
A Case Report of an Orbital Extension of a Necrotizing Otitis Externa
*Corresponding Author(s):
Haifa ZlitniDepartment Of Radiology, Charles Nicolle University Hospital, 9 April Bd, 1006, Bab Souika, Tunis, Tunisia
Tel:00216 29 67 98 97,
Email:zlitnihaifa03@gmail.com
Abstract
Necrotizing otitis externa (NOE), also known as malignant otitis externa, is an invasive infection of the external auditory canal (EAC) with extension to the skull base. Infrequently, it may cause an orbital apex syndrome (OAS) due to the involvement of ocular motor nerves in the anatomical region of the orbital apex.
We describe below a rare case of orbital apex syndrome complicating a necrotizing otitis extern of a 62-year-old man with poorly controlled diabetes and hypertension. He has been suffering from a refractory otitis externa with a left cord paralysis, a grade V left facial palsy, a left-sided axial proptosis and mild optic disc pallor. Computed tomography (CT) revealed a skull base osteomyelitis, an inflammatory process of the left parapharyngeal, masticator and retropharyngeal spaces. Magnetic resonance imaging (MRI) confirmed the results of the Computed tomography (CT) and showed an inflammatory mass of nasopharyngeal wall reaching the left orbit through the inferior orbital fissure. The patient was treated by local debridement of granulation tissue and oral antibiotics and antifungal treatment. Recovery was obtained after 20 weeks of treatment with a free disease follow up of 5 months.
To the best of our knowledge, an orbital apex syndrome has been previously reported once in medical literature. Skull base infection with orbital extension particularly in poorly controlled diabetic patient is potentially lethal.
Introduction
A 62 year-old man presented on 10th, January 2018 to the otolaryngology department with a 3-month history of severe otalgia and otorrhea of the left ear with a deterioration of the general status. The patient had long-standing, poorly-controlled diabetes and hypertension. He had been diagnosed with otitis externa at 3 weeks prior. Though oral antibiotics had been prescribed, he had been experiencing worsening and unbearable ear pain. He reported developing hoarseness and dysphagia recently. Admission to the otolaryngology department of our hospital was decided for suspected necrotizing otitis externa (NOE).
On examination, the patient was apyrexial, and was noted to have periauricular tenderness. Otoscopy demonstrated an edematous external ear canal discharging pus. The tympanic membrane was dull but not perfored. The right ear was normal. An examination of his oral cavity as well as fiber-optic examination of his postnasal space and pharynx showed an inflammatory mass in the posterolateral wall of the nasopharynx. There was a paralysis of the left vocal cord and a grade V left facial palsy. Difficulty with swallowing was also objectified. The protruding of the tongue was normal. On the ocular examination; he had left-sided axial proptosis, no ophthalmoplegia with normal pupils. Dilated funduscopy of the left eye revealed mild optic disc pallor and surrounding normal retina.
Investigations
Laboratory test results showed elevated white blood cell (WBC) count of 14210cells/mm3 with 85% neutrophils, and elevated glucose level (4.8g/l). Inflammatory markers were elevated with a C-reactive protein of 148 mg/l and erythrocyte sedimentation rate of 70mm in the first hour.
Computed tomography (CT) of the skull base revealed a thickened and enhanced wall of the left external auditory canal, with an important erosion of the skull base cortical bone. There was a heterogeneous enhancement of the left parapharyngeal, masticator and retropharyngeal spaces. There was no evidence of orbital extension.
High resolution (3Tesla) magnetic resonance imaging (MRI) of the skull base showed necrotizing otitis externa with marked soft tissue thickening and edema along the left external auditory canal. An abnormal signal showing hyperintense on T2-weighted sequence and enhancing after contrast was noted within the middle ear and mastoid air cells, extending up to the level of the petrous ridge.
It also extended to the temporomandibular joint (TMJ) and infratemporal fossa. Inflammatory changes were also noted in the vascular space with thrombosis of the internal jugular vein.T1-weighted unenhanced MRI showed loss of the normal high marrow signal in the clival bone and left pterygoid process with cortical bone erosions, stating a skull base osteomyelitis.
The infection extended to the pterygopalatine fossa medially. Then, it reached the left orbit via the inferior orbital fissure causing proptosis, intraconal fat stranding and thickening, edema and significant enhancement of the orbital muscles. No enhancement of optic nerve was detected (Figure 1).
