
A Review on the Metal Complex of Nickel (Ii) Salicylhydroxamic Acid and its Aniline Adduct
*Corresponding Author(s):
Aderonke AVDepartment Of Chemistry, Faculty Of Science, University Of Abuja, Nigeria
Tel:+234 8069744094,
Email:victoriaaderonke@gmail.com
Abstract
Metal complexes are fundamentally known to be engendered in biological systems from reaction between a ligand and a metal ion in dynamic equilibrium. This review aims at examining the metal complexes of Nickel (II) salicylhydroxamic acid and its aniline adduct. It is imperative to reiterate that Nickel which is a chemical element with the symbol Ni, and atomic number 28, silvery-white lustrous metal with a slight golden tinge, is the only material magnetic in nature, and as such its combination with other distinct molecules such as aniline produces unique results. As such, this review focuses extensively on studies that revolve around nickel its structure importance, aniline and its derivatives, and the metal complex of both materials. In fact, the potential for nickel and its adduct to serve as an alternative antibiotic therapy as suggested in previous studies was also highlighted as the increasing development of drug resistance, as well as the appearance of undesirable side effects of certain antibiotics has led to the search for new antimicrobial agents with the goal to discover new chemical structures, which overcome the above disadvantages. In conclusion, review of the synthesis, spectroscopy and antimicrobial activity of Nickel (II) Salicylhydroxamic acid, and its aniline adduct unveils the possibility of developing new drugs to improve upon the current resistance-prone antimicrobial therapeutic agents available.
Keywords
Aniline; Adduct; Antimicrobial; Metal complex; Nickel, Salicylhydroxamic acid
INTRODUCTION
Metal complexes are formed in biological systems particularly between a ligand and a metal ion are in dynamic equilibrium with the free metal ion in a more or less aqueous environment [1]. All biologically important metal ions can form complexes and the number of different chemical species which can be coordinated with these metal ions is very large. During the past few decades, a lot of scientist research groups operated through specialization in the direction of drug discovery, by studying the simplest species that use metal ions and researching them as whole compound; for example, they suggested the addition of metal ion to antibiotics to facilitate their spread throughout the body [2]. The development of drug resistance as well as the appearance of undesirable side effects of certain antibiotics has led to the search of new antimicrobial agents with the goal to discover new chemical structures which overcome the above disadvantages. As a ligand with potential oxygen and nitrogen donors, hydroxamic acids are interesting and have gained special attention not only because of the structural chemistry of their coordination modes, but also because of their importance in medical chemistry [3].
The ability of hydroxamic acids to form stable transition metal complexes is the basis of their usefulness as analytical reagent for the sensitive qualitative and quantitative determinations. In addition to the trace elements Mn, Fe, Co, V and Cr, which are required for many forms of life, there are other elements such as nickel whose biological role is not yet clear. It is well known that reactions involving C=O group present a large fraction of chemical and even larger fraction of biological reactions, and since hydroxamic acids are readily associated with both carboxylic acids and hydroxylamine, both structurally and synthetically, thus they used as drugs and they are reported to possess a wide range of biological activities against bacteria, fungi and certain types of tumors [4].
Salicylhydroxamic acid is also known as N, 2-Dihydroxybenzamide, 2- Hydroxybenzenecarbohydroxamic acid and SHAM. SHAM is a brownish crystalline powder with the chemical formula C7H7NO3, molar mass153.14 g·mol−1, and melting point of 175 to 178 °C (347 to 352 °F; 448 to 451 K [5].
This present review focuses on the synthesis, spectroscopy and antimicrobial studies of nickel (II) salicylhydroxamic acid and its aniline adduct in order to orient future investigations towards the finding of new potent and safe antimicrobial drugs.
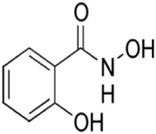 Figure 1: Chemical Structure of Salicylhydroxamic Acid Hydroxamic Acid
Figure 1: Chemical Structure of Salicylhydroxamic Acid Hydroxamic Acid
Hydroxamic acid refers to a class of organic compounds bearing the functional group RC(O)N(OH)R', with R and R' as organic residues and CO as a carbonyl group (Figure 1). They are amides (RC (O) NHR’) wherein the NH center has an OH substitution. They are often used as metal chelators. Hydroxamic acids are usually prepared from either esters or acid chlorides by a reaction with hydroxylamine salts. They were introduced into chemistry when reported that the reaction between diethyl oxalate and hydroxylamine yielded an acidic compound, which he named oxalohydroxamic acid [6]. For the synthesis of benzohydroxamic acid, the overall equations is:
 C6H5CO2Me + NH2OH → C6H5C (O) NHOH + MeOH
C6H5CO2Me + NH2OH → C6H5C (O) NHOH + MeOH
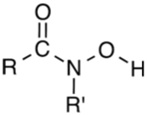
Figure 2: Structure of Hydroxamic Acids
Hydoxamic acids have been classified as primary, secondary and cyclic which has replaced the oxygen atom by sulphur atom to get thio hydroxamic acid (Figure 2). A well-known hydroxamic acid reaction is the Lossen rearrangement. In the area of coordination chemistry, hydroxamic acids are excellent ligands.They deprotonate to give hydroxamates, which bind to metals ions as bidentate ligands. So high is the affinity of hydroxamates for ferric ions that nature has evolved families of hydroxamic acids to function as iron-binding compounds (siderophores) in bacteria. They dissolve insoluble iron (III) compounds. The resulting complexes are transported into the cell, where the iron is extracted and utilized metabolically. Ligands derived from hydroxamic acid and thiohydroxamic acid also form strong complexes with Ni (II) [7].
