
A Split Body Evaluation of a Topical Body Product Applied Pre and Post Liposuction with Helium Plasma and Radio Frequency
*Corresponding Author(s):
Alan D WidgerowDepartment Of Plastic Surgery, Director Center For Tissue Engineering, University Of California, Irvine, United States
Email:alan.widgerow@galderma.com
Abstract
Topic area: Topical adjunct for Liposuction with Helium Plasma and Radio Frequency.
Purpose: Recovery outcomes were compared between a topical body formulation with TriHex Technology® and a bland moisturizer. Products were used 2 weeks prior to upper arm liposuction and 12 weeks after, using helium plasma and radio frequency.
Design: Patients were randomized and blinded to use two topicals on selected right or left arms before and after undergoing liposuction of the upper arms with helium plasma and radiofrequency. Follow-up visits occurred at days 1-3, 5-7, 10-14, 21-25, 28-30, 42-50, and week 12. At follow-up visits, a blinded physician assessed the right and left arms using a 5-point scale examining ecchymosis, edema, skin discoloration, induration, and subcutaneous fibrous banding. Discomfort was analyzed using the VAS pain scale. Patients completed a questionnaire comparing the two sides in respect of sensitivity to the touch, swelling, numbness, bruising/discoloration, pain/discomfort, skin flexibility and texture. Photography was undertaken at every visit.
Findings: Induration and subcutaneous fibrous banding were consistently lower for the topical active side when assessed by the blinded Investigator with subcutaneous banding showing statistical significance (p= 0.025) over the bland moisturizer at week 6 post procedure. Edema improved from week 4 on the active side and trended until week 12. Skin discoloration at weeks 3 and 4 showed mean graded scores lower than the control, and pain/discomfort was lower on the topical active side at each follow-up visit after day 4. Most patients assessed the topical active side as softer and more flexible, less sensitive to the touch, and with less discomfort and swelling. No adverse events were reported.
Summary: Use of a topical body product with TriHex Technology® used with helium plasma liposuction pre and post procedure has shown improved recovery related to induration, subcutaneous fibrous banding, edema, skin discoloration and patient discomfort.
Introduction
Liposuction is one of the most sought-after cosmetic procedures, while postoperative recovery time may last up to 3 months. Surgical body contouring procedures damage fat cells releasing highly inflammatory lipid droplets by way of procedure induced adipocytolysis. The release of these inflammatory molecules in turn can create localized compartments of “inflammasomes,” which can then present as adverse skin induration, fibrous banding, and sometimes as fat necrosis [1]. It has been shown that topical treatment with specific actives used with body contouring procedures can accelerate the healing response and expression of anti-inflammatory genes, resulting in less skin induration, fibrous banding (internal scarring) and patient discomfort [1,4].
A novel topical formulation, ReFORM & RePAIR with TriHex Technology® - R&R (Alastin Skincare, Inc., Carlsbad, CA) has been developed and formulated with actives that have demonstrated efficacy in extracellular matrix remodeling, autophagy, and regenerative macrophage polarization with additional components targeting exaggerated scarring. In a recently published double-blind, split body study [2], R&R was applied topically pre and post body contouring surgery resulting in significant decreases in ecchymosis, induration, and particularly subcutaneous fibrous banding [2].
Renuvion® (Apyx® Medical, Clearwater, FL) is a minimally invasive helium plasma and radiofrequency (RF) energy device. The system uses helium gas and RF energy to generate heat that is applied to tissue through the ionization and rapid neutralization of the helium atoms and by RF energy that heats tissue by passing current through the resistance of the tissue (Joule heating). This allows for subcutaneous soft tissue coagulation and contraction within the Fibroseptal Network (FSN) in a controlled manner [3,4]. Therefore, this clinical study was undertaken to evaluate and compare the recovery outcomes between R&R and a bland moisturizer, when used pre and post liposuction to the arms with Renuvion® helium plasma and radio frequency technology.
Materials & Methods
This double blind, randomized, split body clinical study occurred over 6 months from January 2022 - June 2022. Participants included healthy men and women ages 25-70 who were willing to only use the topical study products and on the designated procedural areas. They agreed to refrain from extended sun exposure, tanning beds, and any other aesthetic procedures to the procedural areas for the duration of the study. Subjects with previous hypersensitivity or known allergy to any of the ingredients in the study product were excluded. Additionally, pregnant or breastfeeding women or those planning pregnancy during the study duration, were excluded.
