
A Review on Chemical and Physical Methods of Controlling Microbial Growth
*Corresponding Author(s):
Alemayehu ChoramoBonga University College Of Agriculture And Natural Resource, Ethiopia
Email:alexchoramo@gmail.com
Abstract
Microorganisms are ubiquitous and many microbes are associated with harmful consequences, like food spoilage and disease. As a result, it is necessary to kill a wide variety of microorganisms or inhibit their growth to reduce their harmful effects. Control of microbes can be achieved by a variety of chemical and physical methods. Agents which destroy bacteria are said to be bactericidal. Agents which inhibit the growth and reproduction of bacteria without bringing about their total destruction are described as bacteriostatic. Desirable qualities of the chemicals like rapid action in low concentration, solubility in water or alcohol, stable, broad spectrum, low toxicity, penetrating, noncorrosive and non-staining, affordable and readily availability are important to choose and use chemical. Therefore, Objectives this review is: To compile different chemical and physical agents used in controlling microbial growth as well as to understand factors that influence activities of chemical agents. Disinfection refers to the use of a chemical agent that destroys or removes all pathogenic microorganisms or agents capable of causing infection. Disinfection destroys vegetative form of pathogens but not bacterial endospores. An antibiotic is a microorganism produced substance (or a synthetic derivative) that has antimicrobial properties. All antibiotics have the common property of interfering in some way with a normal, critical function of the target bacterial cell. Based on their mode of actions antibiotics are classified into four major categories. A limited but increasing number of antibiotics can be used to treat mycotic infections. On the other hand, the virus is a tough creature to kill, as it has no peptidoglycan wall, no ribosomes, and no cell membrane. Resistance to antimicrobial drugs either genetic origin of non-genetic origin of drug resistance. Active replication of bacteria is required for most antibacterial drug actions. Consequently, microorganisms that are metabolically inactive (non-multiplying) may be phenotypically resistant to drugs. Most drug-resistant microbes emerge as a result of genetic change and subsequent selection processes by antimicrobial drug. The physical methods of microbial growth are use temperature fluctuating, filtration and ultraviolet light. Temperatures below the minimum usually have a “static” action on microorganisms and Temperatures above the maximum usually have a “tidal “action. The ultraviolet portion of the light spectrum includes all radiations with wavelengths from 100 nm to 400 nm. It has low wave-length and low energy. The microbicidal activity of ultraviolet (UV) light depends on the length of exposure. Microbiological membrane filters provide a useful way of sterilizing materials such as vaccines, antibiotic solutions, animal sera, enzyme solutions, vitamin solutions, and other solutions that may be damaged or denatured by high temperatures or chemical agents.
Keywords
Chemical; Control; Growth; Microbial; Physical
Introduction
Microbes are ubiquitous and many microorganisms are associated with undesirable consequences, such as food spoilage and disease. Therefore, it is essential to kill a wide variety of microorganisms or inhibit their growth to minimize their destructive effects. The goal is twofold: (a) to destroy pathogens and prevent their transmission and (b) to reduce or eliminate microorganisms responsible for the contamination of water, food, and other substances [1]. Control of microorganisms can be achieved by a variety of chemical and physical methods. Agents which destroy bacteria are said to be bactericidal. Chemical methods, whilst effective at disinfection, are generally not reliable for achieving total sterility. Agents which inhibit the growth and reproduction of bacteria without bringing about their total destruction are described as bacteriostatic [2].
In practice, we speak of “sterilization” as the process of killing all of the organisms in a preparation. Although most sterilization is performed with a physical agent, such as heat, a few chemicals called sterilants can be classified as sterilizing agents because of their ability to destroy spores [3]. In surgery and medicine, the sterilization of instruments, drugs and other supplies is important for the prevention of infection [4].Disinfection processes remove the harmful microorganisms (toxins) from materials. Examples of disinfection include (a) applying a solution of 5% bleach to examining table, (b) boiling food utensils used by a sick person, and (c) immersing thermometers in an isopropyl alcohol solution between uses Products or biocides used to reduce the number of viable microorganisms, or bio burden, on or in a product or surface to a level previously specified as appropriate for its intended further handling or use. Disinfectants are not necessarily sporicidal but are sporostatic, inhibiting germination or outgrowth [3].