Hydroxamic acids are used extensively in flotation of rare earth minerals during the concentration and extraction of ores to be subjected to further processing. Some hydroxamic acids (vorinostat, belinostat, panobinostat, and trichostatin A) are HDAC inhibitors with anticancer properties. Fosmidomycin is a natural hydroxamic acid inhibitor of 1-deoxy-D-xylulose5-phosphate reductoisomerase (DXP reducto-isomerase). Hydroxamic acids have also been investigated for reprocessing of irradiated fuel [8].
PHYSICAL PROPERTIES OF HYDROXAMIC ACIDS
Generally, hydroxamic acids are white solids, soluble in chloroform carbon tetrachloride and some other organic solvents, but sparingly soluble in water .They are weakly acidic compounds which form metal complexes by chelation of metal ion between the two oxygen atoms of the mono-anion to give a five membered ring [9].
Previous Studies to determine the ionization constants (pKa) of ten N-aryl hydroxamic acids in aqueous media, revealed that at 25°C the pka values are in the range of 8.08-8.59, where at 35C° the pka values range from8.01- 8.56. Hydroxamic acids are stronger than phenol (pka=9.89), since they are differ widely in their structure and basicity. The acidity of hydroxamic acids may be attributed mainly to the -OH group and its suppression to intra molecular hydrogen bonding. Cyclic hydroxamic acids in the solid state are capable of intra-molecular and intermolecular (IX) hydrogen-bonding involving the N-hydroxyl and carbonyl groups. Couts and Elizabeth observed that the position and intensity of the carbonyl and the hydroxyl infra-red absorption bonds vary considerably from one hydroxamic acid to another. They found that an intra molecularly hydrogen-bonded carbonyl group absorbs infrared radiation at a lower frequency than the corresponding inter-molecularly hydrogen bonded carbonyl group. In addition, when hydrogen bonding is intra-molecular, the–OH stretching band is usually broad, where as a sharp hydroxyl absorption band is generally obtained when hydrogen bonding is intermolecular [10].
STABILITY OF HYDROXYAMIC ACIDS
The properties of hydroxamic acids strongly depend on the mode of ionization. N-unsubstituted hydroxamic acids have two protons that can be ionized to form hydroxamate ions. The stability of hydroxamate ions has been the topic of many recent scientific investigstions, as it is fundamental to the understanding of their properties. Experimental and theoretical studies in gas phase suggest that hydroxamic acids ionize via N-H bond dissociation, but experimental evidence shows that hydroxamic acids ionize via O-H bond dissociation in solution which is supported by a molecular dynamics simulation [11,12].
HYDROXAMIC ACIDS IN NATURE
The hydroxamic acid bond occurs in products from fungi, yeast, bacteria and plants. The –CO N(OH)bond arises by oxidation of a free or bound amino group in a unit structure which is often closely related to conventional amino acids. Ferrichrome was the first siderphore to be isolated and characterized from the fungi Ustilago sphaerogena in 1952 [13]. The chemistry of ferrichrome type compounds, which are ferric tri hydroxamate containing peptides, has also been worked out in detail and includes a complete crystallographic analysis of the ferrichrome A molecule. Coprogen is a linear tri hydroxamate produced by Penicillium species and Neurospora crassa and was first isolated and characterized by Hesseltine and coworkers. Fusarinines A and B are two naturally occurring hydroxamic acids produced by Fusarium roseum and have been isolated with their structures determined. Several hydroxamic acids in addition fusarinine are found in the culture fluid of fusaria. These compounds represent a new class of naturally occurring hydroxamic acids in which the hydroxamate subunits are joined by ester linkage rather than the usual peptide bonds. The compounds are amorphous hydroscopic and yellow or light brown [14].
SYNTHESIS OF HYDROXYAMIC ACIDS
Several methods for the synthesis of hydroxamic acids have been described, some of the common methods include The coupling of N-aryl or alkyl hydroxyl amine with acid chloride, reactions of carbonyl group with nitroso compounds, reaction of formaldehyde with substituted nitroso benzenes, reaction of pyruvic acid or acetaldehyde with substituted nitroso benzenes, deprotection of O-benzyl hydroxamates, hydroxyamination of esters, reaction of 2-
Nitro so-2-Methyl propane with formaldehyde, glyoxylate and glyoxylic acid, and the Angeli- Rimini reaction [15,16].
IDENTIFICATION OF HYDROXAMIC ACIDS
After the synthesis of a hydroxamic acid by any of the methods out lined above, it can be identified by its melting point and Infra-red spectroscopy (IR). The infra-red spectra of hydroxamic acids show characteristic bands associated with the hydroxamic acid functional grouping due to (OH),
(C=O) and (N-O) stretching vibration. These frequencies are generally assigned in the region of 3200cm-1, 1600 cm-1 and 910cm-1, respectively. Also, the characteristic color reaction is essential. Hydroxamic acids in chloroform with a solution of ferric chloride in acidic medium gives red-blood colour in the Chloroform layer (Efremov et al., 2012). Also hydroxamic acids in chloroform with a solution of vanadium (V) inacidic medium give dee p violet color in thechloroform layer characteristic of hydroxamic acid. Elemental analysis has also been used for identification of hydroxamic acid.