Enrolled participants were provided two blinded topical study products containing either R&R with TriHex Technology® or a bland moisturizer (CeraVe®, NY, NY) and were instructed to apply the product twice daily to the randomized right or left side of the designated surgical procedure area for at least two weeks prior to the planned procedure. Post procedure, participants were instructed to apply the designated product two times a day to the procedural area of the skin until the end of the study. Participants returned for 7 follow-up visits at days 1-3, 5-7, 10-14, 21-25, 28-30, 42-50, and a final visit at 12 weeks postop. Photo release consent was obtained from patients.
Procedure
The upper arms of each participant were marked pre procedure and photographs were taken of the treatment areas. Study participants were then prepped for the procedure and draped. Prior to liposuction of the arms, tumescent anesthesia was infiltrated using Klein solution consisting of lidocaine, epinephrine, and bicarbonate. One or two small incisions were made in the axilla, and one small incision was made at the inferior aspect of the forearm near the elbow. Tumescent anesthesia was infiltrated into the arms until clinical blanching was noted and the patient was numb. Vacuum assisted liposuction was performed on both arms until a clinical endpoint was achieved. After suctioning, the patient was marked for the Renuvion procedure. Renuvion was performed with antegrade and retrograde strokes until 6 passes were performed on the superior and inferior upper arm procedural areas. Following completion of the Renuvion procedure, gas and fluid were removed through the incision sites, and Topifoam® and a garment were applied.
Photography
At every visit, photography of the anterior and posterior procedural arm areas were collected using a Canon Eos Rebel T8i camera (Canon USA Inc., Huntington, NY).
Investigator Assessments
At all follow-up visits, the blinded Investigator completed an assessment of the right and left sides of the procedural areas using a 5-point scale (0-none, 1-barely perceptible, visually or palpably, 2-mild, 3-moderate, 4-severe) for ecchymosis, skin discoloration, edema, induration, and subcutaneous fibrous banding. The VAS, 0-10 pain scale, was used to assess participant pain/discomfort.
Participant Assessments
At all follow-up visits, participants completed an assessment comparing the right and left sides of the procedural area, consisting of 7 questions; less sensitivity to the touch, less swelling, less numbness, less bruising/discoloration, less pain/discomfort, and the side that felt softer and more flexible, and the side that had better skin texture.
Safety
Patients were assessed for adverse events at every visit.
This study was conducted in accordance with the World Medical Association Declaration of Helsinki’s statement of ethical principles for medical research involving human patients, including research on identifiable human material and data. Full informed written consent was acquired from each patient in the study.
Results
- Demographics
Overall, 6 female participants with a mean age of 33.5 years completed the study. Participant Fitzpatrick Skin Type and volume removed from each arm are shown in Table 1.
|
Participant |
Skin Type |
Fat Vol (ml) Removed (LT) |
Fat Vol (ml) Removed (RT) |
Total Fat Vol (ml) Removed |
|
1 |
2 |
325 |
425 |
750 |
|
2 |
4 |
120 |
280 |
400 |
|
3 |
2 |
725 |
675 |
1400 |
|
4 |
4 |
650 |
450 |
1100 |
|
5 |
3 |
500 |
500 |
1000 |
|
7 |
3 |
425 |
125 |
550 |
Table 1: Study participant Fitzpatrick Skin Types, and volume amounts removed during the study procedure.
Blinded Investigator Assessments
Investigator assessments demonstrated greater improvements in the subcutaneous fibrous banding parameter for the R&R treated arms compared to the bland moisturizer for all time points (Figure 1). Similar results were achieved for induration up to week 12, by which time induration had resolved in both groups. Additionally, skin discoloration at weeks 3 and 4 achieved mean graded scores lower on the R&R treated arms compared to the bland moisturizer, and pain/discomfort was also scored lower on the R&R treated arms at each follow-up visit post day 4. Edema scores demonstrated relative equivalence for both treatment arms until week 4 when the active side showed improved resolution (mean score 1.5 for the R&R arm and 2.17 for the bland moisturizer arm) - this trended until week 12. Relative equivalence for ecchymosis scores was also noted for both treatment arms until complete resolution at week 6.
