
A Unique Case Presentation of Methadone Toxicity without QTc Interval Prolongation despite Patient Risk Factors
*Corresponding Author(s):
Keith T VeltriDepartment Of Pharmacy Practice, Touro College Of Pharmacy, New York, United States
Tel:+1 6469814755,
Email:keith.veltri@touro.edu
Abstract
Safe medication practice is a multidisciplinary process involving physicians, nurses, pharmacists and the patients themselves. Over recent years, particularly in health system settings, pharmacists have developed specialist roles in medication safety, working with colleagues in pharmacy and other professions to identify problems with medication use and prevention of errors. In November 2006, the Food and Drug Administration issued a public health advisory titled “Methadone use for pain control may result in death and life-threatening changes in breathing and heart beat”. The report emphasized the importance of clinicians to be mindful of the drug’s ability to cause QTc interval prolongation and is a marker for the risk of developing torsades de pointes, a potentially fatal ventricular arrhythmia. Factors associated with QT prolongation include higher methadone total daily dose, hypokalemia, low prothrombin level (suggestive of reduced liver function) and co-administration of a medication that inhibits the CYP3A4 enzyme system which may increase methadone serum levels. Methadone’s lipophilicity contributes to unpredictable pharmacokinetics with high rates of interpatient variability and unfortunately there is little data to support the development of an accurate risk assessment in patients receiving treatment. Despite there being a valid and reliable risk assessment tool, there are known risk factors for QTc prolongation to be aware of with methadone therapy. Greater awareness of the potential for clinically important interactions when methadone is taken concomitantly with other drugs is substantial.
Keywords
INTRODUCTION
Methadone is widely used and FDA approved for the detoxification and maintenance treatment of opioid dependence. It is a substrate of Cytochrome P450 3A4 (CYP3A4), a metabolizing enzyme in the liver. Co-administration of this medication with other drugs that affect the function of CYP3A4 can change final outcomes [1-7]. Due to fluconazole’s ability to inhibit drug metabolism via this pathway, patients treated simultaneously with fluconazole and methadone may have clinically relevant increases in systemic methadone concentrations [1-7]. Fortunately, most of these Pharmacokinetic (PK) interactions are not life threatening; however they can have important consequences [2].
BACKGROUND
Methadone is a synthetic opioid agonist frequently used in the treatment of opioid dependence. However, secondary to the drug’s long duration of action, efficacy and low cost, its use in the treatment of chronic pain is increasing. Methadone is a racemic mixture of R- and S- methadone; R -methadone is 8-50 times more potent than S-methadone and is responsible for most of it’s action. Methadone is a mu-opioid receptor agonist and it also binds to kappa and delta opioid receptors. Additional mechanisms of action include inhibiting the re-uptake of serotonin and norepinephrine and as an antagonist at the N-Methyl-D-Aspartate (NMDA) receptor, thought to prevent central sensitization and reduce opioid tolerance.
Methadone is extensively metabolized, primarily by N-demethylation to pharmacologically inactive metabolites which are eliminated in the urine and feces. Metabolism takes place primarily in the liver, with some also occurring in the intestines [1-2]. Methadone has large inter-individual variability in its PK, so maintenance doses must be individualized; however, usual doses range from 80 to 120mg/day when there is no drug-drug interaction involved [6]. The Cytochrome P450 (CYP450) enzymes responsible for the metabolism in the liver include CYP3A4, 2B6, and 2C19 (primarily) as well as 2C8, 2C9 and 2D6. The metabolism of methadone is significantly influenced by medications that alter the activity of these enzyme systems resulting in either increased or decreased methadone metabolism in many cases.
Even without the influence of interacting medications, the level of activity of the 3A4 enzyme varies significantly among individuals with up to a 30-fold difference in the liver 3A4 enzymes and an 11-fold difference in the intestinal enzymes [6].