Nuclear magnetic resonance spectroscopy (NMR) and determination of the nitrogen content can also be used to identify hydroxamic acid. There are a large number of structural parameters for NMR of different nuclei and many examples of how they can be applied to the analysis of hydroxamic acids. Hydroxamic acids are made up of hydrogen, carbon, nitrogen and oxygen nuclei (some of their derivatives may also contain, fluorine, phosphorus and silicon). Thus spectra from hydrogen, deuterium, tritium as well as carbon-13, nitrogen-14 and nitrogen-15, oxygen-17 and other nuclei may be observed [17].
ANALYTICAL APPLICATIONS OF HYDROXAMIC ACIDS
The ability of hydroxamic acids to form stable transition metal complexes is the basis of their usefulness as an analytical regent. This is the reason why hydroxamic acids have been used as chelating agents for determination of metals in samples of environmental and industrial importance. The colored and stable solutions of these metal complexes often provide spectrophotometric methods for determination of several metal ions. Hydroxamic acids having the bi-dentate functional grouping fulfill the basic requirements of complex formation with metal ions and therefore form an important family of chelating agents. The complex formation usually takes place with the replacement of the hydroxylamine hydrogen by the metal ion and ring closure through the carbon oxygen.
Hydroxamic acids are used extensively in floatation of rare earth minerals during the concentration and extraction of ores to be subjected to further processing. Hydroxamic also find applications as functionalizing agents for carbon nano tubes and polymer bound chelating agents. Hydroxamic acids also used in the formation of chelating ion exchange resin that gives the best sorption characteristics towards metal ions. All hydroxamic acids in acids solutions, react with ferric chloride to give rust brown complex salts. These colored complexes form the basis for the sensitive qualitative and quantitative determination of carboxylic acids and their derivatives too. A few hydroxamic acids have been found to serve as suitable indicators such as complex metric titration of ferric ion with EDTA [18].
Hydroxamic acids having a phenyl group attached to the side chain have been found to be most useful reagents for gravimetric estimation of metals copper cobalt, nickel, manganese (II), titanium (IV), iron (III), and vanadium (V). Using paper impregnated with a solution of Nphenylbenzohydroxamic acid in 2-octanonc a number of selective separations by paper chromatographic technique can be achieved. N-phenyl benzohydroxamic acids have been used in polarographic determination of tin and antimony.
PHYSIOLOGICAL EFFECTS OF HYDROXAMIC ACIDS
In the past most metabolic reactions on exogenous materials were considered to be detoxification reactions. However, during investigations on adverse effects of aromatic amines and related materials, it was discovered in the last 15 years that certain of these chemicals have pronounced effects on the hematopoietic system, with pharmacological or pathological activities ascribed to a novel toxication reaction. This reaction entails substituting one hydrogen on the amine nitrogen with a hydroxyl group that is N-hydroxylation. Hydroxamic acids also demonstrated cellular mutagenic and antibacterial activities. These activities appear to be dependent on the hydroxamic acid function and are probably due to the interaction with deoxyribonucleic acid. Hydroxamates are essential growth factors, or vitamins, for some microbes. They function as iron binding compounds (siderphores) that solubilise iron and transport it into the cell. Iron is a key component of cytochromes and iron-sulphur proteins (involved in electron transport) and is thus important in cellular respiration.
Simple stable molecules containing the hydroxamic acid functionality have been shown to inhibit 5-Lipoxygenase that block the biosynthesis of leukotriene in vivo. Pulmonary fibrosis represents a fatal stage of interstitial lung diseases. Histone deacetylases inhibitor suberoylanilidehydroxamic acid has anti-fibrotic and anti-inflammatory potential and is an approved anti-cancer drug [19].
PHARMACOLOGICAL POTENTIALS OF SALICYLHYDROXAMIC ACID
Salicylhydroxamic acid is a drug that is a potent and irreversible inhibitor of bacterial and plant urease usually used for urinary tract infections. The molecule is similar to urea but is not hydrolyzable by the urease enzyme. The chemistry and biochemistry of hydroxamic acid and their derivatives have attracted considerable attention, due to their pharmacological, toxicological and pathological properties. Salicylhydroxamic acids generally have low toxicity and have wide spectrum activities in all types of biological systems. As such, they act variously as growth factors, food additives, growth inhibitors, antimicrobial agents, antituberculous, anti-leukemic agents, key pharmacophore, in many important chemotherapeutic agents, pigments and cell division factor. Several of them have been advanced into human clinical trials as pharmaceutical drugs for the treatment of several diseases.
Salicylhydroxamic acid is also a trypanocidal agent. When administered orally, it is metabolized to salicylamide which exerts analgesic, antipyretic and anti-inflammatory effects. Salicylhydroxamic acid is also a common ligand utilized in the synthesis of metal a crowns [20].
