
A Case of Endometrial Stromal Sarcoma Revealed by Lung Metastasis 7 Years after Hysterectomy which Led to Histological Diagnosis of Uterine Fibroid
*Corresponding Author(s):
Dapa A DialloDepartment Of Haematology Medical Oncology, Sickle Cell Disease Research And Control Centre (CRLD), CHU Du Point G, Bamako, Mali
Tel:+223 20223898,
Fax:+223 20223899
Email:dadiallo@icermali.org
Abstract
Endometrial stromal sarcomas are uterine malignant tumours. Recurrences as extragenital locations of these tumours are rare and, occur sometimes long time after the primary tumour treatment. Here we report a case of recurrence of endometrial stromal sarcoma revealed by lung metastasis in a 65-year-old woman who had, 7 years earlier, experienced a total hysterectomy whose histology concluded to the diagnosis of uterine fibroid. Its interest is clinical, diagnostic and prognostic.
Keywords
INTRODUCTION
Sarcomas Developed on Endometrial Stroma Sarcoma (ESS) are rare mesenchymal tumours of the uterus that account for between 0.2% and 1% of all malignant uterine tumours and 15% to 20% of primary uterine sarcoma [1]. The main clinical symptom of ESSs is abnormal vaginal bleeding. ESSs usually originate from the endometrium, but they can also develop from intra- or extra-uterine endometriosis sites [2]. These are tumours characterized by wide anatomopathological heterogeneity. Based on cellular atypia, mitotic index and tumour immunohistochemistry, a distinction is made between high-grade ESSs, also known as ketogenic chorion sarcoma, and low-grade ESSs [3,4]. High-grade malignancies ESSs often have an aggressive outcome and are associated with short survivals [5], while low-grade ESSs have a slow outcome and better prognosis. Recurrences of treated ESSs frequently occur in the vaginal, pelvic or peritoneal cavity and less frequently in certain sites far from the initial site such as the liver, bone marrow and lung [6]; according to several authors, lung metastasis can occur several years after the onset of ESS [1,7]. Here are reporting a case of endometrial stroma sarcoma revealed by lung metastasis in a 65-year-old woman who had, 7 years earlier, experienced a total hysterectomy whose histology led to the diagnosis of uterine fibroid. In our knowledge, it is the first case reported in sub-Saharan Africa. The interest of the case is clinical, diagnostic, prognostic and therapeutic.
OBSERVATION
Mrs. AT, a retired executive secretary aged 65 and residing in Bamako, Mali, was received in April 2017 in the pneumology department in Mali for intermittent chronic cough with whitish sputum. X-ray and Computed Tomography (CT) scans examinations led to a diagnosis of pulmonary metastasis of cancer based on the observation of “balloon-release” pulmonary nodular images (Figures 1 and 2). The pulmonary nodules were also associated with hepatic and abdominal-pelvic nodules. Following the diagnosis, Mrs. AT travelled to Dakar, Senegal, in May 2017, for explorations and treatment. After bronchial fibroscopy with aspiration, the histological examination of bronchial aspiration samples concluded that there was no malignancy. On 25 September 2017, Mrs. AT was referred to our consultation. The clinical examination at this moment found a patient in good general condition with a WHO performance status score evaluated at 1 [8], her conjunctiva were well coloured, abdominal palpation did find no organ hypertrophy and the gynecological examination was normal. Mrs. AT was 6th gesture, 5th pare, had four living children and, an antecedent of abortion; she had a history of oral contraception use in 2007 and the onset of her menopause the same year. On 26 May 2010, she underwent total hysterectomy with bilateral annexectomy, whose histological examination, without an immunohistochemical examination, concluded that she had uterine fibroid. She had hypertension that had been regularly monitored with amlodipine (Amcal® 5mg/day) since 2011. The initial biological tests conducted following our consultation were normal (whole blood cell count, serology of hepatic viruses B and C as well as HIV, carcinoembryogenic antigen, creatinine and serum electrolytes). In October 2017, we decided to conduct a biopsy of the right lung nodules for a histological examination. Using biopsy specimens embedded in paraffin, an immunohistochemical staining has completed the histological examination by using a panel of antibodies including polyclonal antibodies AE1/AE3, polyclonal antibodies anti-desmin, monoclonal antibodies against EMA (Epithelial Membrane Antigen), vimentin, estrogen receptors, progesterone receptors, TTF-1 (Thyroid Transcription Factor-1), Ki-67, CD34, CD10, chromogranin and synaptophysin. Morphological aspect and immuno-histochemical staining authorized to consider the diagnosis of endometrial stroma sarcoma. Indeed, in the lung parenchyma, under the pleura, a lesion has been found consisting of small cells with a regular round nucleus and fine chromatin; cytoplasm of these cells was pale and its limits were discrete. Labelling by antibodies AE1/AE3 was positive, EMA (Epithelial Membrane Antigen) and TTF-1 (Thyroid Transcription Factor-1) were labelled by specific monoclonal antibodies on the epithelium lining and, negative within the tumour; chromogranin, synaptophysin and CD34 were negative. Actin and desmin were focally positive and there were some positive cells for CD10. Progesterone receptors were highly positive and there was a low positivity of lesions for oestrogen receptors. Ki-67 was ? 