Fluconazole inhibits the metabolism of methadone in the liver. Therefore, it increases the methadone blood concentration and risk for methadone-related side effects and toxicity, including CNS depression [2]. In general, the concomitant use of methadone and fluconazole should be avoided. However, if concomitant use is required, the dose of methadone should generally be decreased and signs of methadone side effects should be monitored [5-6]. Due to methadone’s long and unpredictable elimination half-life, ranging from 5 to 130 hours with a mean of about 20-35 hours, fluconazole mediated enzyme inhibition may continue for 4 to 10 days on average after discontinuation [2-6]. As with any other drug, increased knowledge of methadone's metabolism and potential interactions with other agents enables the clinician to use it more safely and effectively and underscores the need to be vigilant for such possible interactions [1-7].
Safe medication practice is a multidisciplinary process involving physicians, nurses, pharmacists and the patients themselves. Of all the health professionals involved in medication usage, pharmacists have the most knowledge about the drugs administered to patients both in the hospital as well as ambulatory care clinics. Over recent years, particularly in health system settings, pharmacists have developed specialist roles in medication safety, working with colleagues in pharmacy and other professions to identify problems with medication use and prevention of errors [8].
In November 2006, the Food and Drug Administration issued a public health advisory titled “Methadone use for pain control may result in death and life-threatening changes in breathing and heart beat”. The report emphasized the importance of clinicians to be mindful of the drug’s ability to cause QTc interval prolongation and is a marker for the risk of developing torsades de pointes, a potentially fatal ventricular arrhythmia [9]. Factors associated with QT prolongation include higher methadone total daily dose, hypokalemia, low prothrombin level (suggestive of reduced liver function) and co-administration of a medication that inhibits the CYP3A4 enzyme system which may increase methadone serum levels [9].
A normal QTc interval is ≤430 msec for men and ≤450 msec for women [10]. Borderline QTc prolongation for men is classified as 431-450 msec and 451-470 msec for women, while QTc prolongation is defined as >450 msec for men and >470 msec for women. As the QTc interval increases, so does the risk for life-threatening arrhythmias such as polymorphic ventricular tachycardia or Torsades de Pointes (TdP). The risk of sudden cardiac death increases 4-fold when QTc is ≥500 msec [11].
CASE REPORT
This case involves a 58-year-old male who presented to the Moses Division Emergency Department (ED) with a 3 week history of difficulty swallowing. Past medical history includes HIV (CD4 23cells/mm3, VL 867, 878copies/mL), noncompliance to antiretroviral therapy secondary to depression, active poly-substance abuse issues including cocaine and on methadone maintenance treatment for heroin and Chronic Obstructive Pulmonary Disease (COPD). The patient reported “feeling food getting stuck in his throat” and “had to drink something to get it down”. Vitals on admission were otherwise unremarkable (Table 1).
|
Vital Signs |
On Admission |
|
Blood Pressure (mmHg) |
136/76 |
|
Pulse (BPM) |
101 |
|
Respirations (BPM) |
20 |
|
Temperature (0F) |
98.2 |
Table 1: Vital signs on admission.
Upon admission, according to the Gastrointestinal (GI) team’s recommendation, the patient was treated for presumed esophageal candidiasis with fluconazole 100mg PO daily for 14 days and was considered for an Esophagogastroduodenoscopy (EGD) if symptoms persisted. The maintenance dose of methadone, 100mg PO daily, according to the patient’s treatment program was continued and baseline QTc interval reported was 420 msec. The Infectious Disease (ID) team was also consulted and suggested 100mg IV fluconazole daily, which began on hospital day 2.
The dysphagia continued to improve, and the patient was mistakenly switched back after 3 days of IV to fluconazole 400mg oral suspension daily as he felt less retrosternal pain. On hospital day 5, the patient reported feeling Shortness of Breath (SOB) and had decreased O2 saturation at rest (93-94%). Walking O2saturation decreased to 87-88% and he was placed on 2L O2. Arterial Blood Gases (ABG) revealed respiratory acidosis (pH 7.353/PaCO2 47.1/PaO2 55/HCO325.5).
