
Journal of Neonatology & Clinical Pediatrics Category: Clinical
Type: Case Report
A Drug Reaction Mimics Perinatal Sepsis
*Corresponding Author(s):
Deanne Wilson-CostelloDepartment Of Pediatrics, Center Of Portage Medical, University Hospitals Of Cleveland, Ravenna, Ohio, United States
Tel:+1 2168443387,
Email:Deanne.Wilson-Costello@UHhospitals.org, drfjcmd@aol.com
Received Date: Jul 04, 2019
Accepted Date: Jul 12, 2019
Published Date: Jul 19, 2019
Abstract
Multiple risk factors contribute to the pathogenesis of early-onset sepsis in the neonate (e.g. maternal GBS status, intrapartum fever, prolonged rupture of membranes, chorioamnionitis). Traditional neonatal treatment with empiric intravenous antibiotics for 5-7 days is not without risk. Furthermore, the majority of newborns with perinatal risk factors do not progress to develop clinical illness. Unique obstetric situations, such as an intrapartum fever generated by a maternal epidural for labor anesthesia, may beguile the neonatal clinician. We present an interesting case report from a community nursery where intrapartum Cytotec exposure resulted in maternal fever, prompting concerns for perinatal sepsis. This report provides a discussion of the mechanism of Cytotec induced fever along with an overview of the scientific merit and potential benefits achieved by the application of the sepsis risk calculator in the community nursery setting. Clinicians must be aware that drug reactions to commonly administered obstetric medications may mimic perinatal sepsis. The application of sepsis risk calculations coupled with open communication between obstetric and neonatal providers may enhance newborn care, avoid antibiotic overuse and shorten lengths of hospital stay.
Keywords
Cytotec; Early-onset sepsis; Prostaglandin; Sepsis calculator; Side effect
INTRODUCTION
In December 2018, the American Academy of Pediatrics (AAP) Committee on Fetus and Newborn (COFN) published two clinical reports on Early-Onset Sepsis (EOS); which are based on gestational age [1,2]. The clinical report pertaining to neonates delivered at ≥ 35.0 weeks presents three approaches for identifying the newborn at increased risk for EOS (e.g. categorical risk, multi-variate risk, risk assessment of newborn condition), with additional discussion of “locally-tailored” risk assessment and clinical management plus ongoing surveillance [2]. These are the latest updates on EOS since publication of “Management of Neonates with Suspected or Proven Early-Onset Bacterial Sepsis” from May 2012; which was reaffirmed in February 2016 [3,4].
Between these publications, at least two expert opinions exist on EOS-Benitz et al. [5], and Hooven and Polin [6]; which may be better summarized by the following statement: “The changing environment and new data require reappraisal of traditional approaches to management of infants at risk for sepsis, with willingness to question and abandon, if necessary, long-held assumptions [5]”.
It follows that current clinical practice for the late preterm and full term newborn remains predicated on the reaffirmed, 2012 COFN clinical report; which is very similar to the 2010 published strategy from the Center for Disease Control and Prevention (CDC), “Prevention of Perinatal Group B Streptococcal Disease Revised Guidelines”.
One of the three algorithms in the 2012 COFN Clinical Report relates to an asymptomatic infant who is ≥ 37 week’s gestation, presenting with chorioamnionitis, one major risk factor for sepsis. While this diagnosis is clinically challenging, a maternal fever greater than 100.4°F (38°C) is the essential clinical criterion for diagnosis in neonatal/pediatric clinical practice [4,7]. Current recommendations for care of the well-appearing newborn whose mother was diagnosed with chorioamnionitis include a “limited evaluation” with an initial blood culture and complete blood cell count/differential, plus empiric treatment with intravenous antibiotics.
Overall, these recommendations have yielded a remarkable reduction in the incidence of EOS: From 3-4 per 1000 live births prior to the 2010 CDC guidelines, to 0.8-1.0 per 1000 live births in 2012 [7]. Nonetheless, with approximately 60% of term newborns requiring admission to the NICU for respiratory or hemodynamic support, the clinical sequelae can be significant; including a mortality rate of 1.6% for infant’s ≥37 weeks gestation [1].
