
Accuracy of Continuous Monitoring of Haemoglobin Concentration after In-Vivo Adjustment
*Corresponding Author(s):
Rosanna Carmela De RosaAorn Dei Colli, Anaesthesia And Intensive Care Monaldi Hospital, Via Leonardo Bianchi, Naples, Italy
Tel:+39 3386623791,
Email:rosderosa.rdr@gmail.com
Abstract
During open aortic aneurysm repair, continuous, non-invasive measurementof Haemoglobin concentration (SpHb), provided by Radical 7 device (Masimo Corp, Irvine, CA, USA) may be advantageous for assessing acute anaemia and improve the ability of the anesthesiologist to decide when to initiate blood transfusion. A strategy to improve the device’s accuracy is the in-vivo adjustment (AdHb) using a haemoglobin value provided by Blood Gas Analyser (BGA).
In this retrospective analysis, we hypothesised that adjustment using the first haemoglobin value provided by BGA could increase the accuracy of Radical 7 device when compared with the standard value provided by the laboratory (Lab). Data were analysed using Pearson’s correlation and Bland-Altman plots and accuracy and precision were calculated. We calculated the percentage of outliers (difference value with Lab greater than ± 1 g/dL) for SpHb, AdHb and BGA. The difference of outliers between SpHb and AdHb were analysed by the chi-squared test. Data were compared and analysed using Student t-test. A p-value
Two hundred-eighty-eight haemoglobin values concentration were obtained for every method of measurements in 72 patients scheduled for open abdominal aortic elective surgery. The proposed adjustment method for SpHb, when compared with Lab, reduced the numbers of the outlier (16.0 % for AdHb vs 77.4% for SpHb, p-value <0.001) and reduced the bias (-1.45 g/dL for SpHb to -0.38 g/dL for AdHb, p-value <0.0001), but it was associated with a deterioration of precision (0.49 g/dL for SpHb to 0.75 g/dL for AdHb) with wide limits of agreement (-2.41 to -0.48 for SpHb and -1.86 to 1.09 g/dL for AdHb).
During an acute bleeding episode, SpHb values should be interpreted with caution and compared with other quickly available methods such as co-oximeter. Further studies are needed.
Keywords
Aortic surgery; Haemoglobin; In-vivo adjustment; Noninvasive monitoring
INTRODUCTION
During open aortic aneurysm repair, patients can develop anaemia due to hemorrhagic episodes and/or hemodilution. Non-invasive, continuous monitoring of Haemoglobin concentration (SpHb) may be advantageous for assessing acute anaemia and may improve the ability of the anesthesiologist to decide when to initiate blood transfusion.
Radical 7 device (Masimo Corp, Irvine, CA, USA) pulse CO-Oximetry, based on multiwavelength technology, provides continuous, non-invasive measurement of SpHb. SpHb technology has been tested in various clinical scenarios and two metanalyses concluded with an alert about clinical decision based on these devices [1,2]. A strategy to improve the accuracy of SpHb is the in-vivo adjustment using a haemoglobin value provided by invasive methods like a laboratory or CO-oximetric method [3-6]. However, central laboratory measurement requires 30-60 min before the result is available, while a rapid Hb value, obtained within 1-2 minutes, can be provided by CO-oximetric method, the Blood Gas Analyser (BGA), and this value could be used to improve SpHb performance after in-vivo adjustment SpHb.
In this retrospective analysis, we hypothesised that adjustment using the Hb value provided by BGA measurement from arterial blood sampled at the first SpHb measurement would increase the accuracy (precision) of the monitor when compared with the value provided by the standard reference laboratory method.
MATERIALS AND METHODS
For this retrospective analysis, we used data collected from 72 patients scheduled for elective open abdominal (sub-renal) aortic aneurysm repair in a single surgery centre (AORN deiColli, Monaldi Hospital, Naples). The local ethics committee approved data collection (University of Naples Luigi Vanvitelli, protocol number 544/2017), and every patient signed written informed consent.
Intraoperative standard monitoring consisted of ECG, SpO2, end-tidal CO2 (EtCO2), non-invasive blood pressure and diuresis. In addition, we monitored invasive blood pressure, Bispectral Index (BIS), neuromuscular blockade (Tof Cuff®), skin temperature, Central Venous Pressure (CVP).
