
A Hospital Based Prospective Study on Functional Change in Cases of Ankylosing Spondylosis in Eastern India
*Corresponding Author(s):
Indranil MukhopadhyayHuman Genetics Unit, Indian Statistical Institute, India
Tel:+919433901325,
Email:indranilm100@gmail.com
Pramit Ghosh
Department Of Community Medicine, Purulia Government MCH, West Bengal, India
Tel:+919433032479,
Email:pramit00@hotmail.com
Abstract
Ankylosing spondylitis is a debilitating condition with gradual worsening of functional capacity over time. Pulmonary involvement is common in the patients either resulting from interstitial lung disease or restrictive lung disease and it eventually affects the overall functional capacity of the person.
This study was designed to estimate the change of some clinical parameters over 9 months of follow up in a teaching hospital, Kolkata amongst early cases of Ankylosing spondylitis and those with mild pulmonary functional abnormality. Periodically, scores like BASDAI was used for reviewing disease activity while BASFI was used for assessing functional status along with routine pulmonary function parameters. The patients were managed as per standard protocol in the hospital and were reviewed every three months over a period of 9 months from the baseline assessment. Thirty subjects were recruited and three cases were lost to follow up.
Rate of change varied as per status at initiation of management. BASFI scores changed gradually from 4.03(+0.55) to 3.37(+0.54) while the disease activity assessed by BASDAI showed decrease from 3.604(+0.65) to 3.23(+0.78). In both the scales baseline to end-line improvement was statistically significant. Maximum decline was noted between initial 3months for BASDAI and 3-6 months in case of BASFI. Baseline scores and vital capacity were two important factors influencing the change in scores. A clustering was noted for BASFI at 3.9 cut off and at score 3.7 for BASDAI. The subjects in two sides of the cut off showed differential rate of improvement.
BASDAI and BASFI showed that ongoing management of newly detected uncomplicated ankylosing spondylitis cases was effective in reducing disease activity and improving functional ability over 9months of follow up. However scores of functional status and disease activity at the outset were critical, influencing the rate of change and final outcome for individuals.
Keywords
Ankylosing; BASDAI; BASFI; Follow-up; Spondylitis
INTRODUCTION
Ankylosing spondylitis is a chronic debilitating inflammatory disease affecting skeletal structure. It is insidious in origin; diagnosis often gets delayed [1] to late stage of the disease. Typically males are more commonly involved and majority of cases gets reported in 3rdor 4thdecade[1,2].A systematic review3of 36 studies indicated prevalence of the disease is highest in North America, followed by Europe, Asia, Latin America and Africa. Adjusted estimate of prevalence in Asian population was 18/10000 [3].
Most common presenting features of ankylosing spondylitis are stiffness and pain. In addition to affecting various axial and other joints including tarsal or calcaneal joints[4] and, extra-articular involvement ma also occur in the subjects. Uveitis, gastrointestinal dysfunction, pulmonary involvement and cardiovascular problems can be associated with this disease [5-7]. Pulmonary function can be affected by skeletal defect or lung parenchyma can be affected directly through inflammatory pathways [6].
For diagnosing ankylosing spondylitis, a set of standard criteria is available [1,8] and along with clinical assessment, imaging plays important role in diagnosing and classifying[8] the disease.
Usually graded approach with various drugs is used to manage the cases with ankylosing spondylitis; physiotherapy is probably the most important intervention, the role of which can never be undermined [1,8]. Evenyoutube [9] may act as an important source of information for patients on physiotherapy techniques. Drugs like NSAIDS, steroids, disease modifying agents, anti-TNF biologics-all are used depending on disease condition. Surgical intervention like arthroplasty, corrective osteotomy and stabilization may also be required in subjects with refractory pain and severely compromised functional status [1,8].
