
A randomized, Double-Blind, Placebo-Controlled Multicenter Study of Kan Jang® Capsules in Patients with URTI Infection with the Primary Outcome of Sick Leave Days
*Corresponding Author(s):
Georg WikmanSwedish Herbal Institute R&D (SHIRD), Vallberga, Sweden
Tel:+46 702-733753,
Email:karl.georg.wikman@gmail.com
Abstract
This randomized, double-blind, and placebo-controlled multicenter study was initiated to evaluate the efficacy of Kan Jang capsules, containing a standardized extract of Andrographis paniculata Burm. Nees, folia (SHA-10) and a standardized hydro-alcoholic extract from the root Eleutherococcus senticosus (Rupr. et Maxim.) Maxim (SHE-3) with the primary outcome of sick leave days.
A total of 86 patients, diagnosed with uncomplicated upper respiratory infection (ICPC code 460), of both sexes were randomized into two groups. They received either Kan Jang capsules (verum) or placebo. The inclusion assessment was based on the doctor's symptom evaluation scores of the patients, which were determined by a Verbal Descripting Scales (VDS).
The ten self-evaluated symptoms, such as cough, expectoration, nasal discharge, headache, fever, sore throat, earache, malaise/fatigue, and sick leave were chosen based on previously performed studies on Kan Jang.
The results showed that the overall common illness symptoms declined faster in the Kan Jang group compared to the placebo group. Reductions of symptom scores were seen in the Kan Jang group from days 2 to 7, especially the symptom of hoarseness and nasal congestion improved in the Kan Jang group. This improvement was maintained for the rest of the treatment period.
In this study, we also found that the sick leave for the placebo group was 1.79 days, while the sick leave in the Kan Jang group was reduced to 0.44 days.
Keywords
Andrographis fixed combination; Common cold; Efficacy; Kan Jang®; Randomized controlled study; Upper Respiratory Tract Infection (URTI)
Introduction
Common cold is one of the most common infections and will affect adults approximately twice per year and in children up to four times per year. In the United States, each adult experience two to four respiratory tract infections annually [1], which accounts for more than 10 % of ambulatory patient encounters with primary care physicians [2]. The figures for USA and Sweden are similar.
Kan Jang is a preparation that has been on the market in the Nordic countries for the past 35 years. Kan Jang in the most trusted trademark in the cough and cold segment [3]. Kan Jang with the fixed combination of the two plants Andrographis paniculata (Burm. F.) (extract SHA-10) and Eleutherococcus senticosus (Rupr. & Maxim.) (Extract SHE-3). This combination is known to exhibit antiviral, immunomodulatory and anti-inflammatory effects [4] and clinical efficacy in the respiratory tract of patients with infectious diseases [5-9].
The objectives of this study were to compare the effect and tolerability of Kan Jang capsules with identical placebo capsules in patients with uncomplicated upper respiratory tract infections/common colds in general open care practice. The study focused on early recovery, with the primary outcome of sick leave days, and improved symptom relief compared to the natural course of the disease.
The efficacy was based on the patients’ daily self-evaluation of symptoms on a symptom scorecard. From an earlier suggestion by a leading epidemiologist, Prof Kjell Alestig, one experimental measurement parameter were introduced, the inhalation capacity via nose, see below, and by their self-measuring of the nasal peak flow using a peak-flow meter.
Materials And Methods
Aim of the study
The aim was to investigate and compare the effect and tolerability of Kan Jang capsules, compared to placebo capsules, in the treatment of symptoms related to uncomplicated respiratory tract infections, with the primary outcome of sick leave days.
The size of the study was based on results from previous studies, which were believed to be sufficient to give a predictive value. Particular attention was given to the assessment of the organoleptic identity between Kan Jang and placebo before the start of the study. An important difference from earlier studies was the inclusion of objective measurements such as nasal congestion and nasal discharge. The study was approved by the local Ethical Committee and by the Medical Products Agency in Sweden.