We present a case where development and rapid postpartum progression of maternal fever was unrelated to sepsis in either the mother or her full-term newborn daughter, who underwent a limited sepsis evaluation.
Between these publications, at least two expert opinions exist on EOS-Benitz et al. [5], and Hooven and Polin [6]; which may be better summarized by the following statement: “The changing environment and new data require reappraisal of traditional approaches to management of infants at risk for sepsis, with willingness to question and abandon, if necessary, long-held assumptions [5]”.
It follows that current clinical practice for the late preterm and full term newborn remains predicated on the reaffirmed, 2012 COFN clinical report; which is very similar to the 2010 published strategy from the Center for Disease Control and Prevention (CDC), “Prevention of Perinatal Group B Streptococcal Disease Revised Guidelines”.
One of the three algorithms in the 2012 COFN Clinical Report relates to an asymptomatic infant who is ≥ 37 week’s gestation, presenting with chorioamnionitis, one major risk factor for sepsis. While this diagnosis is clinically challenging, a maternal fever greater than 100.4°F (38°C) is the essential clinical criterion for diagnosis in neonatal/pediatric clinical practice [4,7]. Current recommendations for care of the well-appearing newborn whose mother was diagnosed with chorioamnionitis include a “limited evaluation” with an initial blood culture and complete blood cell count/differential, plus empiric treatment with intravenous antibiotics.
Overall, these recommendations have yielded a remarkable reduction in the incidence of EOS: From 3-4 per 1000 live births prior to the 2010 CDC guidelines, to 0.8-1.0 per 1000 live births in 2012 [7]. Nonetheless, with approximately 60% of term newborns requiring admission to the NICU for respiratory or hemodynamic support, the clinical sequelae can be significant; including a mortality rate of 1.6% for infant’s ≥37 weeks gestation [1].
We present a case where development and rapid postpartum progression of maternal fever was unrelated to sepsis in either the mother or her full-term newborn daughter, who underwent a limited sepsis evaluation.
CASE REPORT
Shortly after midnight, the on-call pediatrician for a community level I birth center and nursery received report regarding a newborn recently delivered vaginally to a 30 year old G1 now P1 mother, at 40 weeks gestation. Mother had a history of diet-controlled, type 2 diabetes and was Group B Strep screen negative. Thin-meconium fluid was noted after artificial rupture of membranes occurred six hours prior to birth. The mother had an epidural placed for labor analgesia. The APGARs were 8 and 9 for this AGA baby girl weighing 2995 g.
During labor, there were bouts of fetal tachycardia (170’s), and a slightly elevated maternal intrapartum temperature to 100.7°F with a subsequently repeated temperature of 98°F. Postpartum hemorrhage developed after delivery, with a quantitative maternal blood loss of 709 mls. The delivering obstetrician had no clinical concern for chorioamnionitis.
Approximately one hour after delivery, the mother suddenly developed chills, tachycardia, dyspnea and pyrexia to 102.9°F temporal (39.4°C); which rapidly increased to 105.2°F temporal (40.7°C). The mother had a week long history of cough prior to delivery. Additionally, she reported a long standing history of partial hearing loss secondary to a viral illness. There was no dysuria prior to delivery.
In response to the sudden onset of high maternal fever and dyspnea, maternal treatment with intravenous Benadryl (50 mg) was administered along with supplemental oxygen at 10 liters per minute, which resulted in a pulse oximetry reading of 100%, Additional treatment included two IV normal saline boluses, Tylenol 1 gram, Ibuprofen 800 milligrams and facial ice packs. Despite these measures, the mother remained significantly hyperthermic and tachycardic, requiring sepsis evaluation and transfer to the ICU. Given the abrupt and dramatic maternal decompensation, a partial newborn sepsis evaluation including CBC and blood culture was performed along with glucose monitoring and routine admission orders. The maximum infant temperature recorded was 99.7°F (37.6°C). Results of the newborn’s initial CBC, drawn by heel stick were as follows:
|
White blood cell count |
22.8 ×10 E9/L |
Neutrophils |
67 % |
|
Hemoglobin |
20.1 g/dL |
Bands |
6 % |
|
Hematocrit |
66.70 % |
Lymphocytes |
17 % |
|
Platelets |
124 ×10E9/L |
Monocytes |
10 % |
|
Immature forms |
8.20 % |
Enucleated RBCs |
6 % |
Table
The calculated EOS risk via https://neonatalsepsiscalculator.kaiserpermanente.org/ was 0.72/1000 live births, and 0.3/1000 live births after clinical exam. Although the sepsis risk calculator based clinical recommendation was for routine vitals with no antibiotic treatment, ampicillin and gentamicin were initiated for the newborn due to the uncertain etiology of the maternal fever.