Primary aim of management is to reduce pain and stiffness. Studies revealed that perception of doctors and the patients about the disease condition might not be same. A study from Europe [10] followed up 203 subjects over two years in two centers in Netherlands and one in France. Pain, BASFI and BASDAI scores were identified as prominent markers of disease severity by patients, while doctors mostly depended on clinical methods, followed by spinal mobility and laboratory investigation to grade severity of the disease. This difference in outlook towards the disease condition plays important role incompliance to treatment. As mentioned above, the scores BASFI and BASDAI are considered important for patients to understand the disease severity; these and other related Bath Ankylosing Spondylitis indices have become integral components in periodic assessment of subjects suffering from the disease [11,12]. BASFI measures functional ability while BASDAI indicates disease activity as perceived by patients [11]. The indices are very useful to track disease progression in individuals and can act as prognostic markers. These indices have even been treated as gold standard for comparing novel assessment criteria [13]. The scores have been validated and used extensively across the globe [14,15]. These indices play critical role in decision making for case management, especially to identify the need for aggressive intervention with anti-TNF or surgery etc. In addition to being reliable and valid, operational simplicity also made the indices popular for use in out-patient departments, as it requires less than 5minutes to complete the questionnaire.
Lung function parameters deteriorate in ankylosing spondylitis. Vital capacity is primarily compromised and often chest expansion is also affected. Restrictive impairment indicated by decreased vital capacity often persists with apparent improvement in disease activity[16].Compared with 121 controls, for 147 cases of ankylosing spondylitis, various lung function parameters showed significantly reduced values in a study conducted in Norway[17].Evidences from India [1,18] and other Asian countries[19]also supported this findings.
Course of the disease varies among individuals [20] especially for severity and radiological progression. Cases are usually diagnosed at the second phase with standard diagnosing criteria. A longitudinal study [21] among war-veterans diagnosed with ankylosing spondylitis continued for almost four decades since 1947 found that most of the joint restriction occurs within 1st ten years of onset. A comparative study [22] of retrospective review of records showed no differences in clinical features among males and females. During the course of the disease progression, along with changes in radiological or laboratory parameters, the indices like BASFI or BASDAI are also important guide to tailor management [23,24]. Comparative study indicated significant differences in BASFI score between normal control and diseases subjects [25]. The changes in the indices often correlate with disease activity and progression [23,24] they can also act as sensitive markers for treatment effectiveness[26].
A study from Morocco [27] noted that cut off for BASFI for differentiating ankylosing spondylitis subjects from general population would actually increase with age. Another study [28] from United Kingdom reported median age the cohort of 89 subjects with diagnosed ankylosing spondylitis was 50 years. The importance of BASDAI and BASFI was further explained by a study conducted in France [29]. This showed that minimum clinically important difference in these indices after 2 weeks of physical therapy, was independent of baseline scores. A recent study from India [30] reported poor quality of life of ankylosing spondylitis subjects correlating well with BASDAI scores but not with other Bath indices.
With this background, a prospective study was undertaken in a teaching hospital of Kolkata, India to determine the pattern of change of BASDAI and BASFI scores over time. Factors like age, disease duration, and lung function parameters were assessed to identify predictors of change over the follow up period.
METHODS
It was a prospective study with 9 months of follow up. The proposal was approved by institutional ethics committee and tools were predesigned and pretested prior to application. The subjects were recruited from out-patient department. Initial diagnosis was done using modified NY criteria [7,8] (1984). Case in the early stage [8] of the disease not requiring steroids, methotrexate, and anti-TNF drugs were selected. Smokers, those with permanent chest deformity, having cardiac problems who could not tolerate rehabilitation protocols and those having contraindication to therapeutic exercises were excluded. Purpose and process of the study was explained to every subject and informed consent was obtained from those willing to participate.
The subjects were assessed as per regular institutional protocol. Along with clinical assessment, laboratory investigation, lung function tests and necessary imaging were done; treatment was initiated according to standard norms. Physiotherapy remained the mainstay of management in addition to NSAIDs for these subjects. Physiotherapy sessions were held in the hospital on fixed days of the week. For the rest of the days, a home-exercise protocol was made available to each patient and was explained to them in detail. These included a detailed exercise prescription. Aerobic exercise included walking, jogging etc., related to lower extremity, arm ergometry for upper extremity and a combination of both. For problem areas like hip, back, knee- strength training like resisted flexion, abduction, adduction or step up, leg push, hamstring curl etc. were advocated depending on the tolerability of the patients. Thoracolumbar and cervical mobility training were also imparted. To improve pulmonary function, breathing exercises, chest expansion etc., were prescribed.