Study design
The study was performed as a randomized, double-blind, and parallel group study with a Kan Jang group and a placebo group.
The studied product
The studied drug is an approved proprietary herbal medicinal product that has been on the market in the Nordic countries since 1985. The active ingredients in the capsules are a standardized hydro-alcoholic extract of Andrographis paniculata Burm. Nees, folia (SHA-10), and a standardized hydro-alcoholic extract from the roots of Eleutherococcus senticosus Rupr. et Maxim. (SHE-3). Both extracts were developed and produced by Swedish Herbal Institute Ltd (SHI) in Sweden.
The product is standardized to a total daily dose of ≥ 60 mg and rographolide.
The extracts used adhere fully to the EU-cGMP standard for pharmaceuticals including herbal medicinal products. SHI holds a valid license to produce Herbal Active Pharmaceutical Ingredients (API) for herbal medicinal products. All quality control, which includes analysis of solvent residue, heavy metals, pesticide residue and microbial contamination, is performed by contract laboratories in accordance with cGMP. The test medication used in this study is Kan Jang capsules, which was analyzed and released for use by the Swedish Herbal Institute (SHI), Gothenburg. The Kan Jang capsules were supplied in similar jars with 50 capsules. A placebo preparation with identical organoleptic appearance and filled in similar jars with 50 capsules was also supplied by SHI. The active and placebo test medication were filled, packed in glass bottles.
Kan Jang
The active test medication was a fixed combination of standardized extracts as described below. The extract mixture was filled in green colored capsules size 1, containing the following extracts:
Extr. Andrographis paniculata, Nees, folia, siccum (SHA-10): 180 mg
Extr. Acanthopanax senticosus, Maxim, radix, siccum (SHE-3): 23 mg
Microcrystalline cellulose: 92mg
Silica dioxide: 5 mg
Magnesium separate: 3 mg
Placebo
The inactive test medication consisted of green colored capsules identical to the active test medication. It contained a mixture of inactive constituents to achieve the same weight as the active capsules.
Microcrystalline cellulose: 260 mg
Sugar color No. 15684 (E 160): 35 mg
Silica dioxide: 5 mg
Magnesium separate: 3mg
Quinoline yellow (E 104): 0.4 mg
Indigo Carmine (E 132): 0.2 mg
Data management procedures
All data relevant to the study was entered in the Clinical Report Form (CRF). The subjects that received the trial medication were logged in a prepared CRF. Care was taken when entering the data to ensure legibility. Corrections were made by crossing out the error and entering the correct value. All corrections were dated and signed. Statisticians performed the statistical evaluation. According to the accepted policy, the patients were only identified by their patient number and initials, the center, and the trial-ID. The data was entered into the database patient by patient. A clean and labeled database was built before the statistical analysis started.
Statistical analysis
The statistical calculations were carried out using Graph Pad 5 and the Statistica 10.0 for Windows software package. The results were expressed as the mean ± SEM. Student’s t-test was used for statistical analyses of data dichotomy; p-values
Statistics
Eighty-six (86) participants were considered for the final statistical analysis. The patient characteristics and sick leave for the two groups were compared by independent samples using a t-test program from Statistica software.
Individual symptom scores recorded at different time intervals for each group (Placebo and Kan Jang) were analyzed using unpaired Analysis Of Variance (ANOVA). The statistical calculations above were made with the Graph Pad 5.0 software. The subjective data were treated as longitudinal data in the trial in order to evaluate the effect of the treatment over time. The effect size of individual and overall symptoms was treated as non-parametric longitudinal data and calculated as the difference between the Area under the Curve (AUC) for Kan Jang and placebo, respectively. AUC is an acceptable measure for comparing two independent treatment groups over time [10].
Participants
Eighty-six (86) participants of both sexes, aged between 18- and 60-years-old, were enrolled in the study. The study was conducted at eight different centers in Sweden. The study period was chosen due to the self-limiting nature of the illness, and the fact that patients normally treated their illness without contacting healthcare professionals.