1%. The patient was monitored under symptomatic treatments. She was seen again for consultation on 09/08/2018 after which, we noted a WHO performance status still equal to 1, as well as a tumour progression marked by 50% and 30% increase in the size of liver and lung nodules respectively without clinical impact.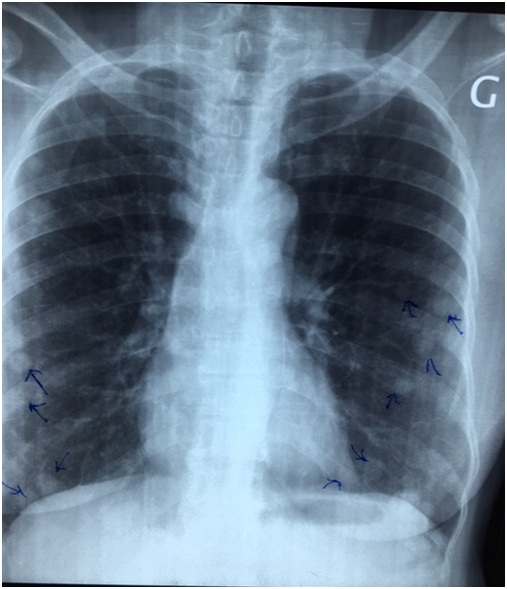 Figure 1: Chest x-ray photograph showing nodules scattered in the two pulmonary fields (arrows).
Figure 1: Chest x-ray photograph showing nodules scattered in the two pulmonary fields (arrows).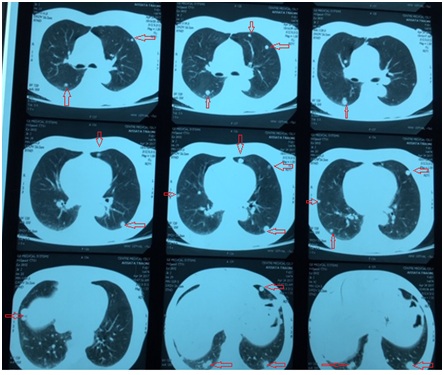 Figure 2: Chest CT scans showing nodules spread to both lungs on different sections (arrows).
Figure 2: Chest CT scans showing nodules spread to both lungs on different sections (arrows).
DISCUSSION
ESS is a rare tumour, accounting for between 0.2% and 1% of all malignant tumours of the uterus. Diagnosis is based on morphological and immunohistochemical or even molecular biology criteria [9]. Our patient had experienced a total hysterectomy with bilateral annexectomy in 2010, the histology of the operating material had not concluded malignancy, but it had not been completed by an immunohistochemical study; we cannot therefore a posteriori eliminate a false anatomopathological diagnosis. The diagnosis of recurrences of sarcomas far from the primary site of the tumour is generally difficult because of morphological aspects that are often not superimposed on those of the primary tumour. Thanks to immunohistochemistry, this diagnosis is facilitated if the right material is available for study. During our patient’s medical treatment, the absence of malignancy of her lung nodules was noted in May 2017 based on the examination of bronchial aspirations. It was necessary to proceed in October 2017 or 5 months later, a biopsy that allow to affirm the malignancy of lung nodules. The false diagnosis made in May 2017 was clearly due to the use of inappropriate material for diagnosis. Most ESS recurrences take place within two years following diagnosis of the primary tumour, but very late recurrences have been described, particularly for low-grade lesions [4,7]. We were unable to obtain the hysterectomy part performed on our patient in 2010 for an immunohistochemical study due to lack of archiving, but two observations allow us to consider that our patient in 2010 had a low-grade ESS undiagnosed due to lack of immunolabelling: (i) the long period (7 years) between the treatment of the primary tumour and the diagnosis of recurrence, (ii) the histological and immunohistochemical characteristics of the lung tumours examined in 2017, revealing, in particular, the positivity of progesterone and oestrogen receptors and the low labelling of Ki-67. Low-grade lung sarcomas is an exceptional tumour whose incidence is estimated at one sarcoma per 500 lung carcinomas, or less than 50 new cases per year in France [10]. These tumours should be investigated for the existence of an extra-thoracic primary tumour, particularly uterine, which usually precedes the development of lung metastases by several years [5]. One of the singularities of the case we are reporting is the absence of gynaecological manifestations at the time of diagnosis of lung metastasis; some authors have reported ESS recurrences in the lungs without pelvic lesions [11]. These data clearly raise the issue of pathophysiology of ESSs recurrences and their extragenital locations, which has not yet been elucidated; the very variable time it takes to recur at extragenital sites, the often indolent course of the recurrences and the limited treatment options call for studies on the risk factors that promote their occurrence. Low-grade sarcomas are characterized by slow growth, with a 5-year survival rate estimated at between 80% and 100%. The specific mortality rate for low-grade ESSs is estimated at 15%-25% [12]. Their treatment is surgical for both primary tumour and metastases where possible. Radiation therapy can be offered for incompletely resected tumours, and it appears to be effective [13]. The case we are reporting could not be operated or irradiable even though the patient had a WHO stable performance status score of 1. Progesterone treatment has resulted in remission in some patients [14,15]; we did not offer this treatment to our patient due to the indolent clinical course of her disease.