In plants, some fungi and some protists with the alternative oxidase (AOX) enzyme in the mitochondrial electron transport chain system, salicylhdroxamic acid acts as an inhibitor of the enzyme, blocking the largely uninhibited flow of electrons through AOX. AOX acts as a "short circuit" of the normal electron chain, dissipating electrons with a much-decreased translocation of protons, and therefore diminished production of ATP by oxidative phosphorylation. When AOX is blocked by SHAM, electrons are forced through the cytochrome pathway and through cytochrome IV, allowing observation of the operation of the cytochrome pathway without AOX activity. The AOX pathway is found to be the exclusive electron transport pathway in Trypanosoma brucei, the organism that causes African sleeping sickness, meaning that SHAM completely shuts down oxygen consumption by this organism [21].
Salicylhydroxamic acid (SHAM) is a drug that is a potent and irreversible inhibitor of bacterial and plant urease usually used for urinary tract infections. It is also trypanocidal agent, when administered orally, it is metabolized to salicyamide which exerts analgesic, antipyretic and antiinflammatory effects. In plants, some fungi and some protists with the alternative oxidase (AOX) enzyme in the mitochondrial electro transport chain system, salicylhydroxamic acid acts as an inhibitor of the enzyme, blocking largely uninhibited flow of electrons through AOX, when AOX is blocked by SHAM, electrons are forced through the cytochrome pathway and through cytochrome iv, allowing observation of the operation of the cytochrome pathway without AOX activity. Aceto-hydroxamic acid (AHA) is a drug that is a potent and irreversible inhibitor of bacterial and plant urease usually used for urinary tract infections. Natural hydroxamic acids and related compounds derived from 1,4-benoxazin-3-one structures inhibited the growth of the alga Chlorella xanthell and the fungus Candida albicans, on the basis of structure-activity relationships. The lipophilic character of the substituent in the aromatic ring and the electrophilic character of the hydroxamic function are suggested to be responsible for anti-algal and anti- fungal activity, respectively. Hydroxamic acids of the type 4-hydroxy-1,4- benzoxazin-3ones constitute one of the most extensively studied secondary metabolites in relation to host plant resistance to pests and diseases. They play a major role in the defense of cereals against insects, fungi and bacteria [22].
A variety of hydroxamic acid derivatives have recently been touted for their potential use as inhibitors of hypertension, tumor growth, inflammation infection agents, asthma, and arthritis. In addition, hydroxamic acid derivatives have been examined as inhibitors of zinc metalloproteases, matrix metalloproteinases, leukotriene A4, hydrolases, ureases, lipoxigenases, cyclooxygenases, as well as peptide deformilases. Lipoxygenase plays an essential role in the biosynthesis of leukotrienes which have been implicated as mediators in the pathophysiology of inflammatory diseases.
NICKEL (II)
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile. Pure nickel, powdered to maximize the reactive surface area, shows a significant chemical activity, but larger pieces are slow to react with air under standard conditions because an oxide layer forms on the surface and prevents further corrosion (passivation). Even so, pure native nickel is found in Earth's crust only in tiny amounts, usually in ultramafic rocks, and in the interiors of larger nickel–iron meteorites that were not exposed to oxygen when outside Earth's atmosphere. Meteoric nickel is found in combination with iron, a reflection of the origin of those elements as major end products of supernova nucleo-synthesis [23]. An iron–nickel mixture is thought to compose Earth's inner core. Use of nickel (as a natural meteoric nickel–iron alloy) has been traced as far back as 3500 BCE. Nickel was first isolated and classified as a chemical element in 1751 by Axel Fredrik Cronstedt, who initially mistook the ore for a copper mineral. The element's name comes from a mischievous sprite of German miner mythology, Nickel (similar to Old Nick), that personified the fact that copper-nickel ores resisted refinement into copper. An economically important source of nickel is the iron ore limonite, which often contains 1–2% nickel [24].
Nickel is slowly oxidized by air at room temperature and is considered corrosion-resistant. Historically, it has been used for plating iron and brass, coating chemistry equipment, and manufacturing certain alloys that retain a high silvery polish, such as German silver. Nickel is one of four elements (iron, cobalt, nickel, and gadolinium) that are ferromagnetic at approximately room temperature. Alnico permanent magnets based partly on nickel are of intermediate strength between iron-based permanent magnets and rare-earth magnets. The metal is valuable in modern times chiefly in alloys; about 60% of world production is used in nickel steels (particularly stainless steel). Other common alloys and some new super alloys comprise most of the remainder of world nickel use, with chemical uses for nickel compounds consuming less than 3% of production. As a compound, nickel has a number of niche chemical manufacturing uses, such as a catalyst for hydrogenation. Nickel is an essential nutrient for some microorganisms and plants that have enzymes with nickel as an active site [25].