Desirable qualities of the chemicals like rapid action in low concentration, solubility in water or alcohol, stable, broad spectrum, low toxicity, penetrating, noncorrosive and nonstaining, affordable and readily availability are important to choose and use chemical [5].
Objectives
- To compile different chemical and physical agents used in controlling microbial growth.
- To understand factors that influence activities of chemical agents.
Definition of Frequently Used Terms
Sterilization: is defined as a process by which an article, surface, or medium is freed of all living microorganisms either in the vegetative or in the spore state. Any material that has been subjected to this process is said to be sterile [4].
A germicide: (microbicide) is any chemical agent that kills pathogenic microorganisms. A germicide can be used on inanimate (nonliving) materials or on living tissue, but it ordinarily cannot kill resistant microbial cells [1].
Biocide: A chemical or physical agent, usually broad spectrum that inactivates microorganisms [3].
Bacteriostatic: A specific term referring to the property by which a biocideis able to inhibit bacterial multiplication [3].
Bactericidal: A specific term referring to the property by which a biocide is able to kill bacteria. The terms “fungicidal,” “sporicidal,” and “virucidal” refer to the property whereby biocides are able to kill fungi, spores, and viruses, respectively [3].
Disinfection: Refers to the use of a chemical agent that destroys or removes all pathogenic organisms or organisms capable of giving rise to infection. This process destroys vegetative pathogens but not bacterial endospores [1].
Aseptic: free of or using methods to keep free of, microorganisms.
Preservation: The prevention of multiplication of microorganisms in formulated products, including pharmaceuticals and foods.
Antibiotics: Naturally occurring and synthetically derived organic compounds that inhibit or destroy selective bacteria, generally at low concentrations.
Sepsis: is defined as the growth of microorganisms in the body or the presence of microbial toxins in blood and other tissue [1].
Disinfectants
Disinfection refers to the use of a chemical agent that destroys or removes all pathogenic organisms or organisms capable of giving rise to infection. This process destroys vegetative pathogens but not bacterial endospores. It is important to note that disinfectants are normally used only on inanimate objects because they can be toxic to human and other animal tissue, when used in higher concentrations [3].
Types of Disinfectants
Disinfectants include the following: phenolic compounds, halogens, alcohols, aldehydes, gases, surface active agents, oxidizing agents, dyes, heavy metals, and acids and alkalis [1].
Phenolic compounds
In 1867, Joseph Lister employed phenolic compounds to reduce the risk of infection during operations. Phenolic compounds are the most widely used antiseptics and disinfectants in laboratories and hospitals worldwide. They are bactericidal or bacteriostatic and some are fungicidal also. They act by denaturing proteins and disrupting cell membranes. They are effective in the presence of organic material and remain active on surfaces long after application. Different phenolic compounds are as follows [2].
Phenol is effective against vegetative forms of bacteria, Mycobacterium tuberculosis, and certain fungi [1]. Cresols are more germicidal and less poisonous than phenol but corrosive to living tissues. They are used for cleaning floors (1% solution), for disinfection of surgical instruments, and for disinfection of contaminated objects. Lysol is a solution of cresols in soap [2].
Halogenated diphenyl compounds
These compounds include hexachlorophene and chlorhexidine. They are highly effective against both Gram-positive and Gram-negative bacteria. They are used as skin antiseptics and for the cleaning of wound surfaces. Hexachlorophene has been one of the most popular antiseptics because once applied it persists on the skin and reduces growth of skin bacteria for longer periods [2]. However, it can cause brain damage and is now used in hospital nurseries only after a staphylococcal outbreak [1].
Halogen
Halogens are fluorine, bromine, chlorine, and iodine, a group of nonmetallic elements that commonly occur in minerals, sea water, and salts. Although they can occur either in the ionic (halide) or nonionic state, most halogens exert their antimicrobial activity primarily in their non-ionic state, but not in the halide state (e.g., chloride, iodide).These agents are highly effective disinfectants and antiseptics, because they are microbicidal and not just microbistatic. They are also sporicidal with longer exposure. For these reasons, halogens are the active ingredients in nearly one-third of all antimicrobial chemicals currently marketed. Chlorine and iodine are the only two routinely used halogens because fluorine and bromine are dangerous to handle [1].
