
A Case of Rebound Due to Brodalumab Discontinuation: The Importance of Therapeutic Continuity
*Corresponding Author(s):
Martina BurlandoUniversita Degli Studi Di Genova Facolta Di Medicina E Chirurgia, Di S Sal Section Of Dermatology, University Of Genoa, Italy
Tel:+39 0105555761,
Email:martinaburlando@hotmail.com
Abstract
Anti-IL-17-biological therapy is actually used for the treatment of moderate to severe plaque psoriasis. Brodalumab, despite other drugs such as Ixekizumab and Secukinumab, targets the IL-17 receptor and not on the circulating cytokine, performing a faster clinical effect than other drugs. However, it is known that, suspending Brodalumab treatment, the disease recurs quickly. Furthermore, the clinical response could be not observed after the re-administration of the drug. The mechanisms of the rebound are not completely known and data in the literature are limited. The case described is an example of rebound due to Brodalumab interruption. Compared to other biological therapies, Physicians should choose carefully the patient to be treated with Brodalumab, to avoid a possible interruption of treatment and the beginning of a rebound.
Keywords
Brodalumab; Psoriasis; Rebound
INTRODUCTION
Psoriasis is a common skin disorder that is associated with both a physical and psychological burden [1]. It is a multifarious disease that is equally prevalent in both sexes. Chronic plaque psoriasis (psoriasis vulgaris) is the most common form of the disease, and accounts for about 90% of cases. To quantify disease severity, the Psoriasis Area and Severity Index (PASI) score is commonly used [2]. It measures erythema, thickness, scaling and the extent of lesions in patients with widespread disease. In the past decade, several biologics have been developed and approved for the treatment of psoriasis. Biologics are used for long-term treatment because there is no evidence of cumulative toxicity or drug-drug interactions [3]. Furthermore, biologics have a good safety profile with only a small increase in opportunistic infections. Among biologics, Brodalumab, a Human monoclonal IgG2 antibody directed against subunit A of the IL-17 receptor (IL-17RA) [4-6], is fast-acting and achieves PASI 75 in 4.2 weeks [5]. However, when abruptly discontinued, psoriasis rebounds quickly [6]. The cause of the rebound is not fully known. We present a patient who experienced severe rebound after Brodalumab discontinuation, but no improvement despite restarting the drug.
CASE REPORT
A 28-year-old patient affected by moderate to severe plaque psoriasis had previously been unsuccessfully treated with topical therapy, UVB narrowband phototherapy and cyclosporine 2.5 mg/kg/daily (his PASI score was 22). In September 2018, after 6 months therapy, as the cyclosporine lost effectiveness, psoriasis worsened (PASI 27). Following European Guidelines, after the failure of a traditional therapy, we started a biologic therapy. We chose brodalumab for its fast action and for its dosage, 210 mg/day once every 2 weeks (Figures 1A & 2A). Within only 4 weeks, the patient achieved clinical improvement (PASI 5) (Figures 1B & 2B). In April 2019, the patient, due to work commitments, skipped his medication and, as a result, psoriasis relapsed within one month in a guttate-plaque form mainly involving the face. No trigger factors could be found. Brodalumab was again administered (210 mg/day once every 2 weeks) but the patient presented no improvement after 3 months (PASI 15) (Figures 1C & 2C).
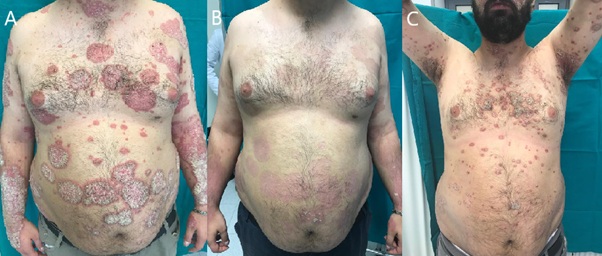
Figure 1: A) Large plaque psoriasis lesions involving the trunk. B) After 4 weeks of brodalumab administration, resolution of the lesions with minor erythema. C) Despite 3 months of Brodalumab re-administration, guttate-plaque psoriasis is still observed.
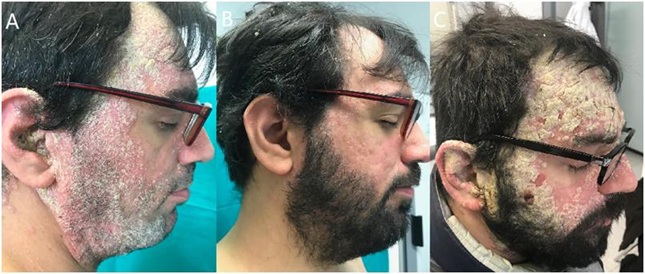
Figure 2: A) Large plaque psoriasis involving the face (right side). B) After 4 weeks of Brodalumab administration. C) Massive hyperkeratosis psoriasis involving the face (right side) despite 3 months of Brodalumab administration.