On hospital day 6, the patient developed an acutely altered mental status, with alternating agitation and somnolence as well as confusion, on a non-focal neurological exam. The patient was disoriented and restless throughout the day. A stat head computerized tomography revealed no acute intracranial pathology. Later that day, it was reported by the clinical pharmacist on medical rounds that the fluconazole order was deescalated incorrectly from IV to PO antifungal therapy (100mg orally daily should have been prescribed) and that the antifungal agent suppressed the hepatic metabolism of methadone potentiating its effect. The patient was referred to addiction psychiatry who subsequently recommended decreasing the methadone dose to 30mg daily and fluconazole was later discontinued.
Despite furthering respiratory depression and hypotension risks in the setting of methadone co-administration, a total dose of 4mg lorazepam was administered on hospital day 7 as the patient presented with seizures. Subsequent improvement in Electroencephalography (EEG) was noted and a lumbar puncture revealed no evidence of acute infection. Levetiracetam 500mg twice daily was later initiated for short term seizure prophylaxis again recognizing the potential of a pharmacodynamic interaction with methadone. Vital signs were monitored closely.
A Magnetic Resonance Imaging (MRI) of the brain later revealed pre-existing patchy white matter hypo densities and periventricular matter confirming the altered mentation was likely secondary to methadone toxicity in the setting of a drug-drug interaction and not worsening HIV encephalitis. The patient became asymptomatic after discontinuation of fluconazole on hospital day 10. His mental status slowly improved as the drug concentrations of fluconazole were gradually eliminated and he returned to his baseline of 100% O2 on room air with clear lungs on day 11. Interestingly, no Electrocardiogram (ECG) changes from baseline were noted throughout the admission. He was eventually discharged on hospital day 12 to a nursing home.
DISCUSSION
Methadone is commonly associated as a culprit for QTc prolongation due to its black box warning and known risk for TdP. The concern for this risk arose in 2002 when Krantz and colleagues reported TdP in patients receiving doses of methadone ranging between 65 and 1000mg per day in a retrospective case series of 17 patients. The QTc intervals varied from 522 to 785 msec. Numerous studies (case-control, cross-sectional and prospective cohort) have reported QTc prolongation since then, however, the rate of TdP remains unknown [11]. According to Abramson, Quinn and Stern there are many cases of QTc prolongation associated with higher doses of methadone with concomitant risk factors including lower prothrombin levels, electrolyte abnormalities and comorbid use of CYP3A4 inhibitors [12]. Methadone doses below 40mg per day were associated with less common risk for QTc prolongation. The prevalence of methadone prolonging the QT interval at doses within a normal therapeutic range is between 16-33% [12]. Currently, there is controversial evidence to support that this effect is dose dependent.
Methadone’s lipophilicity contributes to unpredictable pharmacokinetics with high rates of interpatient variability and unfortunately there is little data to support the development of an accurate risk assessment in patients receiving treatment. Despite there being a valid and reliable risk assessment tool, there are known risk factors for QTc prolongation to be aware of with methadone therapy (Table 2) [12-14].
|
Risk Factors Associated with Greater Chance of QT Prolongation12-15 |
|
Heart Disease |
|
Congenital long QT syndrome |
|
Hypertension |
|
Sinus bradycardia |
|
HIV infection (related to drugs prescribed for these patients or to an acquired form of Long QT Syndrome (LQTS) arising from the viral infection) |
|
QT interval is a heritable trait |
|
Female gender |
|
Increasing age (>50) |
|
Diurnal variation in the QT interval |
|
Electrolyte disturbance (hypokalemia and hypomagnesemia) |
|
Hypoglycemia |
|
Hypothyroidism |
|
Obesity |
|
Lower prothrombin level |
|
Concomitant use of CYP3A4 inhibitors and/or medications associated with QTc prolongation |
|
Hepatic impairment |
|
Cocaine use |
|
Alcohol Use |
Table 2: Risk factors associated with greater chance of QT prolongation [12-14].
As reported in the case description above, there are several patient scenarios where no QT change had occurred with methadone toxicity. Contradictory to this patient case, a large majority of cases do not report QTc prolongation prior to methadone overdose. The risk assessment is commonly made from a single ECG reading reported at the time the patient presents to the emergency department for evaluation. Our patient’s QTc interval was considered normal, 50), HIV infection and a DDI between methadone and fluconazole.
