A Palette of Adipose Tissue: Multiple Functionality and Extraordinary Plasticity
*Corresponding Author(s):
José Rodrigo PauliLaboratory Of Molecular Biology Of Exercise, University Of Campinas (UNICAMP), Limeira, São Paulo, Brazil; Laboratory Of Cell Signaling, Obesity And Comorbidities Research Center (OCRC), University Of Campinas, Campinas - SP, Brazil , CEPECE - Center Of Research In Sport Sciences, School Of Applied Sciences, University Of Campinas (UNICAMP), Limeira, São Paulo, 13484-350, Brazil
Email:rodrigopaulifca@gmail.com
Abstract
After the knowledge of adipose tissue as an endocrine organ and its role as a regulator of metabolisms, studies have advanced on its biological function. Previously, only two adipose tissues were identified in mammals, white and brown adipose tissue. White adipocytes store lipids mainly with the function of energy reserve and brown for thermal homeostasis. Due to the plasticity of adipose tissue and its ability to proliferate and differentiate, the third type of adipocyte, beige, emerged. Beige adipocytes originate from white adipocytes that have acquired phenotypic brown characteristics in response to different stimuli, this process is known as browning. More recently, the plastic properties allowed the identification of the fourth type of adipose tissue, the pink. Pink adipocytes are alveolar epithelial cells of the mammary gland originating from white adipocytes. The plastic properties of adipose tissue allow direct transdifferentiation into fully differentiated adipocytes. This extraordinary plasticity can generate new health therapies, specifically metabolic diseases.
Keywords
Adipocytes; Cell differentiation; Cell transdifferentiation; Plasticity
INTRODUCTION
Although the relevant role of adipose tissue is recognized in functions such as thermal insulation, protection, hormone synthesis and especially energy storage, it is only in the last few decades that adipose tissue has received great prominence in the field of anatomy and physiology for its notorious endocrine and metabolic functions in the human body [1,2]. Definitely, its capacity to store and release available energy is considered of crucial importance for human survival and metabolism. Through the lipid stored in the form of triacylglycerol, humans are able to survive periods of food scarcity and this reserve provides energy for traveling long distances in hunting activities, searching for housing, and territorial conquests [3]. However, the new scientific discoveries of its multifunctionality and extraordinary plasticity have led adipose tissue to assume the role of protagonist over immuno-metabolic actions and the control of body energy expenditure.
Adipose is an organ anatomical depot divided into a subcutaneous compartment and visceral adipose depots, located in mediastinal, abdominal, and pelvic regions [4]. Considered of its phenotype, functional role, and gene expression profile, the adipose tissue can be classified as white, brown, beige or brite, and pink [5,6].Thus, adipose tissue has become a crucial target of study, as this tissue is directly associated with obesity and metabolic diseases, but also the functional response and plasticity of the adipose organ the alteration metabolic and environmental [4,6]. We present the evidence of the types of adipose tissue biology and highlight how these differentiation cellular and possible strategies therapeutics.
WHITE ADIPOSE TISSUE
Over the years, it has been observed that White Adipose Tissue (WAT), which in humans appears yellow, is more involved with the storage and release of lipids. Anatomically, approximately 90% of the white adipocytes are filled with a unilocular droplet of triglycerides in the cytoplasm [4]. White adipocytes are responsible to store and release energy during fasting and fed intervals. Besides, white adipose tissue and its endocrine activities that contribute to pathological changes in other organs and implicates in the development of obesity and associated metabolic disorders, and Visceral Adipose Tissue (VAT) appears to be more endocrinologically active than other adipose depots [7,8]. In addition, WAT has shown a significant endocrine action through adipokine secretion, such as leptin and adiponectin, which is important to control food intake and glucose homeostasis. Moreover, adipose tissue hypertrophy is associated with the recruitment of immune cells and pro-inflammatory secretion, which is an important trigger to insulin and leptin signaling resistance in several tissues, and the development of metabolic disorders such as hypertension, diabetes, and different types of cancer [9]. In addition, this inflammatory context, changing the profile of immune cells in the adipose, reduces M2 macrophages (anti-inflammatory) and increases M1 macrophages (pro-inflammatory) [10].