The required sample size for finding a statistically significant difference between the means of the two groups was estimated. The size of the Kan Jang and placebo groups had to be at least 40 subjects each, or a total of 80 for the whole study, since a dropout rate of approximately 10 %is common in clinical trials and therefore had to be considered.
The patients were recruited from seven (7) Open Care Centers and one (1) Occupational Medical Center in Sweden, predominately located on the west coast of Sweden. The following centers were involved:
- • Hallehälsan (Occupational Health Center), Ulricehamn
- • Vårdcentralen (Open Care Center), Angered
- • Partus Kvinnohälsa Female (Open Care Center), Gothenburg
- • Centralhälsan (Open Care Center) Falköping
- • Kronhushälsan (Open Care Center): (under name change to Previa)
- • Previa (Open Care Center), Nässjö
- • Previa (Open Care Center), Jönköping
- • Vårdcentralen (Open Care Center), Sjuntorp
Patient recruitment
When a patient visited any of the centers, they were diagnosed according to a standard scheme to ensure that the patient fulfilled all the inclusion criteria and did not have any of the exclusion criteria. If a patient was found to fulfill the inclusion criteria, and interested in participating, he or she was included into the study after having received written and verbal information about the study.
After informed consent was signed, the subject was assigned a patient number and given a bottle with capsules with the same number. The patient was instructed to follow the recommended dosage for 8 days and to carefully fulfill the task of recording all symptoms using a patient diary with Verbal Descripting Scales (VDS) for each symptom.
The patients were recruited according to the following inclusion and exclusion criteria:
Inclusion criteria
- • Subjects of both sexes must be in the age group of 18-65 years old
- • Subjects who have been informed verbally and in writing and given written informed consent
- • Subjects predominantly suffering from symptoms of common cold according to the inclusion criteria (cough, expectoration, running nose, headache, fever, sore throat, earache, and malaise/fatigue). The patient should not show any sign of abacterial infection
Exclusion criteria
- • Patients who have known allergies to herbs, herbal spices, or bitter substances
- • Patients who were pregnant and lactating or had the intention of getting pregnant
- • Patients on a medication that might affect the course of the disease (anti-inflammatory medication or antibiotics)
- • Patients using narcotics, tobacco (>20cigarettes per day) or alcohol
- • Subjects suffering from cold symptoms for more than 36 hours or having fever higher than 38.5°C
After the inclusion the doctor performed a baseline diagnosis using a Verbal Descripting Scales (VDS) where the symptoms were graded asfollows:0 = symptoms absent; 1 = mild symptoms; 2 = moderate symptoms; 3 = severe symptoms covering the following symptoms:
- • General malaise (tiredness, muscle pains, coldness, etc.)
- • Headache
- • Nasal running
- • Nasal blockage
- • Sore throat
- • Cough
- • Conjunctivitis
- • Hoarseness
The total score for each patient was calculated from each of the diagnosed symptoms above by use of the VDS.
Randomization
The randomization to allocate patients was a total randomization generated by computer. All randomization codes were kept in a sealed envelope. A qualified person at SHI (a pharmacist) was responsible for keeping the blinded randomization codes and dispensing bottles to the different centers.
Dosage regimen and dose rationale
Each patient was handed one bottle containing 50capsules. Each patient was advised to take the medication according to the administration schedule for a period of eight (8) days. The dosage was based on the earlier published clinical trials carried out with Kan Jang products for the same indication.
Administration schedule
The Kan Jang or placebo capsules were administered as follows: Two (2) capsules, three (3) times daily, every day for 8 days. This corresponded to 48 capsules for the duration of the study. The capsules were to be swallowed whole with sufficient liquid.