The subjects were oriented to keep a log of activities and exercises performed at home in predesigned record booklet. Every three months (+1 week), detailed assessment using the study tool was done. In addition to clinical examination, scoring for BASFI and BASDAI, lung function parameters were also assessed for every subject. BASFI and BASDAI were scored based on visual analogue scale on a scale of 0-10 with 0 indicating least no problem and 10 maximum problem. Pulmonary function was assessed using flow sensing spirometer. Pulse-oximeter was used during assessment and also during physiotherapy sessions to ensure safety of subjects with restrictive lung diseases. The lung function parameter values used during analysis were percentage of predicted values. Case record forms included information on basic demographic profile, examination findings and scores of BASFI and BASDAI. The form also recorded and updated relevant information from the logbook maintained by the subject at home. In the log book information was available for home exercise compliance as well as daily activity pattern. This helped ultimately to calculate MET (Metabolic Equivalent) score.
During every quarterly visit, subjects were asked about their willingness to continue further in this study and doubts, if any were clarified during that time or later over phone, if necessary.
RESULTS
The study recruited at the outset 30 subjects of which 3 were lost to follow up. They did not turn up in time and were omitted from final analysis. 24 subjects were male and 3 females. Average age of subjects was 31.3 years (+ 6.91) with a range of 23-48 years; median age being 30years. Average disease duration was 5 years (+2.48) ranging from 1-10years.
Baseline assessment showed that BASDAI indices ranged between 2.70-4.45 and BASFI ranged from3.20 to 4.90. All the lung function parameter values at baseline were lower than reference range.
The indices changed over time as shown in the figure1. Changes in mean BASFI and BASDAI scores from one time point to the next were statistically significant as evident by results obtained from Wilcoxon signed rank test. The p-values for changes in different time points for both BASFI and BASDAI were all <0.0001. The changes however had different slopes and in case of BASFI, scores reduced more drastically. Figure 2 indicates change in individual patient’s score of BASDAI and figure 3 the change of BASFI over the follow up period.
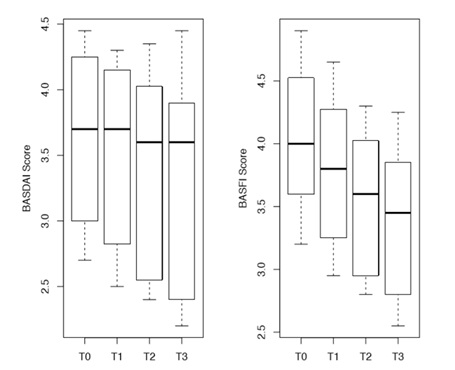 Figure1: Boxplot showing trend of BASDAI and BASFI scores over time (n=27).
Figure1: Boxplot showing trend of BASDAI and BASFI scores over time (n=27).
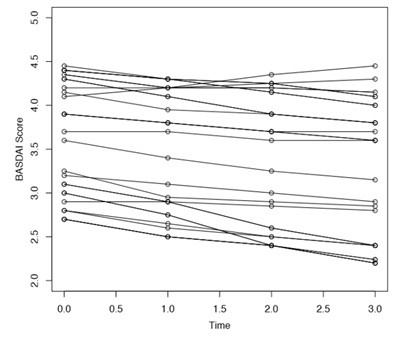 Figure2: Line diagram of changes in BASDAI score over time (n=27).
Figure2: Line diagram of changes in BASDAI score over time (n=27).
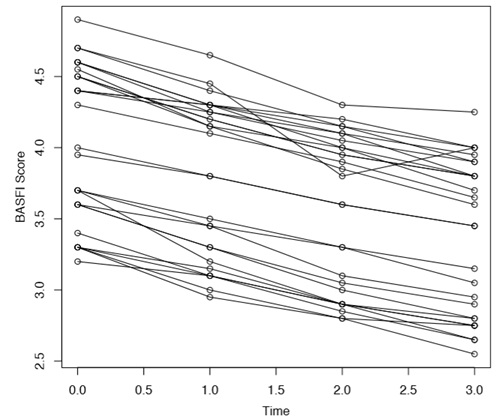 Figure 3:Line diagram of changes in BASFI score over time (n=27).
Figure 3:Line diagram of changes in BASFI score over time (n=27).
Decline in mean scores were not uniform for all the subjects. Line diagram (Figure 2 and figure 3) of changes for individual subjects indicated two groups; one where the indicator score remained above certain value across all time points while another group where decline was more. Cluster analysis identified baseline value of 3.9 for BASDAI and 3.7 for BASFI, which may be considered as watershed line for two groups, with those above the cut off showing persistent higher scores in follow up.