BROWN ADIPOSE TISSUE
On the other hand, in this rainbow of adipose tissue, Brown Adipose Tissue (BAT) has been the topic of many recent studies. The anatomy of BAT cells presents several small multilocular lipid droplets and a higher mitochondria content in their cytoplasm. The brown color is due to elevated numbers of mitochondria. Beyond the mitochondria, BAT expresses an Uncoupling Protein 1 (UCP1) that performs a crucial role as a mediator of uncoupled mitochondria respiration and thus, higher oxidative function [11]. In adult humans, BAT was described in small depots in cervical, supraclavicular, axillary, paraspinal, mediastinal, and abdominal sites [12]. This is important because for a while researcher expected that this kind of adipose would be exclusive to small mammals and newborns. In addition, more recently, an important difference was described in the molecular action activator between humans and mice, which showed that in humans BAT thermogenesis is triggered by β2-adrenergic receptors, while in mice it is triggered by β3-adrenergic receptors [13]. Therefore, new approaches could be investigated in order to increase knowledge of BAT thermogenesis.
Brown adipocytes are also capable of secreting endocrine factors, called "batokines" that act on the glycemic and lipid metabolism [11]. Among the “batokines”, neuregulin 4 (NRG4) derived from adipose tissue can act in the liver and systemic insulin sensitivity. In addition, the anti-inflammatory effects and this adipokine established through local action it is probably relevant to the systemic effects of NRG4. BAT also secretes lipokine, the 12,13-dihydroxy- (9Z) - octadecenoic acid (12,13-diHOME). 12,13-diHOME increased energy expenditure and stimulated the absorption of fatty acids in BAT, seems to be important to supply fatty acids to organs in conditions of high energy demand, the cellular mechanisms still need to be clarified [14] New approaches involving transplantation of embryonic stem cells and Induced Pluripotent Stem Cells (IPSCs) have demonstrated the ability to stimulate the thermogenic activity of BAT. These strategies were recently discussed by Kang-Yun Lu et al., and future gene therapies with the objective of improving metabolic efficiency are still in scientific development [15].
BEIGE ADIPOSE TISSUE
Interestingly, WAT can differentiate and share brown adipocyte characteristics. This process has been characterized as beiging/browning. The morphology of beige cells shows small lipid droplets, increased mitochondrial content, and increased oxidation capacity [6]. Strategies, such as β3-adrenergic stimulators, cold exposure, dietary strategy with specific nutrients and food exposure, and physical exercise, have demonstrated an ability to induce beiging, however clinical investigations are challenging and more studies are necessary [16-19]. The beige adipocytes secrete NRG4 and 12,13-diHOME. This lipokine increases to facilitate thermogenesis [14]. Induction browning is promising for the treatment of obesity [11,18].
PINK ADIPOSE TISSUE
Lastly, during pregnancy and lactation phases, the milk-secreting alveolar cells have a pink color, higher lipid droplets in the cytoplasm, and milk-containing granules. The major difference through the evolution of pregnancy in these cells is the content of lipids [20]. It is postulated that these alveolar milk cells are derived from white adipocytes, and thus, these cells have been denominated pink adipocytes [21]. In addition, pink adipocytes could change to white and brown adipocytes during the post-lactation period [20].
MAJOR POINTS
As discussed here, adipose tissue has a plastic capacity to transdifferentiate and convert into phenotypes with different physiological traits, a phenomenon called rainbow adipocytes [22]. The multifunctionality between the different kind of adipocytes allow the energy storage, burn lipids for thermogenesis, release endocrine factors, and lactation. Thus, the development of studies on the plasticity of adipose tissue, especially on the signs that initiate the plasticity, are relevant to the advancement of therapies that manage to direct the plasticity of adipose tissue. Overnutrition and aging induce alteration in the adipose organ plasticity and promotes metabolically consequences such as diabetes and cancer [4,6,22]. What we have over the rainbow may be of great scientific importance and for the area of anatomy and physiology (Figure 1).
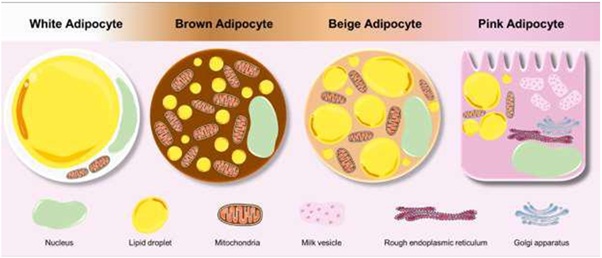 Figure 1: Colorful adipose tissue. Adipocytes present differences in anatomy, physiology, and color. WAT adipocytes are associated with storage and release of energy, while brown and beige adipocytes are involved in thermogenesis. Lastly, pink adipocytes have an ability to store lipids and milk-containing granules. We would like to thank SMART Servier for the images used in the figure of this manuscript (https://smart.servier.com).
Figure 1: Colorful adipose tissue. Adipocytes present differences in anatomy, physiology, and color. WAT adipocytes are associated with storage and release of energy, while brown and beige adipocytes are involved in thermogenesis. Lastly, pink adipocytes have an ability to store lipids and milk-containing granules. We would like to thank SMART Servier for the images used in the figure of this manuscript (https://smart.servier.com).