By ten hours postpartum, both mother and baby were well-appearing and afebrile. The decision was made to transfer the mother back to the postpartum floor with the working diagnosis of a drug reaction to prostaglandins. Additional history suggested that maternal uterine atony had developed following the placental delivery, which was successfully managed with bimanual massage, IV Pitocin, Methergine and buccal Cytotec 800 micrograms, which has been associated with rare cases of anaphylaxis and pyrexia. Based on this additional information, the pediatric plan was modified to include no further serologic testing and discontinuation of antibiotics after 36 hours of negative blood cultures. On day of life two, mother and infant were discharged home in good condition with appropriate follow-up.
DISCUSSION
Though relatively rare in occurrence, drug reactions and side effects are important to consider when perinatal fever occurs. Common drugs which are often associated with fever include atropine, chemotherapeutic agents, allopurinol and minocycline, a widely used acne medication. Since these agents are not clinically germane during labor, clinicians must focus on the medications typically administered during labor. Prior to the clinical manifestation of maternal pyrexia in this case, the following medications were administered: Fentanyl, Lidocaine, Ropivacaine (for epidural insertion and treatment of labor pain), along with Oxytocin, Methergine and Misoprostol (for treatment of postpartum hemorrhage secondary to uterine atony). Although epidural analgesia has been associated with clinical fever during labor, the mechanism is incompletely understood. Altered thermoregulation and inflammation have been incriminated in the etiology. Epidural associated temperature elevations typically occur gradually with an average temperature increase of 1°C within 7 hours. The maternal pyrexia noted in this case report was excessive and rapid, which is typical for Misoprostol, used to treat postpartum hemorrhage.
In their 2017 Practice Bulletin on Postpartum Hemorrhage [8]-a leading cause of worldwide maternal mortality and severe morbidity in the United States, the American College of Obstetricians and Gynecologists (ACOG) reported uterine atony is estimated to cause 70-80% of all postpartum hemorrhage. It should be suspected first in the case of obstetrical hemorrhage. Additionally, they reported that postpartum hemorrhage is also unpredictable, as risk factors alone identify 60-85% of patients who will experience a significant obstetrical hemorrhage. Furthermore, while there are differences between traditional postpartum hemorrhage definitions and the current ACOG definition via the revitalize program, blood loss greater than 500 mLs from a vaginal delivery should be considered abnormal and investigated.
First-line obstetrical medical management begins with uterotonic agents, such as oxytocin (Pitocin). Up to 25% of cases require a second uterotonic agent-such as methylergonovine (Methergine), 15-methyl prostaglandin F 2α or Misoprostol (Cytotec). It is not uncommon for multiple uterotonic agents to be used in rapid succession if there is inadequate uterine response, provided no contraindications exist. The recommended Misoprostol dose is 600-1,000 mcg oral, sublingual or rectal as a one-time dose. Noted adverse effects from Misoprostol include nausea, vomiting, diarrhea, shivering, transient fever and headache, which are dose-dependent [8-12]. Although rare, anaphylaxis to Misoprostol has been reported [13].
Misoprostol is a prostaglandin E1 derivative that is inexpensive, has a long shelf life, does not require refrigeration and is available world-wide [10]. It provides a desirable and reliable resource for community obstetrical units [12] in the United States-where obstetrical services are provided in 92% of rural hospitals [8]; and for similar units in low income/resource countries.
In one study by Leon et al. of Ecuadorian women [14], their incidence of side effects to Misoprostol was considerably higher (36%), than other reports (0-9%) for reasons that were unclear. They found similar fever curves after the administration of Misoprostol 600 mcg and Misoprostol 800 mcg. Maternal body temperature was greater than 39.0°C at 1 hour after medication administration, peaking over 40.0°C between 1-2 hours after administration (Figure 1). Similar to the mother in our case report, the high fevers, greater than 40.0°C were short lived, lasting less than 1 hour.
