, et al. (2010) Characterization of OP9 as authentic mesenchymal stem cell line. J Genet Genomics 37: 475-482.
- Thurley K, Wu LF, Altschuler SJ (2018) Modeling Cell-to-Cell Communication Networks Using Response-Time Distributions. Cell Syst 6: 355-367.
- Hansen NUB, Genovese F, Leeming DJ, Karsdal MA (2015) The importance of extracellular matrix for cell function and in vivo likeness. Exp Mol Pathol 98: 286-294.
- Manning ML, Foty RA, Steinberg MS, Schoetz EM (2010) Coaction of intercellular adhesion and cortical tension specifies tissue surface tension. Proc Natl Acad Sci USA 107: 12517-12522.
- Melissaridou S, Wiechec E, Magan M, Jain MV, Chung MK, et al. (2019) The effect of 2D and 3D cell cultures on treatment response, EMT profile and stem cell features in head and neck cancer. Cancer Cell Int 19: 16.
- Voss MJ, Niggemann B, Zänker KS, Entschladen F (2010) Tumour reactions to hypoxia. Curr Mol Med 10: 381-386.
- Mimeault M, Batra SK (2013) Hypoxia-inducing factors as master regulators of stemness properties and altered metabolism of cancer- and metastasis-initiating cells. J Cell Mol Med 17: 30-54.
- Foty RA, Steinberg MS (2013) Differential adhesion in model systems. Wiley Interdiscip Rev Dev Biol 2: 631-645.
- Mahauad-Fernandez WD, Okeoma CM (2018) B49, a BST-2-based peptide, inhibits adhesion and growth of breast cancer cells. Sci Rep 8: 4305.
- Li X, Seebacher NA, Xiao T, Hornicek FJ, Duan Z (2019) Targeting regulation of cyclin dependent kinase 9 as a novel therapeutic strategy in synovial sarcoma. J Orthop Res 37: 510-521.
- Akamandisa MP, Nie K, Nahta R, Hambardzumyan D, Castellino RC (2019) Inhibition of mutant PPM1D enhances DNA damage response and growth suppressive effects of ionizing radiation in diffuse intrinsic pontine glioma. Neuro Oncol 21: 786-799.
- Mahauad-Fernandez WD, Naushad W, Panzner TD, Bashir A, Lal G, et al. (2018) BST-2 promotes survival in circulation and pulmonary metastatic seeding of breast cancer cells. Sci Rep 8: 17608.
Citation: Huang J (2019) 3D Cell Culture on VitroGel System. J Cytol Tissue Biol S1: 001.
Copyright: © 2019 John Huang, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

Journal Highlights
© 2026, Copyrights Herald Scholarly Open Access. All Rights Reserved!