CONCLUSION
Therefore, adipose tissue plasticity has been an important topic of recent studies and new findings have stimulated scientists to investigate factors associated with adipose tissue transdifferentiation. Through this palette, we have new understanding of adipose tissue organ and function and further discoveries are necessary to increase knowledge about the background of this rainbow. Thus, there is great expectation that the dynamism of adipose tissue will allow the construction of a portfolio of new actions that can contribute to health, specifically obesity and metabolic diseases.
REFERENCES
- Wells JCK (2012) The evolution of human adiposity and obesity: Where did it all go wrong? Dis Model Mech 5: 595-607.
- Ghaben AL, Scherer PE (2019) Adipogenesis and metabolic health. Nat Rev Mol Cell Biol 20: 242-258.
- Sellayah D, Cagampang FR, Cox RD (2014) On the evolutionary origins of obesity: A new hypothesis. Endocrinology 155: 1573-1588.
- Cinti S (2018) Adipose Organ Development and Remodeling. Compr Physiol 8: 1357-1431.
- Oikonomou EK, Antoniades C (2019) The role of adipose tissue in cardiovascular health and disease. Nat Rev Cardiol 16: 83-99.
- Giordano A, Smorlesi A, Frontini A, Barbatelli G, Cint S (2014) White, brown and pink adipocytes: The extraordinary plasticity of the adipose organ. Eur J Endocrinol 170: 159-171.
- Vijay J, Gauthier M-F, Biswell RL, Louiselle DA, Johnston JJ, et al. (2020) Single-cell analysis of human adipose tissue identifies depot- and disease-specific cell types. Nat Metab 2: 97-109.
- McNamara JP, Huber K (2018) Metabolic and Endocrine Role of Adipose Tissue during Lactation. Annu Rev Anim Biosci 6: 177-195.
- Kopelman PG (2000) Obesity as a medical problem. Nature 404: 635-643.
- Gregor MF, Hotamisligi GS (2011) Inflammatory mechanisms in obesity. Annu Rev Immunol 29: 415-445.
- Gaspar RC, Pauli JR, Shulman GI, Muñoz VR (2021) an Update on Brown Adipose Tissue Biology: A Discussion of Recent Findings. Am J Physiol Metab 1-9.
- Leitner BP, Huang S, Brychta RJ, Duckworth CJ, Baskin AS, et al. (2017) Mapping of human brown adipose tissue in lean and obese young men. Proc Natl Acad Sci U S A 114: 8649-8654.
- Blondin DP, Nielsen S, Kuipers EN, Severinsen MC, Jensen VH, et al. (2020) Human Brown Adipocyte Thermogenesis Is Driven by β2-AR Stimulation. Cell Metab 32: 287-300.
- Scheja L, Heeren J (2019) The endocrine function of adipose tissues in health and cardiometabolic disease. Nat Rev Endocrinol 15: 507-524.
- Lu KY, Dass KTP, Lin SZ, Harn HJ, Liu SP (2020) The application of stem cell therapy and brown adipose tissue transplantation in metabolic disorders. Cytotherapy 22: 521-528.
- Nedergaard J, Cannon B (2014) The browning of white adipose tissue: Some burning issues. Cell Metab 20: 396-407.
- Yao L, Cui X, Chen Q, Yang X, Fang F, et al. (2017) Cold-Inducible SIRT6 Regulates Thermogenesis of Brown and Beige Fat. Cell Rep 20: 641-654.
- Okla M, Kim J, Koehler K, Chungn S (2017) Dietary factors promoting brown and beige fat development and thermogenesis. Adv Nutr 8: 473-483.
- Rodrigues KCdC, Pereira RM, de Campos TDP, de Moura RF, da Silva ASR, et al. (2018) The role of physical exercise to improve the browning of white adipose tissue via POMC neurons. Front Cell Neurosci 12: 88.
- Cinti S (2018) Pink Adipocytes. Trends Endocrinol Metab 29: 651-666.
- Cinti S (2019) Anatomy and physiology of the nutritional system. Mol Aspects Med 68: 101-107.
- Corrêa LH, Heyn GS, Magalhaes KG (2019) The Impact of the Adipose Organ Plasticity on Inflammation and Cancer Progression. Cells 8: 662.
Citation: Gaspar RC, Muñoz VR, Macêdo APA, Vieira RFL, Pauli JR (2021) A Palette of Adipose Tissue: Multiple Functionality and Extraordinary Plasticity. Trends Anat Physiol 4: 013.
Copyright: © 2021 Rafael Calais Gaspar, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.