Nickel (II) forms compounds with all common anions, including sulfide, sulfate, carbonate, hydroxide, carboxylates, and halides. Nickel (II) sulfate is produced in large quantities by dissolving nickel metal or oxides in sulfuric acid, forming both a hexa- and heptahydrates useful for electroplating nickel. Common salts of nickel, such as the chloride, nitrate, and sulfate, dissolve in water to give green solutions of the metal aquo complex [Ni(H2O)6]2+. The four halides form nickel compounds, which are solids with molecules that feature octahedral Ni centres. Nickel (II) chloride is most common, and its behavior is illustrative of the other halides. Nickel (II) chloride is produced by dissolving nickel or its oxide in hydrochloric acid [26]. It is usually encountered as the green hexahydrate, the formula of which is usually written NiCl2•6H2O. When dissolved in water, this salt forms the metal aquo complex [Ni(H2O)6]2+. Dehydration of NiCl2•6H2O gives the yellow anhydrous NiCl2. Some tetracoordinate nickel (II) complexes, such as bis (triphenylphosphine) nickel chloride, exist both in tetrahedral and square planar geometries. The tetrahedral complexes are paramagnetic, whereas the square planar complexes are diamagnetic. In having properties of magnetic equilibrium and formation of octahedral complexes, they contrast with the divalent complexes of the heavier group 10 metals, palladium(II) and platinum(II), which form only square-planar geometry [27].
Most transition metals can be bound to a variety of ligands, allowing for a wide variety of transition metal complexes. The general electronic configuration of the d-block elements is [Inert gas] (n −1) d1-10n s0-2. The period 6 and 7 transition metals also add (n − 2) f 0–14 electrons. As implied by the name, all transition metals are metals and thus conductors of electricity. In general, transition metals possess a high density and high melting points and boiling points. These properties are due to metallic bonding by delocalized d electrons, leading to cohesion which increases with the number of shared electrons. However the group 12 metals have much lower melting and boiling points since their full d sub shells prevent d–d bonding, which again tends to differentiate them from the accepted transition metals. Mercury has a melting point of −38.83 °C (−37.89 °F) and is a liquid at room temperature [28].
ATOMIC AND PHYSICAL PROPERTIES OF NICKEL (II)
Nickel is a silvery-white metal with a slight golden tinge that takes a high polish. It is one of only four elements that are magnetic at or near room temperature, the others being iron, cobalt and gadolinium. Its Curie temperature is 355 °C (671 °F), meaning that bulk nickel is non-magnetic above this temperature. The unit cell of nickel is a face-centered cube with the lattice parameter of 0.352 nm, giving an atomic radius of 0.124 nm. This crystal structure is stable to pressures of at least 70 GPa. Nickel belongs to the transition metals and is hard and ductile. It exists in the solidphase, with a melting point of 1728 K (1455 °C, 2651 °F), boiling point of 3003 K (2730 °C, 4946 °F),and density near r.t. 8.908 g/cm3.
ELECTRONIC CONFIGURATION OF NICKEL
The nickel atom has two electron configurations, [Ar] 3d8 4s2 and [Ar] 3d9 4s1, which are very close in energy – the symbol [Ar] refers to the argon-like core structure. There is some disagreement on which configuration has the lowest energy. Chemistry textbooks quote the electron configuration of nickel as [Ar] 4s2 3d8, which can also be written [Ar] 3d8 4s2. This configuration agrees with the Madelung energy ordering rule, which predicts that 4s is filled before 3d. It is supported by the experimental fact that the lowest energy state of the nickel atom is a 3d8 4s2 energy level, specifically the 3d8(3F) 4s23F, J = 4 level [29].
BIOLOGICAL ROLE OF NICKEL (II)
Although not recognized until the 1970s, nickel is known to play an important role in the biology of some plants, eubacteria, archae-bacteria, and fungi. Nickel enzymes such as urease are considered virulence factors in some organisms. Urease catalyzes the hydrolysis of urea to form ammonia and carbamate. The [NiFe]-hydrogenases can catalyze the oxidation of H2 to form protons and electrons, and can also catalyze the reverse reaction, the reduction of protons to form hydrogen gas [30]. A nickel-tetrapyrrole coenzyme, cofactor F430, is present in methyl coenzyme M reductase, which can catalyze the formation of methane, or the reverse reaction, in methanogenic archaea. One of the carbon monoxide dehydrogenase enzymes consists of an FeNiS cluster. Other nickel-bearing enzymes include a rare bacterial class of superoxide dismutase and glyoxalase I enzymes in bacteria and several parasitic eukaryotic trypanosomal parasites (in higher organisms, including yeast and mammals, this enzyme contains divalent Zn2+). Nickel is implicated in the catalytic formation of the hard calcium carbonate plates of the spiny tests on larval sea urchins. Nickel can affect human health through infections by nickel- dependent bacteria. Nickel released from Siberian traps volcanic eruptions (site of the modern city of Norilsk) is suspected of assisting the growth of Methanosarcina, a genus of euryarchaeote archaea that produced methane during the biggest extinction event on record.
ANILINE
Aniline also known as benzenamine, phenylamine, aminobenzene and benzamine, is an organic compound with the formula C6H5NH2. Aniline is a colorless to yellow liquid, with molar mass of 93.13 g·mol−1, 1.0217 g/mL density and a melting point of −6.3°C (20.7 °F; 266.8 K). It consists of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Its main use is in the manufacture of precursors to polyurethane and other industrial chemicals. Like most volatile amines, it possesses the odour of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds. It undergoes oxidation, alkylation, N-acetylation, nitrogenation and hydrogenation [31].