Change of mean values of BASFI and BASDAI from T0 to T3 wasanalyzed using multiple linear regression model considering age of the subject, disease duration, vital capacity, FEV1/FVC, chest expansion, MET score etc. as predictor variable. In case of reduction of BASFI scores (Table 1) of individuals, disease duration was identified as the sole significant predictor.
|
Parameters |
B |
Std. Error |
Beta |
t-statistic |
p-value |
|
(Constant) |
1.645 |
1.814 |
|
0.907 |
0.378 |
|
Vital Capacity |
0.006 |
0.032 |
0.28 |
0.188 |
0.853 |
|
Total Lung Capacity |
-0.009 |
0.025 |
-0.402 |
-0.356 |
0.726 |
|
FEV1 |
-0.005 |
0.015 |
-0.188 |
-0.312 |
0.759 |
|
FEV1/FVC |
0.024 |
0.021 |
0.311 |
1.141 |
0.271 |
|
Chest Expansion |
-0.022 |
0.062 |
-0.07 |
-0.355 |
0.728 |
|
SaO2 |
-0.018 |
0.022 |
-0.185 |
-0.819 |
0.425 |
|
MET |
-0.013 |
0.028 |
-0.085 |
-0.448 |
0.66 |
|
BASFI-baseline |
-0.109 |
0.084 |
-0.361 |
-1.293 |
0.214 |
|
Age |
-0.002 |
0.007 |
-0.082 |
-0.3 |
0.768 |
|
Disease Duration |
-0.057 |
0.019 |
-0.855 |
-3.026 |
.008* |
|
Model |
R |
R Square |
Adjusted R Square |
Std. Error(Estimate) |
|
1 |
0.82 |
0.672 |
0.467 |
0.12154 |
|
Dependent: change in BASFI (T0-T3), Predictors: (Constant), disease duration, SaO2, TLC, Chest Expansion, MET, FVC, Age, baseline BASFI, FEV1/FVC, VC |
||||
Table1: Multiple linear regression model for change in BASFI (T0-T3).
For changes in BASDAI (Table 2), age of subjects, baseline values of FEV1/FVC and BASDAI were identified as significant predictor.Overall, the two Bath indices showed good positive correlation (0.92), but correlation between percentage changes in the scores was less (0.46).
|
Parameters |
B |
Std. Error |
Beta |
t-statistic |
p-value |
|
|
(Constant) |
8 |
2.2 |
|
|
0.002 |
|
|
FEV1/FVC |
-0 |
0 |
-1 |
|
.006* |
|
|
Age |
-0 |
0 |
-1 |
|
.013* |
|
|
Disease Duration |
0 |
0 |
1 |
|
0.061 |
|
|
BASDAI-baseline |
-0 |
0.1 |
-0 |
|
.018* |
|
|
Model Summary |
||||||
|
Model |
R |
R Square |
Adjusted R Square |
Std. Error of the Estimate |
||
|
1 |
.682a |
0.47 |
0.367 |
0.19609 |
||
|
Dependent: change in BASDAI; Predictors: (Constant), baseline BASDAI, Age, FEV1/FVC, disease duration |
||||||
Table 2: Multiple linear regression model for change in BASDAI (T0-T3).
DISCUSSION
In the present study, among subjects in the early stage of the disease were undergoing regular treatment as per standard institutional protocol. The selected subjects were periodically assessed every three months for changes in BASDAI and BASFI over 9-month period. Certain lung function parameters were also recorded to find out association with change in Bath indices. Baseline scores of Bath indices were similar to many other studies [15,23-25,27].
A study among 200 ankylosing spondylitis patients from Morocco [27] reported BASDAI 4.08 ± 2.23 and mean BASFI score 4.5. Cut off of BASDAI for ankylosing spondylitis compared to general population increased gradually from 0.9 in 18-29years, to 1.5 in 30-49years and 2.5 in persons aged more than 50years. In this study mean BASDAI at baseline was 3.6+ 0.65 (Table 3) and BASFI was 4.0.3 +0.55 and comparable to other studies.
|
Parameters |
Average |
Range |
Reference range31 |
|
Vital Capacity (VC) |
70.07+7.71 |
56-82 |
80-120 |
|
Total Lung Capacity(TLC) |
71.44+7.54 |
58-84 |
80-120 |
|
FEV1 |
72.77+6.68 |
64-86 |
|
|
FEV1/FVC |
91.30+2.16 |
88-96 |
Within 5% of predicted |
|
Chest Expansion |
2.79+0.52 |
2.1-3.8 |
|
|
SaO2 |
93.96+1.69 |
91-97 |
|
|
MET |
5.44+1.12 |
7-Mar |
|
|
6MWT |
925.3704 |
850-1060 |
|
|
BASDAI |
3.60+0.65 |
2.7-4.45 |
|
|
BASFI |
4.03+0.55 |
3.2-4.9 |
|
Table 3: Summary values of lung function parameters obtained at baseline for the subjects.