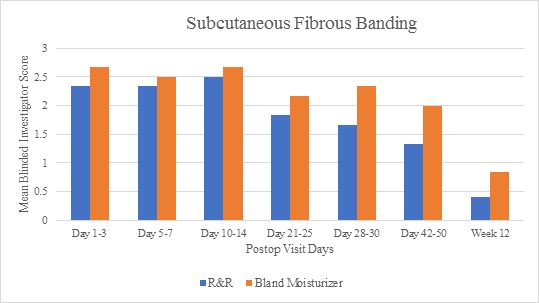 Figure 1: Investigator assessments demonstrated greater improvement in subcutaneous fibrous banding for the R&R treatment arms compared to the bland moisturizer arms at all time points.
Figure 1: Investigator assessments demonstrated greater improvement in subcutaneous fibrous banding for the R&R treatment arms compared to the bland moisturizer arms at all time points.
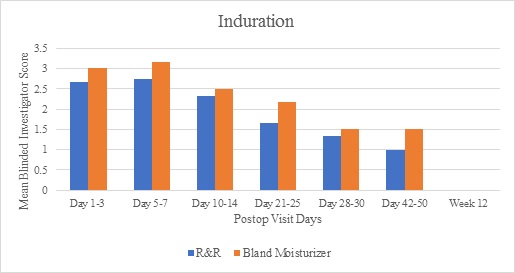 Figure 2: Mean blinded Investigator scores comparing the R&R and bland moisturizer treatment arms for the induration parameter.
Figure 2: Mean blinded Investigator scores comparing the R&R and bland moisturizer treatment arms for the induration parameter.
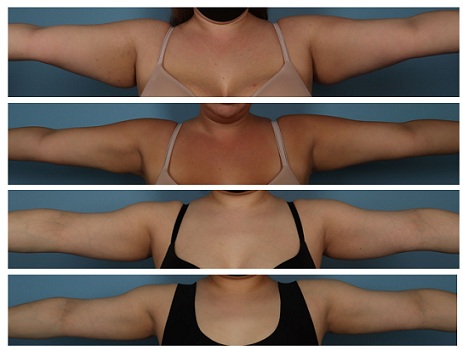 Figure 3: Baseline (BL) before and after (12 weeks- 12W) photos of two participants showing week 12 results following liposuction and Renuvion® helium plasma and radio frequency of the upper arms.
Figure 3: Baseline (BL) before and after (12 weeks- 12W) photos of two participants showing week 12 results following liposuction and Renuvion® helium plasma and radio frequency of the upper arms.
Participant Assessments
Participants returned for 7 follow-up visits post procedure, and a 7-question assessment comparing the right and left sides of the procedural areas was completed. Participants reported the R&R treated arm felt softer and more flexible compared to the bland moisturizer beginning at day 1-3 through week 12. Most notably, at the day 21-25 follow up visit, 83% of participants reported that the R&R treated arm felt softer and more flexible, and no participants reported the bland moisturizer treated arm as feeling softer and more flexible. Participants reported the most marked differences in discomfort, softness and flexibility of the arms at weeks 3 (83%) and 4 (66%) with the R&R treated arm outperforming the comparator at these time points. Additionally, participants reported less pain/discomfort in the R&R treated arm compared to the bland moisturizer at all follow up visits through week 12.
Discussion
Helium Plasma and Radio Frequency technology provides some unique aspects for soft tissue contouring. The energy delivered to the fibroseptal network, and the fat tissue allows for dissolution of the fat, while encouraging skin tightening. In addition, the coagulative nature of the technology minimizes the bleeding and bruising associated with the procedure. The healing profile thus primarily revolves around the breakdown particle created by the fat dissolution.
It has previously been demonstrated that fat tissue breakdown, adipocytolysis, is associated with the release of pro-inflammatory lipid particles and fatty acids, which manifest as skin induration, followed by internal scarring or fibrous banding [1]. This banding creates discomfort and limitation of movement for the patient, hindering full mobility and healing.