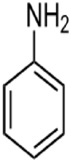 Figure 3: Chemical Structure of Aniline
Figure 3: Chemical Structure of Aniline
Aniline is a planar molecule. The amine is nearly planar owing to conjugation of the lone pair with the aryl substituent. (Figure 3)The C-N distance is correspondingly shorter. In aniline, the C-N and CC distances are close to 1.39 Å, indicating the π-bonding between N and C. Industrial aniline production involves two steps. First, benzene is nitrated with a concentrated mixture of nitric acid and sulfuric acid at 50 to 60°C to yield nitrobenzene. The nitrobenzene is then hydrogenated (typically at 200–300°C) in the presence of metal catalysts [32].
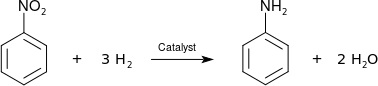 Figure 4: Zinin Reaction
Figure 4: Zinin Reaction
The reduction of nitrobenzene to aniline was first performed by Nikolay Zinin in 1842 using inorganic sulfide as a reductant (Zinin reaction) (Figure 4).
Aniline can alternatively be prepared from ammonia and phenol derived from the cumene process. In commerce, three brands of aniline are distinguished: aniline oil for blue, which is pure aniline; aniline oil for red, a mixture of equimolecular quantities of aniline and ortho- and para-toluidines; and aniline oil for safranine, which contains aniline and ortho-toluidine, and is obtained from the distillate (échappés) of the fuchsine fusion [33]. The product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components is referred to as adduct (from the Latin adductus, "drawn toward" alternatively, a contraction of "addition product"). The resultant is considered a distinct molecular species. Examples include the adduct between hydrogen peroxide and sodium carbonate to give sodium percarbonate, and the addition of sodium bisulfite to an aldehyde to give a sulfonate [34]. It can just be considered as a single product resulting from direct addition of different molecules and constitutes all the reactant molecules' atoms. Compounds or mixtures that cannot form adducts because of steric hindrance are called frustrated Lewis pairs [35]. Adducts are not necessarily molecular in nature. A good example from solid-state chemistry is adducts of ethylene or carbon monoxide of CuAlCl4. The latter is a solid with an extended lattice structure. Upon formation of adduct, a new extended phase is formed in which the gas molecules are incorporated (inserted) as ligands of the copper atoms within the structure. This reaction can also be considered a reaction between a base and a Lewis acid with the copper atom in the electron receiving role and the pi electrons of the gas molecule in the electron-donating role [36].
USES OF ANILINE
Aniline derivatives such as phenylenediamines and diphenylamine, are antioxidants. Illustrative of the drugs prepared from aniline is paracetamol (acetaminophen, Tylenol). The largest application of aniline is for the preparation of methylene dianiline and related compounds by condensation with formaldehyde. The diamines are condensed with phosgene to give methylene diphenyldiisocyanate, a precursor to urethane polymers. Other uses include rubber processing chemicals (9%), herbicides (2%), and dyes and pigments (2%).As additives to rubber.
SOLUBILITY STUDIES
Solubility refers to the property of solid, liquid and gaseous chemical called solute to dissolve in a liquid solvent to form a homogenous solution. The solubility of a compound depends on the used solvent as well as on the temperature and pressure. It is usually expressed as a concentration by mass. Whether or not a solute dissolves in a solvent depends on the balance between the change in the disorder or randomness which occurs during the process and on the bonding interaction which are broken during the dissolving process. However, the usual tendency to heat a solution in order to dissolve more solute works for some solutes but all gases usually become less solute as the temperature of the solution increase and so there is no general rule for the temperature dependence of the solubility of all gases in any solvent as the partial pressure of the gas above solution increase unlike gases, liquid and solid exhibit no change of solubility with change in the pressure. SHA shows great solubilty in ethanol and acetone solution, slightly soluble in chlorofoam and distilled water. Also, Nickel SHA is soluble and insoluble in hexane. Similarly, Nickel SHA adduct showed great affinity for ethanol, acetone and insoluble in chlorofoam, hexane and distilled water, looking at these three compound one can conclude that ethanol (polar solvent) is a common solvent for both the metal complex and aniline adduct.
SPECTROSCOPY
Spectroscopy is the study of the interaction between matter and electromagnetic radiation. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, by a prism. Later the concept was expanded greatly to include any interaction with radioactive energy as a function of its wavelength or frequency [37]. Spectroscopic data is often represented by an emission spectrum, a plot of the response of interest as a function of wavelength or frequency [38].
Daily observations of color can be related to spectroscopy. Neon lighting is a direct application of atomic spectroscopy. Neon and other noble gases have characteristic emission frequencies (colors). Neon lamps use collision of electrons with the gas to excite these emissions. Inks, dyes and paints include chemical compounds selected for their spectral characteristics in order to generate specific colors and hues. A commonly encountered molecular spectrum is that of nitrogen dioxide. Gaseous nitrogen dioxide has a characteristic red absorption feature, and this gives air polluted with nitrogen dioxide a reddish-brown color. Rayleigh scattering is a spectroscopic scattering phenomenon that accounts for the color of the sky.
Spectroscopic studies were central to the development of quantum mechanics and included Max Planck's explanation of blackbody radiation, Albert Einstein's explanation of the photoelectric effect and Niels Bohr's explanation of atomic structure and spectra. Spectroscopy is in physical and analytical chemistry because atoms and molecules have unique spectra. As a result, these spectra can be used to detect, identify and quantify information about the atoms and molecules. Spectroscopy is also used in astronomy and remote sensing on earth. Most research telescopes have spectrographs. The measured spectra are used to determine the chemical composition and physical properties of astronomical objects (such as their temperature and velocity) [39].