In their 2017 Practice Bulletin on Postpartum Hemorrhage [8]-a leading cause of worldwide maternal mortality and severe morbidity in the United States, the American College of Obstetricians and Gynecologists (ACOG) reported uterine atony is estimated to cause 70-80% of all postpartum hemorrhage. It should be suspected first in the case of obstetrical hemorrhage. Additionally, they reported that postpartum hemorrhage is also unpredictable, as risk factors alone identify 60-85% of patients who will experience a significant obstetrical hemorrhage. Furthermore, while there are differences between traditional postpartum hemorrhage definitions and the current ACOG definition via the revitalize program, blood loss greater than 500 mLs from a vaginal delivery should be considered abnormal and investigated.
First-line obstetrical medical management begins with uterotonic agents, such as oxytocin (Pitocin). Up to 25% of cases require a second uterotonic agent-such as methylergonovine (Methergine), 15-methyl prostaglandin F 2α or Misoprostol (Cytotec). It is not uncommon for multiple uterotonic agents to be used in rapid succession if there is inadequate uterine response, provided no contraindications exist. The recommended Misoprostol dose is 600-1,000 mcg oral, sublingual or rectal as a one-time dose. Noted adverse effects from Misoprostol include nausea, vomiting, diarrhea, shivering, transient fever and headache, which are dose-dependent [8-12]. Although rare, anaphylaxis to Misoprostol has been reported [13].
Misoprostol is a prostaglandin E1 derivative that is inexpensive, has a long shelf life, does not require refrigeration and is available world-wide [10]. It provides a desirable and reliable resource for community obstetrical units [12] in the United States-where obstetrical services are provided in 92% of rural hospitals [8]; and for similar units in low income/resource countries.
In one study by Leon et al. of Ecuadorian women [14], their incidence of side effects to Misoprostol was considerably higher (36%), than other reports (0-9%) for reasons that were unclear. They found similar fever curves after the administration of Misoprostol 600 mcg and Misoprostol 800 mcg. Maternal body temperature was greater than 39.0°C at 1 hour after medication administration, peaking over 40.0°C between 1-2 hours after administration (Figure 1). Similar to the mother in our case report, the high fevers, greater than 40.0°C were short lived, lasting less than 1 hour.
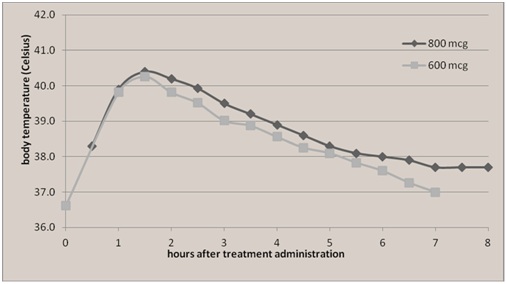
Figure 1: Reprinted with permission, from Leon et al, BMC Pregnancy Childbirth.
While a community hospital in northeastern Ohio is not epidemiologically similar to equator-straddling Ecuador, the resultant time frame of Misoprostol side effects-in particular, pyrexia, provides a tangible, clinical segue for neonatal considerations. This case quickly lends itself to the ongoing and apparently infinite discussion about EOS, highlighted by the COFN’s recommendations [1,2], expert commentaries [5,6,15,16] and AAP reaffirmation [3].
This case is important because it highlights (a) the value of interpersonal communication between obstetricians and pediatricians in solving medical dilemmas in the modern era of technology and electronic medical records and (b) the ongoing and vexing challenge of EOS [17-27] and chorioamnionitis. “Chorio” as neonatologists and pediatricians may call it, carries an increased risk of EOS [7,20] among other known neonatal and pediatric complications; affecting as much as 10% of pregnancies [20].