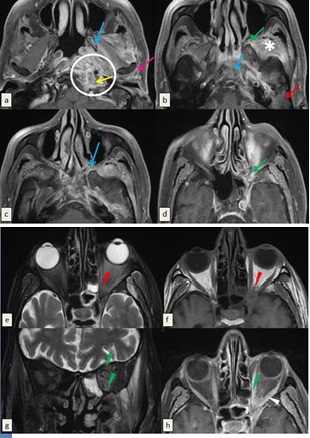
Figure 1: Magnetic resonance imaging (MRI) (3 Tesla) of the skull base performed in T1-weighted sequence before (f) and after saturation of the fat signal and injection of gadolinium (a, b, c, d, h) and T2-weighted sequence after suppression of the fat signal (e, g) ruling out necrotizing otitis externa. The wall of the left external auditory canal is thickened and enhanced after injection of gadolinium (White arrow) associated with inflammatory filling of mastoid cells and middle ear cavities (Red arrow). T1-weighted images show cortical irregularities and enhancement of the bone marrow of the pterygoid process and the clivus related to the osteomylelitis of skull base (Blue arrows).There is synovitis of the left temporomandibular joint attested by the inflammatory changes of periarticular soft tissues(pink arrow). Signal anomalies and enhancement after contrast spread to the ipsilateral infratemporal fossa (Asterix) and to the parapharyngeal, vascular and retropharyngeal spaces (Circle) with several small contiguous collections of parpharyngeal space forming an inflammatory pseudo-mass bulging into the nasopharynx. Extension to the vascular space is accompanied by thrombosis of the ipsilateral jugular vein (Yellow arrow). Demonstration of an obliteration of the fat of the pterygopalatine fossa and the inferior orbital fissure (Green arrows) becoming hypointense in T1-weighted images, hyperintense in T2-weighted images and enhancing after contrast. The intra-orbital extension is attested by the proptosis, intraconal fat stranding (Red arrowhead) and by thickening, edema and significant enhancement of the orbital muscles (Green arrow head). No enhancement of optic nerve in retro-bulbar or intraorbital region was detected.
Treatment
Local treatment based on debridement of granulation tissue of the external auditory canal was initiated. Initially, we medicated the patient with probabilistic antipyocyanic antibiotherapy consisting in ofloxacin 1.2g/day and ceftazidime 6g/day for 12 weeks. Symptoms remained almost stable under antipyocyanic treatment.MRI of the skull base was performed 6 weeks after the initiation of the treatment and concluded that infectious process was stable. We decided to continue the same treatment. In the light of the result of aural swab culture, performed 3 days in a row after a 48h treatment-free interval, yielding to candida parapsilosis as the infectious agent, we switched to systemic antifungal treatment based on fluconazole 400mg/day.
Outcome and follow-up
The duration of antifungal treatment was 6 months of which 2.5 were in the hospital. The patient has been discharged on oral fluconazole. Recovery was obtained after 20 weeks of treatment. It was assessed by total resolution of his pain and otorrhoea. His inflammatory markers have normalized. Partial resolution of the facial nerve palsy was also observed. The duration of follow-up after ending the treatment was 5 months.
Discussion
Necrotizing external otitis is a potentially lethal infection touching the temporal bone and affecting the elderly and diabetics [1]. It’ snow being reported in younger patients with AIDS [2]. Pseudomonas aeruginosa is the most frequent agent responsible for this infection and immunosuppressed patients may be infected by Aspergillus fumigatus [3]. Computed tomography (CT), magnetic resonance imaging (MRI), and nuclear medicine are crucial in the diagnosis and management of patients with this potentially life-threatening disease [4].
The infectious process emanates at the osseous-cartilaginous junction of the external acoustic meatus [5]. Severe otalgia and otorrhea are the main reasons for consultation of patients suffering from malignant external otitis [6]. These symptoms are louder than in a common otitis externa and resist to topical agents as noticed for our patient who has been experiencing worsening otalgia and other symptoms related to the extension of the infection through the deep spaces. On physical examination, the typical finding is granulation tissue in the floor of the ear canal at the bony-cartilaginous junction with normal tympanic membrane [7].
The infection spreads inferiorly through the fissures of Santorini to the infratemporal fossa and the lower cranial nerve foramina [2]. Invasion of cranial nerve VII as described in our case occurs upon involvement of the stylomastoid foramen, the mastoid process or the middle ear, causing facial nerve palsy [8]. Posteriorly, otomastoiditis takes place, erodes the cortex and can be complicated by a Bezold’s abscess, collection around the attachment of sternocleidomastoid muscle on the mastoid process [9]. The infection expands medially to the jugular foramen to affect cranial nerves IX, X, and XI and to the petrous apex invading cranial nerves CN V and VI [2]. For our patient, optic examination of the pharynx showed an inflammatory mass in the posterolateral wall of the nasopharynx related to the medial extension of the infection to the parapharyngeal space. Further extension may result in intracranial collections such as subperiosteal abscesses or epidural or subdural empyemas.