Spectra of atoms and molecules often consist of a series of spectral lines, each one representing a resonance between two different quantum states. The explanation of these series, and the spectral patterns associated with them, were one of the experimental enigmas that drove the development and acceptance of quantum mechanics. The hydrogen spectral series in particular was first successfully explained by the Rutherford-Bohr quantum model of the hydrogen atom.
In some cases spectral lines are well separated and distinguishable, but spectral lines can also overlap and appear to be a single transition if the density of energy states is high enough. Named series of lines include the principal, sharp, diffuse and fundamental series.
ULTRAVIOLET-VISIBLE SPECTROPHOTOMETRY
Ultraviolet–visible spectroscopy refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent ranges. The absorption or reflectance in the visible range directly affects the perceived color of the chemicals involved. In this region of the electromagnetic spectrum, atoms and molecules undergo electronic transitions. Absorption spectroscopy is complementary to fluorescence spectroscopy, in that fluorescence deals with transitions from the excited state to the ground state, while absorption measures transitions from the ground state to the excited state. Molecules containing π-electrons or non-bonding electrons (n-electrons) can absorb the energy in the form of ultraviolet or visible light to excite these electrons to higher anti-bonding molecular orbitals. The more easily excited the electrons (i.e. lower energy gap between the HOMO and the LUMO), the longer the wavelength of light it can absorb. Based on the fact of four type of transition- π-π*, n-π*, σ-σ*, and n-σ*. The energy required for various transitions obey the following order σ-σ*>n-σ*>π-π*>n-π* [40].
BIOLOGICAL SCREENING
Microbial infections are a growing problem in contemporary medicine, and the use of antibiotics is common across the world. Antimicrobials are among the most commonly purchased drugs worldwide. They are essential treatments especially in the developing world where infectious diseases are a common cause of death. The health problem demands to explore and synthesize a novel class of antimicrobial compounds effective against pathogenic microorganisms that developed resistance to the antibiotics used in the current regimen. SHA has been reported to possess significant biological activities against a wide range of microbial organisms including bacteria and fungi organisms. There is therefore great prospect in the use of the potential in this compound as a means of solving the problem menace of antibiotics resistance [41].
CONDUCTIVITY
Electrical conductivity measures the charge carrying ability (conductance) of liquid in a measuring cell of specific dimension. It is important to define the units both length and conductance of Ni(SHA)2C6H5NH2 when taking electrical conductivity. The standard electrical conductivity unit is expressed in siemens permeter (S/m).
CONCLUSION
From the foregoing review a number of things can be gleaned first is the fact that further studies at molecular levels should be carried out to further elucidate on the mode of action of Ni(SHA)2C6H5NH2. Similarly, In vitro studies involving Ni(SHA)2C6H5NH2 and a broad range of gram positive and gram negative bacteria organisms and fungi agents such as yeast and mycrosporum should be carried out to further affirm the antimicrobial efficacy of Ni(SHA)2C6H5NH2. Conclusively, it is imperative to point out that a number of studies have been carried out on the metal complexes of Nickel nonetheless, as pointed out before some vacuum still remain. Nonetheless, the value of Nickel amongst other metal due to its stronger affinity in terms of attaching to ligands cannot be overemphasized and remains a veritable topic of study.
REFERENCES
- Abeer AA, Hapipah MA, Subramaniam P, Ward TR, Seik WNG (2008) Acta Cryst E. Crystallographic Communications 64: 1584.
- Abu Affan M, Siong WF, Ismail J, Sulaiman H , Edward RTT, et al., (2009) Synthesis, characterization and biological studies of organotin(IV) complexes with hydrazone ligand. Inorganica Chimica Acta 362: 5031-5037.
- Abuelghar MF, Abde lghani NT, Badr Y, Elborady OM (2014) Characterization of Mixed Plastic Wastes as a Potential Renewable Energy Resource. ISESCO Science and Technology Vision 5: 2-6.
- Mike F, Lisa I, Sharon W, Henry A, Steven G, et al., (2005) Toxicological Profile for Nickel. Agency for Toxic Substances; Disease Registry.
- Ahmed AE (2012) Inorganica Chimica Acta 362: 1-5278.
- Ahmed I, Beg AZ (2001) Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. Ethnopharmacol 74: 113-123.
- Aljanabi MY (2016) The Physical Methods in Inorganic Iraq: University of Baghdad.
- Angkawijaya AE, Fazary AE, Hernowo E, Ismadji S, Ju YH, et al., (2012) Nickel and cobalt complexes of non-protein l-norvaline and antioxidant ferulic acid: Potentiometric and spectrophotometric studies. J Solution Chem 41: 1156–1164.
- Angkawijaya AE, Fazary AE, Hernowo E, Taha M, Ju YH (2011) Iron (III), chromium (III), and copper (II) complexes of l -norvaline and ferulic acid. J Chem Eng Data 56: 532-540.
- Angkawijaya AE, Fazary AE, Ismadji S, Ju YH (2012) Cu (II), Co (II), and Ni (II)-antioxidative phenolate-glycine peptide systems: an insight into its equilibrium solution study. J Chem Eng Data 57: 3443–3451.