With the aid of a sepsis risk calculator [22]-whose development can be tracked through consecutive decades of scientific method, research and experience, we may be able to better identify newborns at risk with greater accuracy without compromising safety. Some medical conditions encountered in clinical practice may mimic or suggest sepsis-such as side effects of Misoprostol (Cytotec). In such cases where earlier appreciation is possible-including recommendations from the online sepsis calculator, it may be reasonable and safe to monitor these patients through the first 6 hours of life [16,19,25] for the development of symptoms before committing the newborn to a sepsis work up.
The potential exists to reduce unnecessary and painful newborn procedures, promote better bonding and breastfeeding practices by decreasing any separation time, reduce the development of drug-resistant bacteria, as well as decrease the incidence of such neonatal conditions as necrotizing enterocolitis. Moreover, with the judicious use of antibiotics, there will likely be more efficient use of medical resources and less iatrogenic complications Dhudasia et al. [24], observed 82% reduction in laboratory testing in the well-baby nursery after implementing a sepsis risk calculator.
In light of the traditional neonatal approach to early-onset sepsis and current scientific knowledge and understanding, the application of a sepsis calculator-assessing multiple variables or risk factors, may constitute a “win” for the patient in the form of more accurate and safe care; and a “win” for the health care systems in terms of better (i.e. more efficient) utilization of resources. Parents and family members may derive additional satisfaction from improved quality of care for their newest family members.
Not only could these clinical benefits be significant for families, but the associated monetary gains made by enhancing the returns on the $ 1 billion investment already made for scientific research on the modulation of biomarkers [28], and $ 400-500 million associated with neonatal clinical care [24,29], could also be massive.
According to finalized United States government statistics [30], 3,855,500 births were registered in 2017. After accounting for preterm births of < 37 weeks (9.93%), 3,472,648 babies were born in 2017 of at least 37 weeks gestation; just like the baby girl at the center of this case report-an n of 1.
CONCLUSION
Misoprostol, a prostaglandin E1 derivative, is an inexpensive and reliable therapy for maternal postpartum hemorrhage in the community obstetrical setting. It’s rare side effect of high maternal fever typically presents shortly after dosing, reaches peak effect within 60 to 90 minutes and may be mistaken for sepsis. Pediatricians should consider this possibility in the evaluation of any infant born to a mother with postpartum hemorrhage treated with Cytotec. Thorough communication between obstetric and pediatric providers, coupled with the use of sepsis risk calculation tools can enhance the management of affected mothers and infants.
REFERENCES
- Puopolo KM, Benitz WE, Zaoutis TE (2018) Management of neonates born at ≤34 6/7 weeks’ gestation with suspected or proven early-onset bacterial sepsis. Pediatrics 142: 20182896.
- Puopolo KM, Benitz WE, Zaoutis TE (2018) Management of neonates born at ≥ 35 0/7 weeks’ gestation with suspected or proven early-onset bacterial sepsis. Pediatrics 142: 20182894.
- American Academy of Pediatrics (2016) AAP publications reaffirmed or retired. Pediatrics 137: 20160592.
- Polin R (2012) Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics 129: 1006-1015.
- Benitz WE, Wynn JL, Polin RA (2015) Reappraisal of guidelines for management of neonates with suspected early-onset sepsis. J Pediatrics 166: 1070-1074.
- Hooven TA, Polin RA (2017) Time to overhaul the “rule out sepsis” workup. Pediatrics 140: 20171155.
- Mukhopadhyay S, Puopolo KM (2012) Risk Assessment in Neonatal Early Onset Sepsis. Semin Perinatol 36: 408-415.
- Shields LE, Goffman D, Caughey AB (2017) Practice bulletin no. 183: Postpartum hemorrhage. Obstet Gynecol 130: 168-186.
- Hofmeyr GJ, Gulmezoglu AM, Novikova N, Linder V, Ferreira S, et al. (2009) Misoprostol to prevent and treat postpartum haemorrhage: A systematic review and meta-analysis of maternal deaths and dose-related effects. Bulletin of the World Health Organization, Geneva, Switzerland.
- Allen R, O’Brien BM (2009) Uses of misoprostol in obstetrics and gynecology. Rev Obstet Gynecol 2: 159-168.
- Madaan M, Puri M, Sharma R, Sagar S (2012) Hypersensitivity Reaction to Misoprostol—A Case Report. International Journal of Clinical Medicine 3: 223-224.