For SpHb measurement, we used the Masimo Rainbow SET Radical 7®: the model was VKF-RAD7A, and the sensor was composed of Disposable Optical Sensor (DOS-Rainbow R2-25a) and Reusable Optical Sensor (ROS- Rainbow R2-25r). We applied the Masimo platform (with an optical shield to avoid optical interference) to the middle or index finger of the patient's hand, free from venous and arterial lines or monitoring devices, in absence of nail polish or acrylic nails.
All patients, after premedication with intra-muscular Morphine (10 mg), received general anaesthesia. We used Propofol (0.7 mg/kg), Midazolam (0.07 mg/kg), Sufentanil (0.4 mcg/kg) for induction and Rocuronium (0.6 mg/kg) for neuromuscular blockade.
During the maintenance of anaesthesia, we used exhaled Desflurane 3%, keeping BIS value between 40-60%, Remifentanil infusion (0.15 mcg/kg/min), and next Rocuronium administration (0.2 mg/kg) guided by neuromuscular monitoring. We conducted mechanical ventilation (Zeus Drager) in volumetric mode (Tidal Volume 6 mL/kg ideal body weight, PEEP 5cm H2O, Respiratory Rate 12-16/min to obtain a PCO2 35-40 mmHg and FiO2 50% by a closed and Auto Flow system).
To avoid changes in the haemoglobinemia value due to hyper or hypohydration, we followed a Goal-Directed Fluid Therapy Protocol (GDFTP). We used EV 1000™ platform (Edwards Lifesciences) as haemodynamic monitoring. We analysed the parameters with pulse-contour monitoring by FloTrac or Volume View. For FloTrac module, we performed the Allen test to evaluate the state of collateral circulation at the level of the hand and then we placed the intra-arterial catheter in the radial artery. For volume view module, the catheter was placed in the brachial artery and, during the surgery, the anaesthesiologist monitored the presence of distal arterial pulse by pulse oximetry probe and the appearance of signs of ischemia at the level of the hand. The target of our GDFTP was the optimisation of the Stroke Volume Index (SVI) [7].
We administered a basic crystalloid infusion of 1-3 ml/kg/h. After induction of general anaesthesia, in stable haemodynamic condition, we calculated maximal SVI following this protocol: we noted the first value of SVI as baseline, we practised a bolus of colloids in 5-10 min (Fluid challenge 3 ml/kg) and marked the new SVI value so that we calculated the variation respect to the baseline value (ΔSVI%). In case of fluid responsiveness status (ΔSVI% >10%), we repeated the fluid challenge after 10-15 minutes, up to a ΔSVI% <10%.
The last SVI value with fluid responsiveness represented the SVI max and the SVI trigger was SVI max -10% SVmax. We provided a fluid challenge only if the SVI was under the SVI trigger. We administered continuous infusion of Fenoldopam (0.1 mcg/kg/min) for renal protection. If the mean arterial pressure was <65 mmHg (e.g. after declamping of the aorta) despite the optimization of SVI, we used noradrenaline bolus iv (0.01-0.02 mg) to maintain adequate organ perfusion [8].
We used the HOTLINE® blood and fluid warmer to keep the temperature of infusions between 37-39°C. We recorded total volumes of crystalloids, colloids and blood products, the percentage of patients receiving blood products and vasopressors drugs. For each patient, we collected three simultaneous Hb measurements: laboratory (Lab, considered as standard reference method) BGA and SpHb.
For invasive methods, BGA with the GEM Premier 4000 Blood Gas Analyzer (Instrumentation Laboratory) and conventional laboratory analysis with the COULTER® LH 780 (Beckman Coulter, Inc.), we took a sample of 5 mL of blood from the arterial line, after discarding 10 mL of blood. The arterial line was 44 cm in length with a dead volume of about 1 mL. At the same time, we registered the SpHb value.
For each patient, we performed four measurements of Hb concentration at specific times: after the induction of anaesthesia (T0), pre and post aortic cross-clamping (T1 and T2), and at the end of surgery (T3). T0 was also the start time of GDFTP. The volume of blood in the suction chambers represented an estimation of total blood loss. We adopted blood salvage. We performed blood transfusion if Hb value was ≤8 gr/dL in patients without comorbidities or ≤9 gr/dL in patients with cardiac disease or with active bleeding.
The anesthesiologist was the same in all interventions, and the value obtained from BGA and not the value of SpHb was the parameter for intraoperative clinical decision. For in-vivo adjustment, we used the difference between the T0 values of SpHb and BGA. We used a formula to calculate adjusted SpHb (AdHb):
AdHb = SpHb - (T0 SpHb - T0 BGA).