Exceptional is the situation in which the inflammatory process spreads anteriorly through the sphenopalatine fossa, to the orbit via the superior orbital fissure and the optic canal causing an orbital apex syndrome (Ophthalmoplegia, proptosis, ptosis and hypoesthesia of the ipsilateral forehead) [10], via the superior orbital fissure only leading to a superior orbital fissure syndrome (Presenting similarly to the orbital apex syndrome without the involvement of the optic nerve) [11] or via the cavernous sinus causing hypoesthesia of the cheek in addition to the signs seen in orbital apex syndrome due to the involvement of maxillary division of trigeminal nerve (V2) [6]. On the ocular examination of our patient, incomplete orbital apex syndrome was diagnosed because he had only left-sided axial proptosis and dilated funduscopy revealed mild optic disc pallor.
From an imaging perspective, determination of extension to the diseased tissue into the deep facial soft tissues is crucial for the therapeutic approach [12]. Computed tomography (CT) detects early bone erosion and changes in bone density particularly of the clivus, the middle ear, the mastoid, the bony facial nerve canal, the petrous apex and foramina of the skull-base [13]. Infiltration of the retrocondylar fat may be seen as an early sign of temporo mandibular joint infiltration [7]. Effacement and increased density with enhancement on post contrast images of the fat planes of the infra temporal, parotid, vascular and retropharyngeal spaces are also assessed when the infection spreads beyond the EAC [14]. Infrequently, this cellulitis affects the orbital content and computed tomography (CT) demonstrates fat infiltration of the orbit through the superior or inferior fissura or the optic canal, orbital muscle thickening and proptosis [15]. Computed tomography of skull base of our patient revealed marked erosions of the left temporal bone, extension of the infection to the otomastoid cavity, to the left parapharyngeal, masticator and retropharyngeal spaces with no evidence of orbital extension.
Magnetic resonance imaging (MRI)is essential to assess bone marrow involvement resulting in the loss of fatty signal of the bone marrow of the skull base which appears hypointense on T1-weighted images and hyperintense on T2-weighted images and enhances after contrast [13,16]. As bone marrow is lacking in the smaller osseous structures of the external auditory canal, Computed tomography (CT) remains a more useful imaging exam for initial diagnosis by detecting the small cortical erosions [13]. Extension of the infection into the soft tissues of facial deep spaces may effectively be evaluated with Computed tomography (CT). However, edematous signal of nasopharyngeal, parapharyngeal, intracranial spaces and of retrocondylar and intraocular fat is precisely demonstrated with magnetic resonance imaging (MRI) [7]. Soft tissues may appear, as any classic infection, hypointense on T1-weighted images and hyperintense on T2-weighted images, but they are commonly seen hypointense on T1 and T2-weighted images [17]. This is explained by denser matrix, associated fibroses and microangiopathy [18]. Infected tissues enhance after contrast with restricted diffusion and lower values on apparent diffusion coefficient (ADC) [19].
Meningeal, parenchymal, cranial nerve and orbital involvement are optimally visualized [16]. Magnetic resonance imaging (MRI) of the skull base in our case confirmed the inflammatory changes of the external auditory canal and the oto-mastoid cavity and demonstrated an inflammatory pseudo-mass of the nasopharyngeal wall extending to the deep spaces. It also detected an extension of the infection to the pterygopalatine fossa and then to the left orbit through the inferior orbital fissure. However, CT and MRI are limited in determining disease resolution if changes persist despite effective treatment.
Nuclear medicine imaging should be used for the initial diagnosis and follow up of necrotising external otitis. This includes three phase technetium Tc99m methylene diphosphonate scintigraphy (bone scan), technetium-99 hexamethyl propylene amine oxime white cell scintigraphy white cell scintigraphy, and gallium Ga 67 scintigraphy [5].
Necrotizing otitis externa is treated with the quinolones providing high bioavailability in bone and cartilage(20). Ciprofloxacin 750 mg twice daily for at least 6-8 weeks is recommended [20]. In cases of intracranial, intraorbital involvement or affection of cranial nerves, or refractory infection, a third generation of cephalosporin or an aminoglycoside is associated with ciprofloxacin [21]. Surgery is not recommended except for diagnostic biopsy and excision of necrotic tissue of the external auditory canal [22].
Data Availability
All data underlying the results are available as part of the article and no additional source data are required.
Consent
Written informed consent for publication of clinical details and clinical images was obtained from the patient.