Chlorine has been used for disinfection and antisepsis for approximately 200 years. The major forms used in microbial control are (a) liquid and gaseous chlorine and (b) hypochlorite. Treatment of water with chlorine destroys many pathogenic vegetative microorganisms without unduly affecting its taste. Chlorination at a concentration of 0.6-1.0 part of chlorine per million parts of water makes water potable and safe to use [2].
Hypochlorites are perhaps the most extensively used of all chlorine compounds. They are used for: Sanitization and disinfection of food equipment in dairies, restaurants, canneries, Treatment of swimming pools, spas, drinking water, and even fresh foods, Treatment of wounds anddisinfection of equipments, beddings, and instruments [1].
Iodine is a pungent black chemical that forms brown-colored solutions when dissolved in water or alcohol. The two primary iodine preparations are free iodine in solution and iodophors. Free iodine in solution: Aqueous iodine contains 2% free iodine in solution and 2.4% sodium iodide. It is used as a topical antiseptic before surgery and also occasionally as a treatment for burnt and infected skin. A stronger iodine solution (5% iodine and 10% potassium iodide) is used primarily as a disinfectant for plastic items, rubber instruments, cutting blades, and thermometers.
Iodophors: Iodophors are complexes of iodine and a neutral polymer, such as polyvinyl alcohol. This formulation permits the slow release of free iodine and increases its degree of penetration [2].
Alcohols
Alcohols are among the most widely used disinfectants and antiseptics. They are bactericidal and fungicidal but not sporicidal. They have no action against spores and viruses. Ethyl alcohol and isopropyl alcohol are the two most popular alcohol germicides. They are effective at a concentration of 60-70% in water. They act by denaturing bacterial proteins and possibly by dissolving membrane lipids. They are used as skin antiseptics. Isopropyl alcohol is used for disinfection of clinical thermometers. A 10-15 minute soaking is sufficient to disinfect thermometers. Methyl alcohol is effective against fungal spores [1].
Aldehydes
Formaldehyde and glutaraldehyde are the two most commonly used aldehydes that are used as disinfectants. They are highly reactive molecules that combine with nucleic and alkylating molecules. They are sporicidal and can also be used as chemical sterilants.Formaldehyde is usually dissolved in water or alcohol before use. In aqueous solution, it is bactericidal, sporicidal, and also effective against viruses. A 2% buffered solution of glutaraldehyde is an effective disinfectant. It is less irritating than formaldehyde and is used to disinfect hospital and laboratory equipments. Glutaraldehyde usually disinfects objects within time frame of 10 minutes but may require as long as 12 hours to destroy all spores [3].
Gases
Various gaseous agents are used for sterilization of large volume of heat-sensitive disposable items and also instruments. Ethylene oxide, formaldehyde gas, and betapropiolactone are frequently used gaseous agents [1].
Ethylene oxide is a colorless liquid used for gaseous sterilization. It is active against all kinds of bacteria, spores, and viruses. It kills all types of microorganisms by inhibiting proteins and nucleic acids. It is both microbicidal and sporicida [5].
The formaldehyde gas is used for (a) the fumigation of operation theaters, wards, sick rooms, and laboratories; and (b) the sterilization of instruments and heat-sensitive catheters, clothing and bedding, furniture, books, etc. The room to be sterilized is completely closed and sealed at least for 48 hours after fumigation with formalin gas. Sterilization is achieved by condensation of gas on exposed surface [5]. Beta-propiolactone (BPL) is a condensation product of ketone and formaldehyde. It is active against all microorganisms and viruses [1].
Surface active agents
Due to their amphipathic nature, detergents solubilize and are very effective cleansing agents. They are different from soaps, which are derived from fats. Surface active agents are of four types: cationic detergents, anionic surface active agents, nonionic surface active agents, amphoteric or ampholytic compounds [5].
The cationic detergents are effective disinfectants. Cationic detergents like benzalkonium chloride and cetylpyridinium chloride kill most bacteria but not M. tuberculosis, endospores, or viruses. They do have the advantages of being stable and nontoxic, but they are inactivated by hard water and soap Anionic surface active agents include soaps prepared either from saturated or unsaturated fatty acids, which act better at acidic pH. The soaps prepared from saturated fatty acids are more effective against Gram-negative organisms, whereas those prepared from unsaturated fatty acids are more active against Gram-positive bacilli and Neisseria [1]. Nonionic surface active agentsare nontoxic and some of them may even promote the growth of bacteria. Amphoteric or ampholyticcompoundsare active against a wide range of Gram-positive and Gram-negative bacteria and also against a few viruses [1].