Symptomatic assessment of efficacy experimental subjective parameters
The patient assessed symptoms every day for eight (8) consecutive days. The following symptoms were self-evaluated and recorded as Verbal Descripting Scales (VDS) by the patient: general malaise (tiredness, muscle pains, coldness, etc.); headache; nasal running: nasal blockage; sore throat; cough; conjunctivitis and hoarseness.
Objective assessment of efficacy: No of sick leave days
Absence from work in days (number of days): The patient was instructed to record each day absent from work. This is an absolute parameter that gives a clear indication on the patient’s general state of disease and ability to perform.
Symptomatic assessment of efficacy objective parameters
Nasal obstruction measured with a Peak flow meter (mL per minute): For this purpose, each patient was supplied with anasal-inspiratory peak-flow meter and asked to record the nasal potency by making 3 maximal inspirations through the nose with the instrument placed over the nose. The values were recorded in the evenings and written down in the patient diary. The patients made rhinomanometric measurements each day by means of a Nasal Peak Inspiratory Flow Rate Meter (NPIFR) according to instructions [11]. These measurements were performed to complement the subjective evaluations by the patients of their nasal congestion. The reason for this was that some cold preparations contain substances that stimulate the cold receptors in the nose and thus gives a feeling of relief of congestion – but, the flow of air through the nostrils is unchanged. Especially substances like menthol, eucalyptus oil, pine oil and peppermint oil have been attributed this effect, (geraniol, hydroxycitronella and linalool).
Safety evaluation
Study events: The study events were defined as any relevant event experienced by the patient during the study, such as unwanted effects and intercurrent illness.
Procedures in case of serious/unexpected study events: The patients were informed at the start of the study to take immediate contact with the investigator if intercurrent illnesses developed or if side-effects occurred. As mentioned previously, no unexpected or serious side-effects were expected. The patients were informed to stop the treatment immediately if side-effects appeared that possibly were linked to the treatment with the study medication and then report back to the investigator. All tolerability problems were recorded in the proper CRF. In case of unexpected and/or serious unwanted effects, these were to be reported to the Medical Products Agency according to current regulations and in the proper form. No side-effects or adverse reactions were recorded during the study.
Emergency code envelopes: An emergency code envelope was prepared for each patient. This envelope contained information about the treatment that the patient received during the study. The envelope was to be opened only in case of emergency, such as if severe side-effects would occur. In this way, proper treatment could be given to the specific patient without affecting the blinding of the study.
In case of emergency, the responsible investigator had an Emergency Code Envelope for each bottle of Kan Jang and placebo capsules. The ID number was written on the envelope. If an unexpected event and/or serious unexpected event occurred, the envelope should be opened and the patient or doctor could then take the proper action, depending on if it was Kan Jang or placebo that the patient had received.
A record of adverse events was also kept in the patient diary. During the treatment period, the patients were instructed to report to the investigators if any adverse reactions occurred. The investigators were also instructed to report any adverse events that were brought to their knowledge.
Concomitant medications
The study restricted all concomitant administration of other drugs, such as anti-inflammatory drugs, antibiotics, cough remedies, antihistamines, or any other herbal/OTC product for the treatment of URTI. All use of other concomitant medicine during the study period had to be recorded in the CRF.
Drug inventory and unused medication Compliance
Patient compliance was censured by the patient’s record in the patient diary and by the investigator collecting the bottles of unused medication. Deviations from the protocol had to be recorded by the patient and in the CRF.
Results
A total of 86 patients, fulfilled the selection criteria, and willingly gave informed consent to be enrolled in the study and randomized to either placebo (n=43) or Kan Jang (n=43). The demographic characteristic (age) of all available patients was summarized in table 1. The mean characteristics of both groups, at baseline, were found to be similar in a comparison (Figures 1-3).
|
t-test for independent samples Spread sheet in compared placebo/verum data. Note variables were treated as independent samples. |
|||||||||||
|
|
Mean |
Mean |
t-value |
df |
p |
Valid N |
Valid N |
Std. Dev |
Std. Dev |
F-ratio |
P |
|
Age placebo vs Age Kan Jang Caps. |
44.04651 |
46.16279 |
-1.05684 |
84 |
0.293614 |
43 |
43 |
9.418709 |
9.14937 |
1.059743 |
0.851741 |
Table 1: Age of patients.