Most studies that did display a positive correlation between dose and QTc prolongation reported patients on methadone doses greater than 100mg. Vieweg et al., performed a literature review and found 31 adult cases and one newborn case of methadone-associated QTc interval prolongation and/or TdP. Out of the 32 cases, 21 had a correlation between QTc interval prolongation and methadone dose. Out of the 21 cases the following similarities were noted: six (28.6%) had methadone doses ≥ 200mg, eight (38.1%) had heart disease, seven (33.33%) had electrolyte abnormalities, 12 (57.14%) were on either a CYP inhibitor or QTc prolonging agent, six (28.6%) had hepatic impairment (all six of these patients experienced QTc prolongation) and six (28.6%) had other risk factors (i.e., sinus bradycardia or cocaine use). There was an average of 3.19 risk factors per patient case for a positive correlation between QT interval prolongation and methadone dose [14]. Out of these 32 cases, 11 did not have a correlation between QTc interval prolongation and methadone dose. Out of the 11 cases the following similarities were noted: seven (63.64%) had doses ≥ 200mg, three (27.27%) had heart disease, one (9.09%) had electrolyte abnormalities, four (36.36%) were on either a CYP inhibitor or QTc prolonging agent, zero had hepatic impairment and five (45.45%) had other risk factors. There was an average of 1.82 risk factors per patient case that did not have a positive correction between QT interval prolongation and methadone dose. Multiple risk factors, most likely three or more, may be associated with a higher likelihood of QT prolongation [14].
There have also been multiple cases (21) of methadone-induced TdP without any correlation between QT prolongation and dose. Roy et al., also concluded after studying 180 patients (69.1% men) with an average QTc of 420.9 ± 21.1 msec and average methadone dose of 80.4 ± 27.7mg that there was no significant correlation between dose and QT interval. Presently, there is not enough evidence to fully state whether there is an apparent relationship between methadone dose and QT interval prolongation [11,12,14].
Combination drug therapy is common in patients with human or Acquired Immunodeficiency Virus (HIV/AIDS) and opioid dependence [1]. Drug-drug interactions can result in clinical response variations following the co-administration of two or more therapeutic agents especially in this population [1]. The patient discussed in the case description did not experience QTc prolongation with his methadone treatment despite having two defined risk factors. He was prescribed fluconazole, a known CYP inhibitor and had a past medical history of uncontrolled HIV infection. Our patient’s methadone dose never exceeded 100mg throughout the hospital stay. However, he did experience several toxic methadone manifestations most notably on day 5 including altered mental status, hypotension, bradycardia and poor respirations (Figures 1-4). This patient case strengthens the notion that the risk of QTc prolongation may be increased when methadone doses are greater than 100mg when multiple risk factors are present but not in all instances . Two risk factors to potentially monitor with greater importance in many hospitalized patients include hepatic impairment, concomitant CYP3A4 inhibitor(s) and the addition QTc prolongating agents throughout admission.
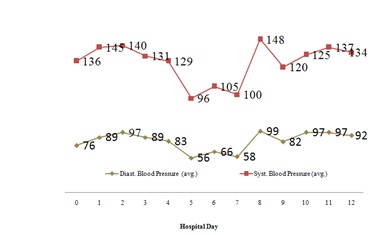 Figure 1: Average blood pressure throughout hospitalization.
Figure 1: Average blood pressure throughout hospitalization.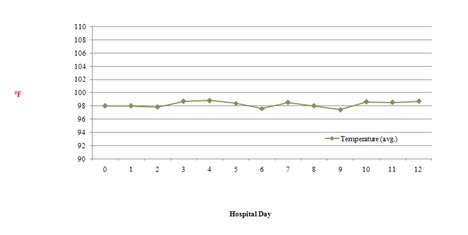 Figure 2: Average body temperature throughout hospitalization.
Figure 2: Average body temperature throughout hospitalization.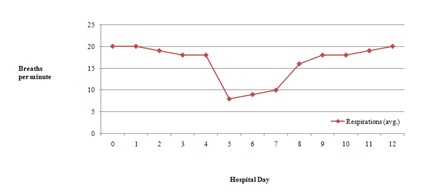 Figure 3: Average respiration rate throughout hospitalization.