Oxidizing agents
This group includes halogens, hydrogen peroxide, potassium-permanganate, and sodium perborate. They are good disinfectants and antiseptics but are less effective in the presence of organic matter [5].
Dyes
The dyes that have been used extensively as skin and wound antiseptics include (a) acridine dyes and (b) aniline dyes. The acridine dyes include acriflavine, euflavine, proflavine, and aminacrine. They show more activity against Gram-positive bacteria than against Gram-negative organisms. They act by interfering with the synthesis of nucleic acids and proteins in bacterial cells. Aniline dyes (such as gentian violent, crystal violet, and malachite green) are also more active against Gram-positive bacteria than against Gram-negative organisms. They are also effective against various fungi, hence are incorporated into solutions and ointments to treat fungal skin infections, such as ringworm [1].
Heavy metals
Soluble salts of mercury, silver, copper, arsenic, and other heavy metals have antibacterial activity, both bactericidal and bacteriostatic. They combine with proteins, often with their sulfhydryl groups and inactivate them. They may also precipitate cell proteins. Silver compounds are widely used as antiseptics. Silver sulfadiazine is used for burns. Silver nitrate is used as a prophylactic agent in ophthalmic neonatorum in newborn infants. Copper sulfate is an effective algaecide in lakes and swimming pools. Mercuric chloride is used as disinfectant. These compounds, however, are increasingly replaced by other less toxic and more effective germicides [1].
Acids and alkalis
Acids (such as sulfuric acid, nitric acid, hydrochloric acid, and benzoic acid) and alkalis (like potassium and sodium hydroxide and ammonium hydroxide) are germicidal in nature. They kill microorganisms by hydrolysis and altering the pH of the medium. They are rarely used as disinfectants. Organic acids are widely used in food preservation because they prevent spore germination and bacterial and fungal growth, and because they are generally regarded as safe to eat [1].
Action of disinfectants
Disinfectants act in many ways .They produce damage to the cell wall and alter permeability of the cell membrane, resulting in exposure, damage, or loss of the cellular contents. Disinfectants alter proteins and form protein salts or cause coagulation of proteins. They inhibit enzyme action and inhibit nucleic acid synthesis or alter nucleic acid molecules and cause oxidation or hydrolysis [3].
Factors influencing activity of disinfectants
Various conditions like temperature, type of microorganism, Physiological state of the cell, environment influencing the efficiency of disinfectant. Temperature: increase in temperature increases the efficiency of disinfectants. Type of microorganism affects efficiency of disinfectants; Vegetative cells are more susceptible than spores, Spores may be resistant to the action of disinfectants (Table 1). Based on physiological state of the cell: young and metabolically active cells are more sensitive than old dormant cells, Non growing cells may not be affected. Environment: The physical or chemical properties of the medium or substance influence rate as well as efficiency of disinfectants, e.g., pH of the medium and presence of extraneous materials [1].
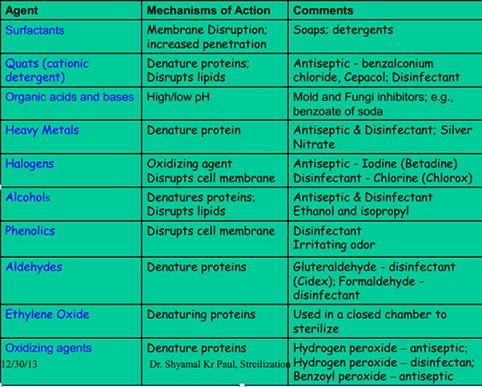 Table 1: Summary of disinfectants and their mechanism of actions.
Table 1: Summary of disinfectants and their mechanism of actions.
Source: [5].