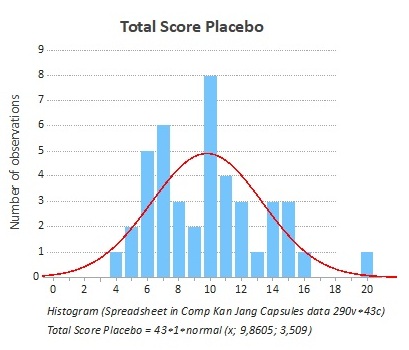 Figure 1: The frequency of the distribution of the total score at the start of the study was the same for both groups (Placebo).
Figure 1: The frequency of the distribution of the total score at the start of the study was the same for both groups (Placebo).
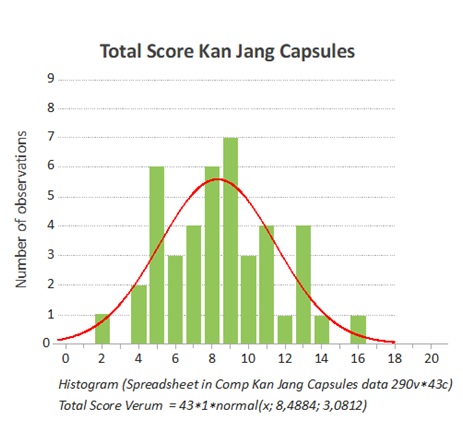 Figure 2: The frequency of the distribution of the total score at the start of the study was the same for both groups (Kan Jang).
Figure 2: The frequency of the distribution of the total score at the start of the study was the same for both groups (Kan Jang).
The total score includes the doctors’ evaluation of the symptom scores for all investigated subjective parameters such as general malaise (tiredness, muscle pains, coldness, etc.); headache; nasal discharge; sore throat; cough; conjunctivitis and hoarseness.
On day 1 (baseline), there was no significant difference between the individual symptom scores inthe Kan Jang group compared to the placebo group. The total symptom scores of all included patients, both placebo and Kan Jang, are summarized in figure 3. During the treatment period, the participants were instructed to record their subjective symptoms very thoroughly in a symptom diary.
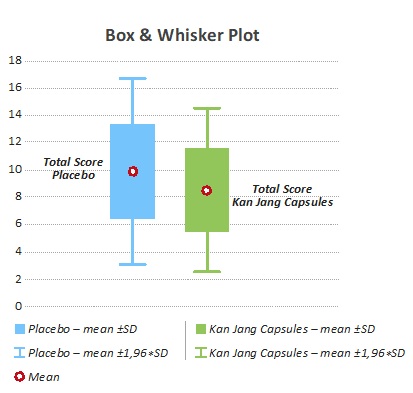 Figure 3: Box & Whisker lot Total Symptom Score baseline for p<0.200.
Figure 3: Box & Whisker lot Total Symptom Score baseline for p<0.200.
The measurement of symptom score was evaluated for each patient and the two groups were compared for each symptom as well as for total score. Observations in each group show that common illness, hoarseness, and nasal congestion, subjectively evaluated by the patient, was improving faster in the Kan Jang group compared to the placebo group.
The overall common illness symptom scores declined faster in the Kan Jang group, in comparison to the placebo group (Figure 4). The reduction in symptom score for the Kan Jang group was seen from days 2 to day 7. 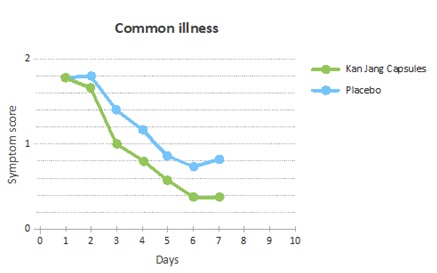
Figure 4: Improvement of the common illness scores measured as the decline of the scores each day relative to the baseline.