Study from United Kingdom [28] reported median age the cohort of 89 subjects with diagnosed ankylosing spondylitis was 50 years (inter-quartile range while median age of disease onset was 25 years and the median disease duration was 18 years. In this study median age was 30 years with disease duration varying from 1-10 years. More than half (47/89) participants in the UK based study [28] had BASDAI score of 4 or higher on the first assessment, of whom 45 (51%) continued to have 4 or higher on all subsequent assessments. In this study 10 out of 27 subjects (37%) had baseline BASDAI score of 4 or above. BASDAI scores correlated strongly with Bath Ankylosing Spondylitis Functional Index (BASFI) scores. Participants with persistently higher BASDAI scores showed higher scores for anxiety and depression compared to the other group of subjects with persistently lower BASDAI score. In the current study also some subjects were performing sub-optimally beyond a cut off value of 3.9 for BASDAI.
A study [15] was conducted in China among two sets of patients receiving two different anti TNF-α agents. The baseline BASDAI scores were 4.92 ± 1.75 and 4.31 ± 1.6 in two groups. After 6 weeks, BASDAI scores came down to 2.82 ± 1.65 and 0.96 ± 1.1. In this study it was 3.60+0.65 at baseline and after 3 months, of treatment with physiotherapy and NSAIDs, score was down to 3.46+ 0.69. Later the scores went down to 3.24+0.79. Similarly BASFI scores went down from 3.99±2.34 to 2.15±2.03 in one and from 2.40±2.26 to 0.67±1.11 in 6 weeks. In this study BASFI scores at baseline was 4.03+0.55 and later gradually reduced to 3.37+0.54. BASFI and BASDAI showed15 significant positive correlation in that study with r=0.587 and 0.590 in two groups. In the current study also at baseline correlation between two scores was very high (r=0.9805). However, correlation between changes in two scores over time showed only a mild positive correlation with r= 0.2349. In the study mentioned above, similarly, correlation between changes of scores was quite low in one group[15] 0.345.Maximum decline for BASDAI in the current study was noted during the initial 3 months while that for BASFI was noted during 3rd to 6th month. Overall, the two Bath indices showed good positive correlation (0.92), but correlation between percentage changes in the scores was not that high (0.46).
Changes in the scores over different time points were statistically significant. The pattern of change depended upon respective baseline values. Score of3.7 for BASDAI and 3.9 for BASFI split the cohort in two distinct groups where end-line scores vary.
Pulmonary function test results and some other parameters were assessed during the study to identify predictors of change in Bath indices. In a study [17] conducted in 2011 at University of Oslo, Norway among 147 subjects, mean FEV1 was 89.8% and FEV1/FVC were 76.5%. The results were similar in another study from Uttar Pradesh, India [18].In the present study values of FEV1 at baseline was 72.7 and FEV1/FVC was 91.3%.
A longitudinal study from UK carried out between 1996 to 2001, reported [24] that in cases of sever ankylosing spondylitis BASDAI scores remained practically unaltered during the course of follow up while BASFI score actually worsened. In the current study however improvement in both the scores over shorter follow up period was noted in both the indices. A systematic review [32] on effect of TNF-α inhibitor used synthesis model to estimate conditional change in BASDAI and BASFI scores among responder and non-responders. It was predicted that for BASDAI, from overall score of 6.08, it will come down to 3.45 and from 5.24 to 3.6 in case of BASFI. In the present study the baseline value was much less compared to this study; still a statistically significant change in scores was observed.
A longitudinal study conducted earlier in France noted that lung function parameters like vital capacity did not change much over time with therapy [16]. In active phase of disease chest expansion was correlated with vital capacity but neither was related to diseases activity. Another study from Taiwan [19] showed vital capacity, chest expansion to be correlated with BASFI. In this study also Bath indices were correlated with lung function values at baseline.