Antibiotics
An antibiotic is a microbially produced substance (or a synthetic derivative) that has antimicrobial properties. Examples of bacteriostatic antibiotics are tetracycline, spectinomycin, sulphonamides, macrolides, chloramphenicol, and trimethoprim. Examples of bactericidal antibiotics are penicillin, cephalosporins, fluroquinolones, and monobactams. New antibiotics are still being sought today. Of the thousands isolated so far, only a small proportion has proved to be of any real therapeutic or commercial value. This is because, like the actinomycin, most of the substances isolated harm not only bacteria but humans too. A key prerequisite for any chemotherapeutic agent is selective toxicity. Obvious way of achieving this is for a compound to direct its effect against a metabolic or physiological function found in microbial cells but not in the host [2]. All antibiotics have the common property of interfering in some way with a normal, critical function of the target bacterial cell. Based on their mode of actions antibiotics are classified into four major categories. The most commonly used antibiotics exert their effect by one of the following methods: Inhibition of cell wall synthesis (group I), disruption of cell membranes (group ii), interference with protein synthesis (group iii), and interference with nucleic acid synthesis (group IV).
Inhibitors of cell wall synthesis (Group I)
The main groups which work in this way are the β-lactamantibiotics, so-called because they contain a β-lactam ring in their structure. Included among this group are the penicillin and the cephalosporin. The β-lactams also act by preventing the natural regulation of enzymes called autolysins. These enzymes function by breaking down peptidoglycan in a controlled fashion, causing breaks to allow for the addition of new peptidoglycan as the cell grows, and are normally regulated by naturally occurring inhibitors. The β-lactams neutralize the activity of these inhibitors, leading to further breakdown of the cell wall [3].
Antibiotics that disrupt cell membranes (Group II)
Polymixins are a class of antibiotic that act by disrupting the phospholipids of the cytoplasmic membrane and causing leakage of cell contents. Produced naturally by a species of Bacillus, polymixins are effective against pseudomonad infections of wounds and burns, often in combination with bacitracin and neomycin (an inhibitor of protein synthesis; see below). Their toxicity makes them unsuitable for internal use. Polyene antibiotics such as amphotericin and nystatin are antifungal agents that act on the sterol components of membranes [3].
Inhibitors of protein synthesis (Group III)
Antibiotics that act by affecting protein synthesis generally have a relatively broad spectrum of action. Members of this group are gentamicin, kanamycin, neomycin, chloramphenicol, macrolide (Erythromycin), aminoglycosides and tetracyclines. Streptomycin belongs to a group of antibiotics called aminoglycosides, which act by binding to the 30S subunit of the bacterial ribosome, preventing attachment of the 50S subunit to the initiation complex (They can thus discriminate between procaryotic (70S) and eucaryotic (80S) ribosomes, and consequently have a relatively high therapeutic index (although not as high as cell wall inhibitors). Tetracyclines also work by binding to the 30S ribosomal subunit, preventing the attachment of aminoacylt RNA, and therefore extension of the peptide chain. Erythromycin is the best known of the macrolide group of antibioticsit has a large hydrophobic molecule and is unable to gain access to most Gram-negative bacteria, thus restricting its spectrum of activity [2].
Inhibitors of nucleic acid synthesis (Group IV)
Examples of drugs acting by inhibition of nucleic acid synthesis are the quinolones, pyrimethamine, rifampin, sulfonamides, trimethoprim, and trimetrexate. Rifampin inhibits bacterial growth by binding strongly to the DNA-dependent RNA polymerase of bacteria. Thus, it inhibits bacterial RNA synthesis. Rifampin resistance results from a change in RNA polymerase because of a chromosomal mutation that occurs with high frequency. The mechanism of rifampin action on viruses is different. It blocks a late stage in the assembly of poxviruses [3].
Anti-Fungal Drugs
A limited but increasing number of antibiotics can be used to treat mycotic infections. Most have one or more limitations, such as profound side effects, a narrow antifungal spectrum, poor penetration of certain tissues, and the selection of resistant fungi. Finding suitable fungal targets is difficult because fungi, like humans, are eukaryotes. Many of the cellular and molecular processes are similar, and there is often extensive homology among the genes and proteins. Therefore, anything that damages fungal cells is likely to damage human cells too [2].
The classes of currently available drugs include the polyenes (amphotericin B and nystatin), which bind to ergosterolin the cell membrane; flu cytosine, a pyrimidine analog; the azoles and other inhibitors of ergosterol synthesis, such as the allylamines; the echinocandins, which inhibit the synthesis of cell wall β-glucan; and griseofulvin, which interferes with microtubule assembly. Currently under investigation are inhibitors of cell wall synthesis, such as nikkomycin and pradimicin, and sordarin, which inhibits elongation factor 2 [3].