Competing Interests
No competing interests were disclosed.
Grant Information
The authors declared that no grants were involved in supporting this work.
References
- Yang TH, Kuo ST, Young YH (2006) Necrotizing external otitis in a patient caused by Klebsiella Eur Arch Otorhinolaryngol 263: 344-346.
- Grandis JR, Branstetter BF, Yu VL (2004) The changing face of malignant (necrotising) external otitis: clinical, radiological, and anatomic correlations. Lancet Infect Dis 4: 34-39.
- Ong Y, Chee G (2005) Infections of the External Ear. Ann Acad Med Singap 34: 330-334.
- Morales RE, Eisenman DJ, Raghavan P (2019) Imaging Necrotizing Otitis Externa. Semin Roentgenol 54: 215-226.
- Mehrotra P, Elbadawey MR, Zammit-Maempel I (2011) Spectrum of radiological appearances of necrotising external otitis: a pictorial review. J Laryngol Otol 125: 1109-1115.
- Handzel O, Halperin D (2003) Necrotizing (Malignant) External Otitis. Am Fam Physician 68: 309-312.
- Kwon BJ, Han MH, Oh SH, Song JJ, Chang KH (2006) MRI findings and spreading patterns of necrotizing external otitis: Is a poor outcome predictable? Clin Radiol 61: 495-504.
- Mani N, Sudhoff H, Rajagopal S, Moffat D, Axon PR (2007) Cranial nerve involvement in malignant external otitis: implications for clinical outcome. Laryngoscope 117: 907-910.
- Vazquez E, Castellote A, Piqueras J, Mauleon S, Creixell S, et al. (2003) Imaging of Complications of Acute Mastoiditis in Children. Radiographics 23: 359-372.
- Yeh S, Foroozan R (2004) Orbital apex syndrome. Curr Opin Ophthalmol 15: 490-498.
- Goyal P, Lee S, Gupta N, Kumar Y, Mangla M, et al. (2018) Orbital apex disorders: Imaging findings and management. Neuroradiol J 31: 104-125.
- Amorosa L, Modugno GC, Pirodda A (1996) Malignant external otitis: review and personal experience. Acta Otolaryngol Suppl 521: 3-16.
- Sreepada GS, Kwartler JA (2003) Skull base osteomyelitis secondary to malignant otitis externa. Curr Opin Otolaryngol Head Neck Surg 11: 316-323.
- Sudhoff H, Rajagopal S, Mani N, Moumoulidis I, Axon PR, et al. (2008) Usefulness of CT scans in malignant external otitis: effective tool for the diagnosis, but of limited value in predicting outcome. Eur Arch Otorhinolaryngol 265: 53-56.
- Baig R, Assad Khan Q, Sadiq M, Awan S, Khabir A (2013) A case of orbital apex syndrome in a patient with malignant otits externa. J Pak Med Assoc 63:271-273.
- van der Meer WL, Waterval JJ, Kunst HPM, Mitea C, Pegge SAH, et al. (2021) Diagnosing necrotizing external otitis on CT and MRI: assessment of pattern of extension. Eur Arch Otorhinolaryngol 25.
- Kohut RI, Lindsay JR (1979) Necrotizing (“malignant”) external otitis histopathologic processes. Ann Otol Rhinol Laryngol 88: 714-720.
- Sando I, Harada T, Saito R, Okano Y, Caparosa RJ (1981) Temporal Bone Histopathology of Necrotizing External Otitis: A Case Report. Ann Otol Rhinol Laryngol 90: 109-115.
- Razek AA, Mahmoud W (2020) Prediction of skull base osteomyelitis in necrotizing otitis externa with diffusion-weighted imaging. J Laryngol Otol 134: 404-408.
- Levenson MJ, Parisier SC, Dolitsky J, Bindra G (1991) Ciprofloxacin: Drug of choice in the treatment of malignant external otitis (MEO). Laryngoscope 101: 821-824.
- Pedersen HB, Rosborg J (1997) Necrotizing external otitis: aminoglycoside and beta-lactam antibiotic treatment combined with surgical treatment. Clin Otolaryngol Allied Sci 22: 271-274.
- BerenholzL, Katzenell U, Harell M (2021) Evolving resistant pseudomonas to ciprofloxacin in malignant otitis externa. Laryngoscope 112: 1619-1622.
Citation: Jrad M, Zlitni H, Soussi I, Boumediene M, Zouari H, et al. (2021) A Case Report of an Orbital Extension of a Necrotizing Otitis Externa. J Otolaryng Head Neck Surg 7: 62
Copyright: © 2021 Myriam Jrad, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