Figure 3: Average respiration rate throughout hospitalization.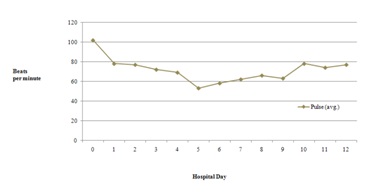 Figure 4: Average pulse rate throughout hospitalization.
Figure 4: Average pulse rate throughout hospitalization.
In the literature review conducted by Vieweg et al., those patients that experienced QTc prolongation with methadone doses had no direct correlation explaining this prolongation [14]. Despite their possibly being a positive association between methadone dose and QTc prolongation, the clinical significance of this remains uncertain and warrants further study. Regardless, methadone can be safely administered as long as appropriate monitoring precautions are taken.
CONCLUSION
Greater awareness of the potential for clinically important interactions when methadone is taken concomitantly with other drugs is substantial. Fortunately, most of these pharmacokinetic interactions are not life-threatening if identified and managed appropriately. Per the consensus guidelines for therapeutic drug monitoring it is recommended to obtain methadone levels for safety reasons, however, optimal drug levels may vary markedly due to variable levels of pharmacokinetics and tolerance. Serum methadone levels typically do not offer clinical significance but can be beneficial in determining a need for a dosage increase or patients needing split daily dosing. There is no established toxic level of methadone, however, in general a target through level of 400-600ng/mL is desired. A serum level was not drawn for our patient due to the reasons above [16,17].
Clinical pharmacists who attend medical rounds have a better sense of the patients’ current medical problems and are able to make recommendations at the point of prescribing, rather than retrospectively. This has potential patient safety benefits since there are no delays in correcting any erroneous errors in medication orders. It was acknowledged based on the time-event relationship that a highly probable adverse drug event did occur in our patient according to the Naranjo algorithm with a score of 9 (Table 3). The clinical stability and improvement of objective findings and the temporal association between the resolutions of symptomatology following the discontinuation of fluconazole further supports our assumption that a drug interaction between the antifungal agent and methadone was more likely the cause of the relative overdose.
|
S.No |
Naranjo’s Algorithm: Determination of Adverse Drug Reaction Probability |
Yes |
No |
Do not know |
Score |
|
1 |
Are there previous conclusive reports on this reaction? |
+1 |
0 |
0 |
+1 |
|
2 |
Did the adverse event occur after the suspected drug was administered? |
+2 |
-1 |
0 |
+2 |
|
3 |
Did the adverse reaction improve when the drug was discontinued or a specific antagonist was administered? |
+2 |
0 |
0 |
+2 |
|
4 |
Did the adverse reaction reappear when the drug was re-administered? |
+2 |
-1 |
0 |
0 |
|
5 |
Are there alternative causes (other than the drug) that could have on their own caused the reaction? |
-1 |
+2 |
0 |
+2 |
|
6 |
Did the reaction reappear when a placebo was given? |
-1 |
+1 |
0 |
+1 |
|
7 |
Was the drug detected in the blood (or other fluids) in concentrations known to be toxic? |
+1 |
0 |
0 |
0 |
|
8 |
Was the reaction more severe when the dose was increased or less severe when the dose was decreased? |
+1 |
0 |
0 |
0 |
|
9 |
Did the patient have a similar reaction to the same or similar drugs in any previous exposure? |
+1 |
0 |
0 |
0 |
|
10 |
Was the adverse event confirmed by any objective evidence? |
+1 |
0 |
0 |
+1 |
|
Total Score |
9 |
Table 3: Naranjo’s Algorithm.