Antivirals
The virus is a tough creature to kill. It has no peptidoglycan wall, no ribosomes, and no cell membrane. All it has is a protein coat, nucleic acid strand, and a few simple enzymes. The only things these critters do is replicate and then hang out in a latent state. The current antiviral agents attack steps in viral replication much like the chemotherapeutic agents attack replicating tumour cells [6].
In spite of the looming threat of resistant strains, there is no doubt that antibiotics have been hugely successful in the control of bacterial diseases. We have, however, been a lot less successful when it comes to finding a treatment for diseases caused by viruses. Most antiviral agents target nucleic acid synthesis, usually by acting as base analogues. These are molecules that are incorporated into viral nucleotides instead of the normal deoxynucleosides, disrupting synthesis because DNA polymerase is unable to act on them. The majority of viruses encode their own DNA polymerases, and the base analogues exert their effect by selectively inhibiting these, thus having little effect on that of the host cell. An example is acyclovir, which is an analogue of guanosine; it is converted to the nucleoside triphosphate by the action of thymidine kinase and then in this form acts as a competitive inhibitor of the ‘correct’ version. When the acyclovir nucleotide is incorporated into the viral DNA, there is no attachment point for the next nucleotide, so further elongation of the chain is prevented [2].
So some examples anti-viral agents are nucleoside reverse transcriptase inhibitors like zidovudine (AZT, ZDV), didanosine (ddl), zalcitabine (ddC), stavudine (d4T), lamivudine (3TC) and abacavirl. Non-nucleoside reverse transcriptase inhibitors (nevirapine, delaviridine and efavirenz); and protease inhibitors (saquinavir, indinavir, ritonavir, nelfinavir and amprenavir [6].
Resistance To Antimicrobial Drugs
Resistance to antimicrobial drugs either genetic origin of non-genetic origin of drug resistance. Active replication of bacteria is required for most antibacterial drug actions. Consequently, microorganisms that are metabolically inactive (non-multiplying) may be phenotypically resistant to drugs [3]. Most drug-resistant microbes emerge as a result of genetic change and subsequent selection processes by antimicrobial drug. There are many different mechanisms by which microorganisms might exhibit resistance to drugs [3] as discussed below.
- Microorganisms produce enzymes that destroy the active drug. Examples: Staphylococci resistant to penicillin G produce a β-lactamase that destroys the drug [1].
- Microorganisms change their permeability to the drug. Examples: Tetracyclines accumulate in susceptible bacteria but not in resistant bacteria. Resistance to polymyxins is also associated with a change in permeability to the drugs.
- Microorganisms develop an altered structural target for the drug (see also 5). Examples: Erythromycin-resistant.
- Microorganisms develop an altered metabolic pathway that bypasses the reaction inhibited by the drug. Example: Some sulfonamide-resistant bacteria do not require extracellular PABA but, similar to mammalian cells, can use preformed folic acid [2] (Figure 1).
- Microorganisms develop an altered enzyme that can still perform its metabolic function but is much less affected by the drug. Example: In trimethoprim-resistant bacteria, the dihydrofolic acid reductase is inhibited far less efficiently than in trimethoprim-susceptible bacteria [2].
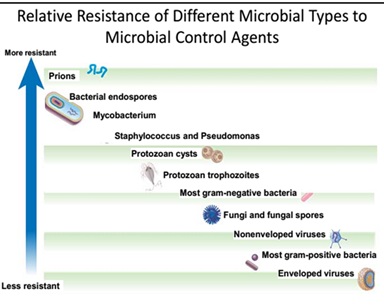
Figure 1.Relative resistance of different microbes to anti-microbial agents.
Source: [5].
Physical Methods Of Microbial Control
Temperature
Microorganisms have a minimum, an optimum, and a maximum temperature for growth. Temperatures below the minimum usually have a static action on microorganisms. They inhibit microbial growth by slowing down metabolism but do not necessarily kill the organism. Temperatures above the maximum usually have a cidal action, since they denature microbial enzymes and other proteins. Temperature is a very common and effective way of controlling microorganisms.
High temperature
Vegetative microorganisms can generally be killed at temperatures from 50°C to 70°C with moist heat. Bacterial endospores, however, are very resistant to heat and extended exposure to much higher temperature is necessary for their destruction. High temperature may be applied as either moist heat or dry heat.