A multi-country study [33] involving 283 subjects from Europe reported the overall change in BASDAI and BASFI scores after 4 weeks of NSAID therapy in terms of Minimum Clinically Important Improvement (MCII) &Patient Accepted Symptom State (PASS). Cut off for MCII were found to be 0.7 and 0.4 respectively for BASDAI and BASFI, while 4.1 & 3.8 for PASS. Baseline values and disease duration were important predictors for PASS and MCII scores. In the current study also, change in BASFI was dependent on disease duration and for BASDAI-age and baseline score were found to be important predictors.
CONCLUSION
It may be concluded from the study that over short period of follow up, BASFI and BASDAI for subjects in early stages of ankylosing spondylitis did improve with statistically significant change from one time point to next. The change in scores from baseline to score during last follow up, indicated BASFI to be associated with disease duration and BASDAI with baseline value of BASDAI, age and FEV1/FVC.Long term follow up may provide more information on pattern of change in indices and possible role of pulmonary functions on functional impairment or disease activity in the disease.
LIMITATIONS
The study aimed to document changes in the Bath indices over time, however the study only focused on subjects in early phase of the disease and naturally the result obtained cannot be generalized for severe forms of the disease. Moreover indices like BASMI or BAS-G, if measured, would have provided more comprehensive insight into the problem.
ACKNOWLEDGEMENT
Research reported in this publication was supported by the Fogarty International Center of the National Institutes of Health under Award Number R25TW009717. We also acknowledge the support from Prof A. Talukder, department of Medicine, Medical College, Kolkata in guiding us. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- McVeigh CM, Cairns AP (2006) Diagnosis and management of ankylosing spondylitis. BMJ 333:581-585.
- Prakash S, Mehra NK, Bhargava S, Vaidya MC, Malaviya AN (1984) Ankylosing spondylitis in North India: a clinical and immunogenetic study. Ann Rheum Dis 43:381-385.
- Dean LE, Jones GT, MacDonald AG, Downham C, Sturrock RD, et al. (2014) Global prevalence of ankylosing spondylitis. Rheumatology (Oxford) 53:650-657.
- Koca TT, Gö?ebakan H, Koçyi?it BF, Nacitarhan V, Yildir CZ(2019) Foot functions in ankylosing spondylitis. ClinRheumatol 38:1083-1088.
- El Maghraoui A (2011) Extra-articular manifestations of ankylosing spondylitis: prevalence, characteristics and therapeutic implications. Eur J Intern Med 22:554-560.
- Kanathur N, Lee-Chiong T (2010) Pulmonary manifestations of ankylosingspondylitis.Clin Chest Med 31:547-554.
- Narsimulu G, Suresh K. Management of AnkylosingSpondylitis, chap 62.
- Braun J, van der Heijde D, Dougados M, Emery P, Khan MA, et al. (2002) Staging of patients with ankylosingspondylitis: a preliminary proposal. Ann Rheum Dis 61:19-23.
- Kocyigit BF, Nacitarhan V, Koca TT, BerkE (2019)YouTube as a source of patient information for ankylosing spondylitis exercises.ClinRheumatol. 38:1747-1751.
- https://nass.co.uk/wp-content/uploads/2018/09/Bath-Indices.pdf accessed on September-2018
- Zochling J, Braun J (2005) Assessment of ankylosingspondylitis. ClinExpRheumatol 23: 133-141.
- Spoorenberg A, van Tubergen A, Landewé R, Dougados M, van der Linden S, et al.(2005) Measuring disease activity in ankylosing spondylitis: patient and physician have different perspectives.Rheumatology (Oxford). 44:789-795.
- Bansal N, Duggal L, Jain N (2017) Validity of Simplified Ankylosing Spondylitis Disease Activity Scores (SASDAS) in Indian Ankylosing Spondylitis Patients. J ClinDiagn Res 11:6-9.
- Kemal NAS, Remzi ÇEV?K, Mehtap BOZKURT, Ali GÜR, Ay?egül Jale SARAÇ (2011) Relationship Between Clinical Findings, Quality of Life and Functional Disability Related to Disease Activity in Patients with Ankylosing Spondylitis. Turk J Rheumatol 26: 29-37.
- Lin Z, Gu J, He P, Gao J, Zuo X, et al (2009) Multicenter validation of the value of BASFI and BASDAI in Chinese ankylosing spondylitis and undifferentiated spondyloarthropathy patients. Rheumatol Int 31:233-238.