Total Score: ≥9 Highly probable, 5-8 Probable, 1-4 Possible, ≤0 Doubtful
Despite the fact that our patient did not experience ECG changes throughout the admission and the variable pharmacokinetic clinical course of methadone in any given individual, inpatients on methadone should obtain a baseline ECG and repeat after either dose escalation to an oral methadone dose of 60mg/day, if started on IV methadone or when drugs that can increase the risk for QTc prolongation are added to a regimen. Stable outpatients should receive a baseline ECG before treatment, 30 days after treatment initiation and annually [11]. More frequent ECG monitoring should be considered for patients using ≥60mg/day of oral methadone. In addition an ECG should be obtained immediately in patients with unexplained syncope or seizures especially in the suspicion of methadone toxicity [11].
CONFLICT OF INTEREST STATEMENT
The authors whose names are listed immediately above certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements) or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
REFERENCES
- Cobb MN, Desai J, Brown LS Jr, Zannikos PN, Rainey PM (1998) The Effect of Fluconazole on the Clinical Pharmacokinetics of Methadone. Clin Pharmacol Ther 63: 655-662.
- Ferrari A, Coccia CP, Bertolini A, Sternieri E (2004) Methadone--metabolism, pharmacokinetics and Interactions. Pharmacol Res 50: 551-559.
- Moody DE, Liu F, Fang WB (2015) Azole antifungal inhibition of buprenorphine, methadone and oxycodone in vitro metabolism. J Anal Toxicol 39: 374-386.
- Tarumi Y, Pereira J, Watanabe S (2002) Methadone and fluconazole: Respiratory depression by drug interaction. Journal of Pain and Symptom Management 23: 148-153.
- Dhananjay P, Mitra AK (2006) CYP3A4 and MDR Mediated Interactions in Drug Therapy. Clin Res Regul Aff 23: 125-163.
- Eap CB, Buclin T, Baumann P (2002) Interindividual Variability of the Clinical Pharmacokinetics of Methadone: Implications for the treatment of opioid dependence. Clin Pharmacokinet 41: 1153-1193.
- Lee HY, Li JH, Wu LT, Wu JS, Yen CF, et al. (2012) Survey of methadone-drug interactions among patients of methadone maintenance treatment program in Taiwan. Subst Abuse Treat Prev Policy 7: 11.
- Kaboli PJ, Hoth AB, McClimon BJ, Schnipper JL (2006) Clinical pharmacists and inpatient medical care: A systematic review. Arch Intern Med 166: 955-964.
- FDA (2006) Methadone use for pain control may result in death and life-threatening changes in breathing and heart beat, FDA, Maryland, USA.
- Cruciani RA (2008) Methadone: To ECG or Not to ECG…That is still the question. J Pain Symptom Manage 36: 545-52.
- Krantz MJ, Martin J, Stimmel B, Mehta D, Haigney MC (2009) QTc Interval Screening in Methadone Treatment. Ann Intern Med 150: 387-395.
- Abramson DW, Quinn DK, Stern TA (2008) Methadone-associated QTc prolongation: A case report and review of the literature. Prim Care Companion J Clin Psychiatry 10: 470-476.
- Isblster GK, Page CB (2013) Drug induced QT prolongation: The measurement and assessment of the QT interval in clinical practice. Br J Clin Pharmacol 76: 48-57.
- Vieweg VR, Hasnain M, Howland RH, Clausen T, Koneru JN, et al. (2013) Methadone, QTc interval prolongation and torsade de pointes: Case reports offer the best understanding of this problem. Ther Adv Psychopharmacol 3: 219-232.
- Vallejo CN, Rodriguez PD, Sanchez HA, Tornos Mas MP, Ribera E, et al. (2002) Ventricular tachycardia and long QT associated with clarithromycin administration in a patient with HIV infection. Rev Esp Cardiol 55: 878-881.
- Hiemke C, Bergemann N, Clement HW, Conca A, Deckert J, et al. (2018) Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: Update 2017. Pharmacopsychiatry 51: 9-62.
- Leavitt SB (2003) Addiction treatment forum: Methadone dosing and safety in the treatment of opioid addiction. Clinco Communications Inc, Illinois, USA.
Citation: Veltri KT, Olsufka WA (2018) A Unique Case Presentation of Methadone Toxicity without QTc Interval Prolongation despite Patient Risk Factors. J Clin Stud Med Case Rep 5: 057.
Copyright: © 2018 Keith T Veltri, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