Moist heat
Moist heat is generally more effective than dry heat for killing microorganisms because of its ability to penetrate microbial cells. Moist heat kills microorganisms by denaturing their proteins (causes proteins and enzymes to lose their three-dimensional functional shape). It also may melt lipids in cytoplasmic membranes.
Autoclaving
Autoclaving employs steam under pressure. Water normally boils at 100°C; however, when put under pressure, water boils at a higher temperature. During autoclaving, the materials to be sterilized are placed under 15 pounds per square inch of pressure in a pressure-cooker type of apparatus. When placed under 15 pounds of pressure, the boiling point of water is raised to 121°C, a temperature sufficient to kill bacterial endospores. The time the material is left in the autoclave varies with the nature and amount of material being sterilized. Given sufficient time (generally 15-45 minutes), autoclaving is cidal for both vegetative organisms and endospores, and is the most common method of sterilization for materials not damaged by heat.
Boiling water
Boiling water (100°C) will generally kill vegetative cells after about 10 minutes of exposure. However, certain viruses, such as the hepatitis viruses, may survive exposure to boiling water for up to 30 minutes, and endospores of certain Clostridium and Bacillus species may survive even hours of boiling.
Dry heat
Dry heat kills microorganisms through a process of protein oxidation rather than protein Coagulation. Examples of dry heat include.
Hot air sterilization
Microbiological ovens employ very high dry temperatures: 171°C for 1 hour; 160°C for 2 hours or longer; or 121°C for 16 hours or longer depending on the volume. They are generally used only for sterilizing glassware, metal instruments, and other inert materials like oils and powders that are not damaged by excessive temperature.
Incineration
Incinerators are used to destroy disposable or expendable materials by burning. We also sterilize our inoculating loops by incineration.
Pasteurization
Pasteurization is the mild heating of milk and other materials to kill particular spoilage organisms or pathogens. It does not, however, kill all organisms. Milk is usually pasteurized by heating to 71.6°C for at least 15 seconds in the flash method or 62.9°C for 30 minutes in the holding method.
Low temperature
Low temperature inhibits microbial growth by slowing down microbial metabolism. Examples include refrigeration and freezing. Refrigeration at 5°C slows the growth of microorganisms and keeps food fresh for a few days. Freezing at -10°C stops microbial growth, but generally does not kill microorganisms, and keeps food fresh for several months.
Desiccation
Desiccation, or drying, generally has a static effect on microorganisms. Lack of water inhibits the action of microbial enzymes. Dehydrated and freeze-dried foods, for example, do not require refrigeration because the absence of water inhibits microbial growth.
Osmotic pressure
Microorganisms, in their natural environments, are constantly faced with alterations in osmotic pressure. Water tends to flow through semipermeable membranes, such as the cytoplasmic membrane of microorganisms, towards the side with a higher concentration of dissolved materials (solute). In other words, water moves from greater water (lower solute) concentration to lesser water (greater solute) concentration. When the concentration of dissolved materials or solute is higher inside the cell than it is outside, the cell is said to be in a hypotonic environment and water will flow into the cell. The rigid cell walls of bacteria and fungi, however, prevent bursting or plasmoptysis. If the concentration of solute is the same both inside and outside the cell, the cell is said to be in an isotonic environment. Water flows equally in and out of the cell. Hypotonic and isotonic environments are not usually harmful to microorganisms. However, if the concentration of dissolved materials or solute is higher outside of the cell than inside, then the cell is in a hypertonic environment. Under this condition, water flows out of the cell, resulting in shrinkage of the cytoplasmic membrane or plasmolysis. Under such conditions, the cell becomes dehydrated and its growth is inhibited. The canning of jams or preserves with a high sugar concentration inhibits bacterial growth through hypertonicity. The same effect is obtained by salt-curing meats or placing foods in a salt brine. This static action of osmotic pressure thus prevents bacterial decomposition of the food. Molds, on the other hand, are more tolerant of hypertonicity. Foods, such as those mentioned above, tend to become overgrown with molds unless they are first sealed to exclude oxygen (Molds are aerobic.).