To this end, this study was conducted with particular interest in assessing how the application of a topical product could impact this longer-term side effect. The efficacy of the technology on end result was obvious, as all cases experienced significant improvements in volume and aesthetic outcome. Typical cases are demonstrated in Figure 3 showing baseline photos vs 12-week outcomes. As far as healing parameters are concerned, as anticipated, early response ecchymoses and edema were minimal, but around 3 weeks post procedure the induration was noted by both investigators and participants. This heralded the period of edema, induration and subcutaneous fibrous banding typically cascading over the 3-6-week period until resolution. Thus, day 21 to day 50 is the critical time period when significant differences are evident and reported both by participant and investigator. Interestingly, by the end of the study 12-week point, a substantial difference in subcutaneous fibrous banding was still present.
These study results are consistent with what has been demonstrated in previous studies with almost identical findings at the 4-6-week period [2,5,6]. This is when the topical appears to be most effective at creating resolution of internal scarring and soft tissue remodeling. At a gene transcription level, this activity has been validated in previous studies [7].
Conclusion
This study demonstrates the efficacy of the Renuvion® helium plasma and Radiofrequency (RF) energy device technology as an effective method for removing excess adipose tissue and treating skin laxity, and the adjunctive synergy of the topical formulation ReFORM & RePAIR with TriHex Technology® which aids in reducing internal scarring, subcutaneous fibrous banding, and patient discomfort.
Alastin Skincare, Inc., provided the blinded topical products and funded the study. Alastin Skincare took no part in the conducting of the study or data collection. They were involved in submitting independent statistical analysis and aiding in manuscript compilation
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Disclosures
Ms. Bell is the Director of Clinical Research at Alastin Skincare, Inc., Ms. Robison is the Clinical Research Manager at Alastin Skincare, Inc., and Dr. Widgerow is the Chief Scientific Officer, Galderma.
References
- Widgerow AD, Ziegler ME, Casas LA (2021) Topical Skin Treatment and Its Influence on Surgical Healing: Review of Literature and Underlying Physiology. Aesthet Surg J Open Forum 3: 29.
- Casas LA, Claytor RB, Zeidler KR, Shridharani SM, Cohen Sr, et al. (2022) A Multicenter, Randomized, Double-Blind, Split-Body Clinical Trial Evaluating the Efficacy and Outcomes of a Topical Product Pre and Post Aesthetic Surgical Body Procedures. Aesthet Surg J Open Forum. 4: 54.
- Duncan DI, Roman S (2020) Roman S. Helium Plasma Subdermal Tissue Contraction Method of Action. Biomedical Journal of Scientific & Technical Research.
- Tanzi E, Capelli C, Robertson D, LaTowsky B, Jacob C, et al. (2021) Improvement in the appearance of cellulite and skin laxity resulting from a single treatment with acoustic subcision: Findings from a multicenter pivotal clinical trial. Lasers Surg Med 54: 121-128.
- Casas LA, Bell M, Claytor B, Ziegler ME, Widgerow AD (2021) An Analysis of Patient-Reported Recovery Outcomes of Topical Tripeptide/Hexapeptide Formulations Utilized in a Prospective Randomized Double-Blind Split Neck and Body Study. Aesthet Surg J Open Forum 3: 52.
- Claytor B, Casas L, Ziegler M, Widgerow AD, Bell M (2020) Evaluating the Efficacy, Tolerability, and Outcomes of Topical Tripeptide/Hexapeptide Formulations before and After Liposuction of the Medial Thighs. Aesthet Surg J Open Forum 3: 55.
- Ziegler ME, Claytor B, Bell M, Casas L, Widgerow AD (2020) Gene Expression Changes in the Skin of Patients Undergoing Medial Thigh Liposuction With Pre-Surgical and Post-Surgical Application of Topical Products. Aesthetic Surgery Journal Open Forum 2: 33.
Citation: Shantharam R, Behr K, Robison T, Bell M, Widgerow AD0 (2023) A Split Body Evaluation of a Topical Body Product Applied Pre and Post Liposuction with Helium Plasma and Radio Frequency. J Clin Dermatol Ther 9: 0128.
Copyright: © 2023 Rohini Shantharam, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