- Barceloux D (2014) Nickel. Clinical Toxicology 37: 239-258.
- Bates RG, Vijh AK (1975) Determination of pH Theory and Practice. J Chem Rev 46: 381.
- Bjerrum, J (1999) Metal Ammine Formation in Aqueous Solution: Theory of the Reversible Step Reactions. Copenhagen: Haase and Son.
- Buttice C (2015) Nickel Compounds. The Sage Encyclopedia of Cancer and Society.
- Capracotta MD, Sullivan RM, Martin JD (2006) Sorptive Reconstruction of CuMCl4 (M = Al and Ga) upon Small-Molecule Binding and the Competitive binding of CO and ethylene. Journal of the American Chemical Society 41: 13463-13473.
- CDC-NIOSH Pocket Guide to Chemical Hazards-Nickel metal and other compounds (as Ni)" (2015).
- Cowan MM (1999) Plant Products as Antimicrobial Agents. Clin Microbiol Rev 4: 564582.
- Davies J (1994) Inactivation of antibiotics and the dissemination of resistance genes. Science 264: 375-382.
- Deepak S, Lokesh K, Sulekh C (2008) Spectroscopic studies on chromium (III), manganese (II), cobalt (II), nickel (II) and copper (II) complexes with hexadentate nitrogen-sulfur donor [N2S4] macrocyclic ligand”. Spectrochim Acta 71: 746-750.
- Douglas XW, Heloisa B, Amal AN, Fathy AE, Mohammed IA (2009) Trans Met. Chem 24: 421d.
- Dunnick JK, Elwell MR, Radovsky AE, Benson JM (2015) Comparative carcinogenic effects of nickel subsulfide, nickel oxide, or nickel sulfate hexahydratechronic exposures in the lung. Cancer Research 55: 5251-5256.
- Dyer RJ (1995) Application of Absorption Spectroscopy of Organic Compounds. New Jersey: Prentice Hall.
- Efremov VA, Potolokov VN, Nikolashin SV, Fedorov VA (2002) Interaction of Water with GeCl4 , SnCl4 , and AsCl3. Inorg Mater 38: 837-846.
- Eksperiandova LP, Blank AB, Makarovskaya YN (2010) X-Ray Spectrom 31: 259.
- Fazary A (2010) Metal Complexes of Hydroxamic Acids, Germany: Lambert Academic Publishing.
- Fazary AE, Taha M (2009) Iron complexation studies of gallic acid. J Chem Eng Data 54: 35-42.
- Hernowo E, Angkawijaya AE, Fazary AE, Smadji S, Ju YH (2011) Complex stability and molecular structure studies of divalent metal ion with L-norleucine and vitamin b3. J Chem Eng Data 56: 4549-4555.
- Housecroft CE, Sharpe AG (2008) Acids, bases and ions in aqueous Inorganic Chemistry.
- Huang YL, Tsai YF, Lin TH (2017) Anal Sci. 15: 79.
- Irving HM, Rossotti HSJ (2014) The calculation of formation curves of metal complexes from pH titration curves in mixed solvents. Chem Soc Pg No: 2904-2910.
- Kamerud KL, Hobbie KA, Anderson KA (2013) Stainless Steel Leaches Nickel and Chromium into foods during cooking. Journal of Agricultural and Food Chemistry 61: 9495–501.
- Lever ABP (1968) Inorganic Electronic Spectroscopy. New York: Elsevier.
- Lin PH, Danadurai KSK, Huang SDJ. (2011) Anal At Spectrom 16: 409.
- NIOSH "Aniline" (2014) Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- Poole KJ (2001) Overcoming antimicrobial resistance by targeting resistance mechanisms. J Pharm Pharmacol 53: 283-294.
- Thomas K, Kai Wilfrid Schroder FR, Lawrence WJ, Marshall HRJ (2007) "Aniline" Ullmann's Encyclopedia of Industrial Chemistry. New York: John Wiley & Sons.
- Thirugnanam T, Tamilvendan D, Vishnu vardhanaraj G, Amaladasan M (2013) Synthesis, characterization, antibacterial and antifungal activities of some new mannich bases. World Journal of Pharmacy and Pharmaceutical Sciences 2: 5863-5870.
- Trumbo P, Yates AA, Schlicker S, Poos M (2001) Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and Journal of the American Dietetic Association 101: 294-301.
- Wojcik GM (2007) Structural Chemistry of Anilines. Patai's Chemistry of Functional Groups.
- Yabu Y, Yoshida A, Suzuki T, Nihei C, Kawai K, et al., (2003) The efficacy of ascofuranone in a consecutive treatment on Trypanosomabruceibrucei in mice. Parasitol Int 52: 155–164.
- Yoshikazu S, Murata H, Nakanishi T, Inatomi YJ (2001) Inhibitory effect of plant extracts on production of verotoxin by enterohemorrhagic Escherichia coli O157: H7. J Health Sci 47: 473-477.
Citation: Aderonke AV, Dede AH, Oluwatobi AI, Stephen NE (2019) A Review on the Metal Complex of Nickel (Ii) Salicylhydroxamic Acid and its Aniline Adduct. J Transl Sci Res 2: 006.
Copyright: © 2019 Aderonke AV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