In particular, it was the symptom of hoarseness and nasal congestion that improved in the Kan Jang group (Figure 5).
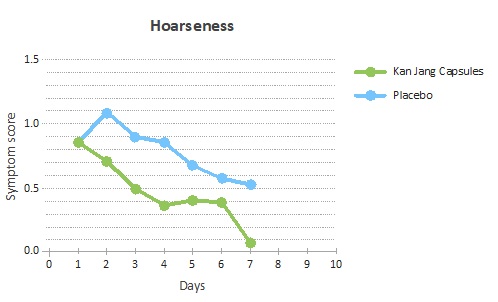 Figure 5: Improvement of the hoarseness scores measured as the decline of the scores each day relative to the baseline.
Figure 5: Improvement of the hoarseness scores measured as the decline of the scores each day relative to the baseline.
The difference between placebo and verum continued throughout the treatment, although the grade of congestion could not be confirmed by rhinomanometry, and no significant difference could be seen between the two groups. However, the actual experience of feeling a better flow in the nasal cavity might be attributed to the treatment (Figure 6).
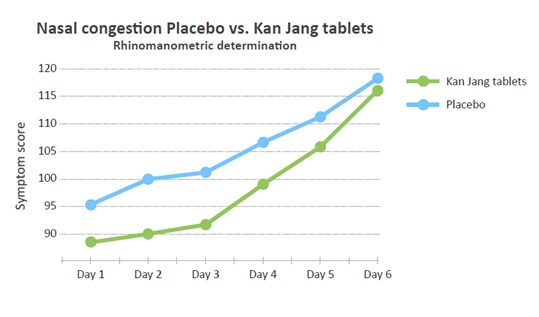 Figure 6: Development of the nasal congestion scores measured as the symptom scores each day.
Figure 6: Development of the nasal congestion scores measured as the symptom scores each day.
Since the Kan Jang does not contain any volatile oils or menthol-like substances, there could be no “menthol effect” [12] on the cold receptors postulated for the Kan Jang treatment. Nasal decongestion as experienced by the patients in the study and evaluated subjectively was not possible to correlate to the rhinomanometric measurements performed by the patients. It is a relatively novel field of research. The rhinomanometric data did not correlate to the grade of nasal congestion and were not significantly different between the two groups.
The use of the Pnif instrument was demonstrated, but it turned out to be insufficient training for adequate use, ie more learning of use is necessary (Figure 7).

Figure 7: Nasal decongestion measured as improved rhinomanometric flow [mL/min].
As for the experimental measurement parameters it became clear that this need further development (Table 2 & Figure 8).
|
Sick Leave Absence from work |
|||||||||
|
|
Mean placebo |
Mean Kan Jang |
t-value |
df |
p |
Valid N |
Valid N |
std. Dev. |
std. Dev. |
|
Total sick leave Age placebo vs Total sick leave Age Kan Jang Caps. |
1.790698 |
0.44186 |
3.322108 |
84 |
0.001 |
43 |
43 |
2.502933 |
0.907701 |
Table 2: Sick leave number of days absent from work.
Number of sick leave days
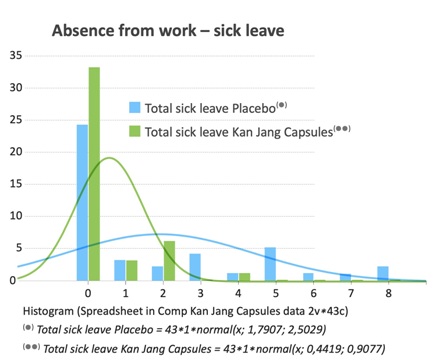 Figure 8: Sick leave number of days absent from work.