- Hofmery GJ, Gulmezoglu AM, Novikova N, Lawrie TA (2013) Postpartum misoprostol for preventing maternal mortality and morbidity. Cochrane Database Syst Rev 15: 008982.
- Schoen C, Campbell S, Maratas A, Kim C (2014) Anaphylaxis to buccal misoprostol for labor induction. Obstet Gynecol 124: 466-468.
- Leon W, Durocher J, Barrera G, Pinto E, Winikoff B (2012) Dose and side effects of sublingual misoprostol for treatment of postpartum hemorrhage: What difference do they make? BMC Pregnancy Childbirth 12: 65.
- Brady MT, Polin RA (2013) Prevention and management of Infants with suspected or proven neonatal sepsis. Pediatrics 132: 166-168.
- Polin RA, Watterberg K, Benitz W, Eichenwald E (2014) The conundrum of early-onset sepsis. Pediatrics 133: 1122-1123.
- Escobar GJ, Li D, Armstrong MA, Gardner MN, Folck BF, et al. (2000) Neonatal sepsis workups in infants ≥ 2000 grams at birth: A population-based study. Pediatrics 106: 256-263.
- Puopolo KM, Madoff LC, Eichenwald EC (2005) Early-Onset Group B Streptococcal disease in the era of maternal screening. Pediatrics 115: 1240-1246.
- Escobar GJ, Puopolo KM, Wi S, Turk BJ, Kunziewicz MW, et al. (2014) Stratification of risk of early-onset sepsis in newborns ≥34 weeks’ gestation. Pediatrics 133: 30-36.
- Kiser C, Nawab U, McKenna K, Aghai ZH (2014) Role of guidelines on length of therapy in chorioamnionitis and neonatal sepsis. Pediatrics 133: 992-998.
- Cantey JB, Pozniak PS, Pruszynski JE, Sanchez PJ (2016) Reducing unnecessary antibiotic use in the neonatal intensive care unit (scout): A prospective interrupted time-series study. Lancet Infect Dis 16: 1178-1184.
- Kuzniewicz MW, Puopolo KM, Fischer A, Walsh EM, Li S, et al. (2017) A quantitative, risk-based approach to the management of neonatal early-onset sepsis. JAMA Pediatr 171: 365-371.
- Warren S, Garcia M, Hankins C (2017) Impact of neonatal early-onset sepsis calculator on antibiotic use within two tertiary healthcare centers. J Perinatol 37: 394-397.
- Dhudasia M, Mukhopadhyay S, Puopolo KM (2018) Implementation of the sepsis risk calculator at an academic birth hospital. Hosp Pediatr 8: 243-250.
- Simonsen KA, Anderson-Berry AL, Delair SF, Davies D (2014) Early-onset neonatal sepsis. Clin Microbiol Rev 27: 21-47.
- Kerste M, Corver J, Sonnevelt MC, van Brakel M, van der Linden PD, et al. (2016) Application of sepsis calculator in newborns with suspected infection. J Matern Fetal Neonatal Med 29: 3860-3865.
- Barber EL, Zhao G, Buhimschi IA, Illuzzi JL (2008) Duration of intrapartum prophylaxis and concentration of penicillin G in fetal serum at delivery. Obstet Gynecol 112: 265-270.
- Geneviève Du Pont-Thibodeau, Jean-Sébastien Joyal and Jacques Lacroix (2014) Management of neonatal sepsis in term newborns. F1000Prime Reports, London, United Kingdom.
- Mukhopadyay S, Dukhovny D, Mao W, Wichernwald EC, Puopolo KM, et al. (2014) 2010 perinatal GBS prevention guideline and resource utilization. Pediatrics 133: 196-203.
- Martin JA, Hamilton BE, Osterman MK, Driscoll AK, Drake P (2018) Births: Final data for 2017. Natl Vital Stat Rep 67: 1-50.
Citation: Tatka J, Carroll J, Wilson-Costello D (2019) A Drug Reaction Mimics Perinatal Sepsis. J Neonatol Clin Pediatr 6: 033.
Copyright: © 2019 Jason Tatka, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
© 2026, Copyrights Herald Scholarly Open Access. All Rights Reserved!