DISCUSSION
Rebound due to Brodalumab has been reported in the literature. Regnault, et al. found that all 77 patients enrolled in the AMAGINE I, AMAGINE II and AMAGINE III studies presented rebound a median of 46 days after drug discontinuation [7]. The patients were treated with new therapies, but 36% of patients still presented a PASI score >10 after 36 weeks of follow-up. There is no agreement in the literature regarding the rebound pattern. Regnault, et al. reported 73 cases of plaque psoriasis, 3 of pustular psoriasis and 1 of the erythrodermic type [7], where as Kemis, et al. reported 19 cases of pustular psoriasis 12 weeks after Brodalumab discontinuation [8].
The reason for rebound is still unknown but some hypotheses have been put forth. According to Cheuk, et al. [9], psoriasis relapse may be triggered by the rapid recruitment of circulating T lymphocytes supported by high IL-17 and IL-22 levels that are produced by tissue-resident memory T cells (CD4 and CD8 cells) in the healed psoriasis lesion. The second hypothesis concerns IL-17 levels in the serum of psoriatic patients which, according to registration studies [10,11], increase after Brodalumab administration and lead to an over-saturation of IL-17RA when the drug is no longer circulating. This effect would appear to be dose-dependent [10,11]. We believe that the lack of an elimination pathway (i.e., the internalization of the cytokine-receptor complex in the cell) due to Brodalumab antagonism may cause an increase in cytokineconcentration. When Brodalumab is discontinued, all the cytokines over expressed, bind the receptor, thus resulting in a rebound. We have also hypothesized that when Brodalumab is discontinued, IL-17 may bind more quickly and easily to a larger number of receptors due to IL-17RA up-regulation on the keratinocyte, thus stimulating the keratinocytes to differentiate. This could explain why exacerbation occurs so quickly and why no improvement of the lesions is observed thereafter despite restarting treatment. Besides, the drug would be under dosed to antagonize more receptors. To date, it is not clear whether the risk of rebound is higher only in the first months of therapy or even when therapy has been on-going for years. Our hypotheses support both these options. However, literature data are discordant, and discontinuation of the drug reportedly does not always result in rebound. Thus, there is surely still a great deal to be understood in this regard. As a result, when we evaluate a patient, this aspect is taken into consideration, and if the patient is deemed to be unreliable, another drug with a low rebound rate is chosen, even though it may not be as fast-acting.
REFERENCES
- Mrowietz U, Kragballe K, Reich K, Spuls P, Griffiths CE, et al. (2011) Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res 303:1-10.
- Schmitt J, Wozel G (2005) The psoriasis area and severity index is the adequate criterion to define severity in chronic plaque-type psoriasis. Dermatology 210: 194-199.
- Cozzani E, Wei Y, Burlando M, Signori A, Parodi A (2020) Serial biologic therapies in psoriasis patients: A 12-year, single-center, retrospective observational study. J Am Acad Dermatol 82: 37-44.
- Roostaeyan O, Kivelevitch D, Menter A (2017) A review article on brodalumab in the treatment of moderate-to-severe plaque psoriasis. Immunotherapy 9: 963-978.
- Blauvelt A, Papp KA, Lebwohl MG, Green LJ, Hsu S, et al. (2017) Rapid onset of action in patients with moderate-to-severe psoriasis treated with brodalumab: A pooled analysis of data from two phase 3 randomized clinical trials (AMAGINE-2 and AMAGINE-3). J Am Acad Dermatol 77: 372-374.
- Papp KA, Reich K, Paul C, Blauvelt A, Baran W, et al (2016) A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol 175: 273-286.
- Masson Regnault M, Konstantinou MP, Khemis A, Poulin Y, Bourcier M, et al. (2017) Early relapse of psoriasis after stopping brodalumab: a retrospective cohort study in 77 patients. J Eur Acad Dermatol Venereol 31:1491-1496.
- Khemis A, Cavalié M, Montaudié H, Lacour JP, Passeron T (2016) Rebound pustular psoriasis after brodalumab discontinuation. Br J Dermatol 175: 1065-1066.
- Cheuk S, Wikén M, Blomqvist L, Nylén S, Talme T, et al. (2014) Epidermal Th22 and Tc17 cells form a localized disease memory in clinically healed psoriasis. J Immunol 192: 3111-3120.
- US Food and Drug Administration (2017) Center for Drug Evaluation and Research. Application number 761032orig1s000: Clinical pharmacology and biopharmaceutics review(s). FDA, Maryland, USA.
- Valeant Pharmaceuticals. Siliq (brodalumab) injection prescribing information. Bridgewater, NJ; 2017.
Citation: Burlando M, Muracchioli A, Cozzani E, Parodi A (2020) A Case of Rebound Due to Brodalumab Discontinuation: The Importance of Therapeutic Continuity. J Clin Dermatol Ther 6: 041.
Copyright: © 2020 Martina Burlando, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