Figure 8: Sick leave number of days absent from work.
The parameter “sick leave –absence from work” was calculated over eight days and is probably the most important parameter for diseases such as URTI and common cold. The sick leave for the placebo group was 1.79 days (100 %), while the sick leave for patients in the Kan Jang group was reduced to 0.44 days (25 %). This difference is highly significant.
There were no adverse reactions reported in any of the groups during the study period.
Discussion
Viral Upper Respiratory Tract Infections (URTIs) are one of the most common diseases among humans. Adults are reported to have, on average, two to five common colds per year. This figure is higher among school children with seven to ten colds per years [13]. These figures may be reduced with herbal medicines that are beneficial for preventing and treating common colds. The active ingredients in the proprietary herbal medicinal product Kan Jang have been used in both modern and traditional medicine to promote a healthy immune system.
In the present study, we evaluated the effect of Kan Jang, which contains standardized extracts of Andrographis paniculata (SHA-10) and Eleutherococcus senticosus (SHE-3), using Verbal Descripting Scales (VDS) for each symptom of uncomplicated URTIs, as most treatments are symptomatic. Indeed, changes in symptom scores are, in general, the main parameter of efficacy in clinical studies.
The findings in this study confirm that the symptom scores in the Kan Jang group got progressively better during the treatment, compared to placebo. There was a significant decrease in the severity of the general sickness, hoarseness and nasal congestion. In addition, there was an observed reduction in the severity scores for the nasal discharge due to the treatment with Kan Jang, which is in line with previous findings [5,14].
A recent meta-analysis of clinical trials based on Kan Jang showed that Kan Jang was more effective than placebo and can be considered an alternative when treating uncomplicated URTIs [15]. There were also significant improvements for patients with URTI (the overall effect) as regards short-term treatment with Kan Jang [7,1314].
Other clinical studies have looked at the intensity/frequency of cough as one of the primary outcome measures of the efficacy of Kan Jang and reported that Kan Jang was superior to placebo [6,7,14]. Indeed, Kan Jang has shown a beneficial effect in reducing the severity of sore throats in two earlier clinical studies [5]. Gabrielian et al., also showed that general malaise significantly improved for patients treated with Kan Jang tablets [5]. Kan Jang showed a quick recovery [8] with reduced duration of sick leave [6,9].
The estimated total number of workdays for a small business with 20 employees is estimated to be 4400 days per year, while an average sick leave for the industrial workforce is estimated to be 5.5 %. This gives 242 days per year lost due to sick leave. In monetary terms, this translates to a cost in the region of EUR 72 600 for a small business (the sick leave cost is on average EUR 200 per day).
In a health economic study performed in Sweden [16] it was found that common colds and allergic rhinitis pose a global health problem that affects social life, sleep, school and work performance and that this is likely to impose a substantial economic burden on society due to the absence from work and reduced working capacity. The study assessed the loss of productivity in the Swedish working population. Four thousand questionnaires were sent to a randomized adult population, aged 18-65 years, in Sweden, stratified by gender and area of residence (metropolitan area vs. the rest of the country).
One thousand two hundred and thirteen individuals responded (1213), response rate 32 %. The mean productivity loss was estimated at 5.1 days or EUR 653 per worker and year, yielding a total productivity loss in Sweden of EUR 2.7 billion a year.
In this study, we demonstrated that the sick leave for the placebo group was 1.79 days. This figure was reduced by approximately 75 % in the Kan Jang group to 0.44. Thus, theoretically Kan Jang may have a substantial cost-saving impact on the economy and on the public health system. Kan Jang has been found to be well tolerated in patients with URTIs. There were no reported side-effects in our study, which were in accordance with results from other published literature [4,5,6,7,8,9,14].
Our findings showed that Kan Jang treatment significantly (p<0.001) decreased several symptom parameters between days 2 to and 7, such as general sickness, hoarseness, and nasal congestion, in comparison to placebo. The most important parameter was days of sick leaving, which was significantly, reduced. This means that the absence from work/school can be positively affected and reduced using Kan Jang.