Radiation
Ultraviolet radiation
The ultraviolet portion of the light spectrum includes all radiations with wavelengths from 100 nm to 400 nm. It has low wave-length and low energy. The microbicidal activity of ultraviolet (UV) light depends on the length of exposure: the longer the exposure the greater the cidal activity (Figure2). It also depends on the wavelength of UV used. The most cidal wavelengths of UV light lie in the 260 nm - 270 nm range where it is absorbed by nucleic acid. In terms of its mode of action, UV light is absorbed by microbial DNA and causes adjacent thymine bases on the same DNA strand to covalently bond together, forming what are called thymine-thymine dimers.
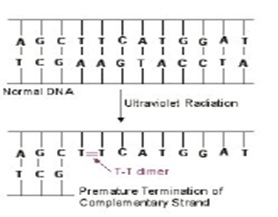 Figure 2: DNA: UV radiation, UT-T dimer.
Figure 2: DNA: UV radiation, UT-T dimer.
As the DNA replicates, nucleotides do not complementary base pair with the thymine dimers and this terminates the replication of that DNA strand. However, most of the damage from UV radiation actually comes from the cell trying to repair the damage to the DNA by a process called SOS repair. In very heavily damaged DNA containing large numbers of thymine dimers, a process called SOS repair is activated as kind of a last ditch effort to repair the DNA. In this process, a gene product of the SOS system binds to DNA polymerase allowing it to synthesize new DNA across the damaged DNA. However, this altered DNA polymerase loses its proofreading ability resulting in the synthesis of DNA that its self now contains many misincorporated bases. In other words, UV radiation causes mutation and can lead to faulty protein synthesis. With sufficient mutation, bacterial metabolism is blocked and the organism dies. Agents such as UV radiation that cause high rates of mutation are called mutagens.
The effect of this inproper base pairing may be reversed to some extent by exposing the bacteria to strong visible light immediately after exposure to the UV light. The visible light activates an enzyme that breaks the bond that joins the thymine bases, thus enabling correct complementary base pairing to again take place. This process is called photo reactivation. UV lights are frequently used to reduce the microbial populations in hospital operating rooms and sinks, aseptic filling rooms of pharmaceutical companies, in microbiological hoods, and in the processing equipment used by the food and dairy industries. An important consideration when using UV light is that it has very poor penetrating power. Only microorganisms on the surface of a material that are exposed directly to the radiation are susceptible to destruction. UV light can also damage the eyes, cause burns, and cause mutation in cells of the skin.
Ionizing radiation
Ionizing radiation, such as X-rays and gamma rays, has much more energy and penetrating Power than ultraviolet radiation. It ionizes water and other molecules to form radicals (molecular fragments with unpaired electrons) that can disrupt DNA molecules and proteins. It is often used to sterilize pharmaceuticals and disposable medical supplies such as syringes, surgical gloves, catheters, sutures, and petridish plates. It can also be used to retard spoilage in sea foods, meats, poultry, and fruits.
Filtration
Microbiological membrane filters provide a useful way of sterilizing materials such as vaccines, antibiotic solutions, animal sera, enzyme solutions, vitamin solutions, and other solutions that may be damaged or denatured by high temperatures or chemical agents. The filters contain pores small enough to prevent the passage of microbes but large enough to allow the organism-free fluid to pass through. The liquid is then collected in a sterile flask.
 Figure 3: Filtration.
Figure 3: Filtration.
Filters with a pore diameter from 25 nm to 0.45 µm are usually used in this procedure. Filters can also be used to remove microorganisms from water and air for microbiological testing.
References
- Parija SC (2012) Text Book of Microbiology and Immunology 2nd 1-682.
- Hogg S (2005) Essential Microbiology (Wily AndSon,Ltd.) Chichester 1-481.
- Books GF, Caroll KC, Butel JS, Morse SA, Mietzner TA (2013) Medical microbiology, 26th McGraw Hill Companies,Inc. 877.
- Sundararaj T, Athoniraj S, Kannan N, Muthukaruppan SM (2004) MICROBIOLOGY: Text Book Corporation 1-248.
- Paul SK (2013) Physical and Chemical Agent for Microbial Control. 1-64.
- Gladwin M, Trattler B (2001) Clinical microbiology made the ridiculously simple 3rded. 1-281.
- https://books.google.co.in/books/about/Microbiological_Applications.html?id=sQy5DWGTQZ4C.
Citation: Choramo A (2022) A Review on Chemical and Physical Methods of Controlling Microbial Growth. J Community Med Public Health Care 9: 107.
Copyright: © 2022 Alemayehu Choramo, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