This observation calls for systematic search for uterine sarcoma in front of low-grade lung sarcoma in a woman, as well as systematic immunohistochemical study of uterine tumours; it also calls for close monitoring of any woman who has experienced uterine tumour hysterectomy regardless of the initial histology. The observation also raises the fundamental issue of risk factors for the spread of ESSs, which requires multicentre research studies.
REFERENCES
- Koss LG, Spiro RH, Brunschwing A (1985) Endometrial stromal sarcoma. Surg Gynecol Obstet 121: 531-537.
- Hendrickson MR, Kempson R (1995) Pure mesenchymal neoplasm of the uterine corpus (4th edn). In: Fox H, Wells M (eds.). Haines and Taylor, Obstetrical and Gynecological Pathology, Churchill Linvingston, New York, USA, Pg no: 519-586.
- Chang K, Crabtree G, Lim-Tan S, Kempson R, Hendrickson M (1990) Primary uterine endometrial stromal neoplasms. A clinicopathologic study of 117 cases. Am J Surg Pathol 14: 415-438.
- Inayama Y, Shoji A, Odagiri S, Hirahara F, Ito T, et al. (2000) Detection of pulmonary metastasis of low-grade endometrial stromal sarcoma 25 years after hysterectomy. Pathol Res Pract 196: 129-34.
- Michel G, Pfeiffer F, Duvillard P, Prade M, Castaigne D, et al. (1989) Sarcoma of the uterus. A clinical study apropos of 50 surgically treated cases at the Gustave Roussy Institute. Review of the literature J Gynecol Obstet Biol Reprod 18: 1024-1030.
- Masand RP, Euscher ED, Deavers MT, Malpica A (2013) Endometrioid stromal sarcoma: A cliniopathologic study of 63 cases. Am J Surg Pathol 37: 1635-47.
- Abrams J, Talcott J, Corson JM (1989) Pulmonary metastases in patient with low-grade endometrial stromal sarcoma: Clinicopathologic findings with immunohistochemical characterization. Am J Surg Pathol 13: 133-140.
- Oken MM, Creech RH, Tormey DC, Horton J, Carbone PP, Davis TE, et al. (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5: 649-55.
- Tsuyoshi H, Yoshida Y (2018). Molecular biomarkers for uterine leiomyosarcoma and endometrial stromal sarcoma. Cancer Sci 109: 1743-52.
- Nascimento AG, Uni KK, Bernatz PE (1982) Sarcomas of the lung. Mayo Clin Proc 57: 355-359.
- Tuyaerts S and Amant F (2018) Endometrial Stromal Sarcomas: A revision of their potential as targets for immunotherapy. Vaccines (Basel) 6: 56.
- Gadducci A, Sartori E, Landoni F, Zola P, Maggino T, et al. (1996) Endometrial stromal sarcoma: analysis of treatment failures and survival. Gynecol Oncol 63: 247-253.
- Weitmann HD, Knoocke T, Kucera H, Potter R (2001) Radiation therapy in the tratment of endometrial stromal sarcoma. Int Radiat Oncol Biol Phys 49: 739-748.
- Nakamura K, Nakayama K, Ishikawa M, Ishikawa N, Katagiri H, et al. ( 2016) Letrozole as second-line hormonal treatment for recurrent low-grade endometrial stromal sarcoma: A case report and review of the literature.Oncol Lett 12: 3856-3860.
- Dupont NC, Disaia PJ (2010) Recurrent endometrial stromal sarcoma: treatment with a progestin and gonadotropin releasing hormone agonist. Sarcoma 2010: 353679.
Citation: Bathily M, Dembélé AK, Touré BA, Badiaga Y, Samaké CO, et al. (2019) A Case of Endometrial Stromal Sarcoma Revealed by Lung Metastasis 7 Years after Hysterectomy which Led to Histological Diagnosis of Uterine Fibroid. J Clin Stud Med Case Rep 6: 064.
Copyright: © 2019 Moussa Bathily, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