References
- Garibaldi RA (1985) Epidemiology of community acquired respiratory tract infections in adults.Incidence, etiology and impact. Am J Med 78: 32-37.
- Dixon RE (1985) Economic costs of respiratory tract infections in the United States. Am J Med 78: 45-51.
- Digest R (2007) Trusted brands comparison of winning brands over 5 years. Cough and cold segment: Sweden - Kan Jang.
- Panossian A, Davtyan T, Gukassyan N, Gukasova G, Mamikonyan G, et al. (2002) Effect of Andrographolide and Kan Jang fixed combination of extracts HA-10 and extract SHE-3-on proliferation of humanlymphocytes, production of cytokines and immune activation markers in the whole blood cells culture. Phytomedicine 9: 598-605.
- Gabrielian ES, Shukarian AK, Goukasova GI, Chandanian GL, Panossian AG, et al. (2002) A double blind, placebo-controlledstudy of Andrographis paniculatafixed combination Kan Jang in the treatment ofacute upper respiratory tract infections including sinusitis. Phytomedicine 9: 589-597.
- Kulichenko LL, Kireyeva L, Malyshkina EN, Wikman G (2003) A randomised, controlled study of Kan Jang® versus amantadine in the treatment of influenza in Volgograd. J Herb Pharmacother 173: 188-194.
- Melchior J, Spasov AA, Ostrovskij OV, Bulanov AE, Wikman G (2000) Double-blind,placebo-controlled pilot and Phase III study ofactivity of standardized Andrographis paniculataHerbaNees extract fixed combination (Kan Jang)in the treatment of uncomplicated upperrespiratorytract infection. Phytomedicine 7: 341-350.
- Spasov AA, Ostrovskij OV, Chernikov MV, Wikman G (2004) Comparative controlled study of Andrographis paniculata fixed combination, Kan Jang and an Echinacea preparation as adjuvant, in the treatment of uncomplicated respiratory disease in children. Phytother Res 18: 47-53.
- Melchior J, Palm S, Wikman G (1997) Controlled clinical study of standardizedAndrographis paniculata dried extract* incommon cold – a pilot trial. Phytomedicine 3: 315-318.
- Albert PS (2000) Longitudinal data analysis (repeatedmeasures) in clinical trials. Stat Med 19:1821.
- Wihl JÅ, Malm L (1988) Rhinomanometry and nasal peak expiratory and inspiratory flow rate. Ann Allergy 61: 50-55.
- Eccles R (1992) Nasal airway resistance and nasalsensation of airflow. Rhinol Suppl 14: 86-90.
- Eccles R (2005) Understanding the symptoms of thecommon cold and influenza. Lancet 2005; Infect Dis 5: 718-725.
- Cáceres DD, Hancke JL, Burgos RA, Sandberg F, Wikman GK (1999) Use of visual analogue scale measurements (VAS) to assess theeffectiveness of standardized Andrographispaniculata extract SHA-10 in reducing thesymptoms of common cold. A randomizeddouble blind-placebo study.
Phytomedicine 6: 217-223. - Coon JT, Ernst E (2004) Andrographis paniculata in the treatment of upper respiratory tract infections: a systematic review of safety and efficacy. Planta medica 70: 293-298.
- Hellgren J, Cervin A, Nordling S, Bergman A, Cardell LO (2009) Allergic rhinitis and the commoncold – high cost to society. Allergy 65: 776-783.
Citation: Melchior J, Barth A, Hoheisel O, Palm S, Wikman G, et al. (2021) A randomized, Double-Blind, Placebo-Controlled Multicenter Study of Kan Jang® Capsules in Patients with URTI Infection with the Primary Outcome of Sick Leave Days. J Altern Complement Integr Med 7: 161.
Copyright: © 2021 Jan Melchiorre, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