- Franssen MJ, van Herwaarden CL, van de Putte LB, Gribnau FW(1986) Lung function in patients with ankylosing spondylitis. A study of the influence of disease activity and treatment with nonsteroidal antiinflammatory drugs. J Rheumatol 13:936-940.
- Berdal G (2011) Pulmonary Function in Patients with Ankylosing Spondylitis A cross sectional controlled. University of Oslo, Norway.
- Singh AK, Lochab K (2017) Assessment of Pulmonary Function Test (Pft) In Patients of Ankylosing Spondylitis. IOSR Journal of Dental and Medical Sciences 16: 63-68.
- Hsieh LF, Wei JC, Lee HY, Chuang CC, Jiang JS, et al. (2016) Aerobic capacity and its correlates in patients with ankylosing spondylitis. Int J Rheum Dis 19:490-499.
- Pradeep DJ, Keat A, Gaffney K (2008)Predicting outcome in ankylosing spondylitis. Rheumatology 47: 942-945.
- Carette S, Graham D, Little H, Rubenstein J, Rosen P (1983) The natural disease course of ankylosing spondylitis. Arthritis Rheum 26:186-190.
- Jiménez-Balderas FJ, Mintz G (1993) Ankylosing spondylitis: Clinical course in women and men. The Journal of rheumatology 20: 2069-2072.
- da Costa IP, Bortoluzzo AB, Gonçalves CR, da Silva JA, Ximenes AC, et al. (2014) Evaluation of performance of BASDAI (Bath Ankylosing Spondylitis Disease Activity Index) in a Brazilian cohort of 1,492 patients with spondyloarthritis: data from the Brazilian Registry of Spondyloarthritides (RBE).Rev Bras Reumatol 5: 48-54.
- Robertson LP, Davis MJ (2004) A longitudinal study of disease activity and functional status in a hospital cohort of patients with ankylosing spondylitis. Rheumatology 43:1565-1568.
- Brophy S, Cooksey R, Davies H, Dennis MS, Zhou SM, et al. (2013). The effect of physical activity and motivation on function in ankylosing spondylitis: a cohort study. Semin Arthritis Rheum 42:619-626.
- Vastesaeger N, van der Heijde D, Inman RD, Wang Y, Deodhar A, et al. (2011) Predicting the outcome of ankylosing spondylitis therapy. Ann Rheum Dis70:973-981.
- Wariaghli G, Allali F, Berrada K, Idrissi Z, Hmamouchi I, et al. (2012) Normative values for the bath ankylosing spondylitis functional index in the general population compared with ankylosing spondylitis patients in Morocco. BMC Musculoskeletal Disorders 13:2-5.
- Martindale J, Smith J, Grennan D, Goodacre L, GoodacreJA (2010) Outcome of active disease in ankylosing spondylitis: a prospective study.Musculoskeletal Care8:10-7.
- Pavy S, Brophy S, CalinA (2005) Establishment of the minimum clinically important difference for the bath ankylosing spondylitis indices: a prospective study.JRheumatol 32:80-85.
- Gupta L, Ahmed S, Choudhury GD,Misra DP, Agarwal V (2018). Poor quality of life in Indian ankylosing spondylitis patients. Indian Journal of Rheumatology 13: 101-106.
- Barreiro TJ, Perillo I (2004) An Approach to Interpreting Spirometry. American Family Physician (69):1107-1115.
- Corbett M, Soares M, Jhuti G, Rice S, Spackman E, et al. (2016) Tumour necrosis factor-α inhibitors for ankylosing spondylitis and non-radiographic axial spondyloarthritis: a systematic review and economic evaluation. Health Technol Assess 20:1-334.
- Kviatkovsky MJ, Ramiro S, Landewé R, Dougados M, Tubach F, et al. (2016) The Minimum Clinically Important Improvement and Patient-acceptable Symptom State in the BASDAI and BASFI for Patients with Ankylosing Spondylitis. J Rheumatol. 43:1680-1686.
Citation: MisraSK, MondalPK, Faiheng AL, Jami J, Langstieh BT, et al.(2019) A Hospital Based Prospective Study on Functional Change in Cases of Ankylosing Spondylosis in Eastern India. J Phys Med Rehabil Disabil 5: 033.
Copyright: © 2019 Swapan Kumar Misra, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