The values of SpHb and AdHb were compared with Lab values. The objective was to compare Hb value provided by standard referment method (Lab) with, respectively, SpHb, AdHb and BGA. Data were analysed using Pearson’s correlation and Bland-Altman plots.Bias and precision were calculated. The sample size was set to a minimum of 150 measurements according to Bland and Altman [9-11] recommendations for precision of 0.3 SD of the 95% CI of the limits of agreement. We calculated the percentage of outliers for SpHb, AdHb and BGA, which were defined as difference value with Lab greater than ± 1 g/dL. We used the chi-squared test to analysethe difference of outliers between SpHb and AdHb. Data were compared and analysed using Student t-test. A p-value <0.05 was considered significant.
RESULTS
We included in the present study seventy-two patients scheduled for open abdominal aortic elective surgery.We presented the main patients characteristics, surgical and intraoperative data in table 1.
|
Patient characteristics, surgical and intraoperative data |
|
|
Patients |
72 |
|
Sex (M/F) |
65/7 (90.3/9.7%) |
|
Age (years) |
68.8 (7.7) |
|
Weight (kg) |
75.9 (12.9) |
|
Height (cm) |
169.4 (6.8) |
|
Coronarydisease |
28 (38.9%) |
|
CABG*/PTCA† |
20 (27.8%) |
|
Cardiomyophaty |
15 (20.8%) |
|
Hypertension |
59 (81.9%) |
|
Diabetes |
16 (22.2%) |
|
Chronicrenal failure |
11 (15.3%) |
|
COPD‡ |
32 (44.4%) |
|
TIA§/Ictus |
7 (9.7%) |
|
ASA I |
0 (0%) |
|
ASA II |
4 (5.5%) |
|
ASA III |
68 (94.5%) |
|
Duration of surgery (min) |
231 (61) |
|
Crystalloids IV (mL) |
647.2 (238.8) |
|
Colloids IV (mL) |
795.1 (282.5) |
|
Vasopressors use in patients |
42 (58.3%) |
|
Patients transfused with PRBCs¶ |
12 (16.7%) |
|
PRBCs volume transfused (mL) |
700 (432.8) |
|
Patients transfused with intraoperative blood salvage |
69 (95.8%) |
|
Intraoperative blood salvage transfused (mL) |
623.2 (276.2) |
|
Patients transfused with FFP** |
3 (4.2%) |
|
FFP volume transfused (mL) |
695 (335.9) |
|
Total blood loss (mL) |
1103.5 (498.8) |
|
Diuresis (mL) |
627.1 (257.4) |
|
Final Fluid Balance (mL) |
328.9 (372.8) |
Table 1: Patients characteristics, surgical and intraoperative data. Values expressed as number (proportion) and mean (SD).
*Coronary Artery Bypass Graft; †Percutaneous transluminal coronary angioplasty; ‡ Chronic obstructive pulmonary disease; § Transient Ischemic Attack; ¶ Packed red blood cells; ** Fresh Frozen Plasma
We used the Vascular-POSSUM score (V-POSSUM, http://www.riskprediction.org.uk/vasc-index.php) and ASA status to provide information on the risk of morbidity and mortality of all patients.
Two hundred-eighty-eight Hb values concentration were obtained for every methods of measurements. The Hb measurements ranged from 8.3 g/dL to 16.0 g/dL for Lab, from 7.5 g/dL to 16.5 g/dL for BGA, from 6.9 g/dL to 15.0 g/dL for SpHb and from 6.6 g/dL to 17.0 g/dL for AdHb. The mean and SD for methods were 12.62±1.59 g/dL for Lab, 12.01±1.61 for BGA, 11.16±1.56 g/dL for SpHb and 12.23±1.78 g/dL for AdHb.
Figures 1 and 2 showed, respectively the scatterplots, linear regression statistics and Bland-Altman bias analysis for SpHb and AdHb versus Lab measurements.Plot for BGA was not reported.
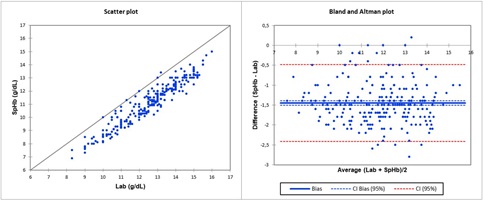
Figure 1: Laboratory total haemoglobin concentration (Lab) and continuous non-invasive Haemoglobin concentration (SpHb): scatter and Bland-Altman plots. Equation regression line is SpHb = -0.6156+0.9338*Lab.
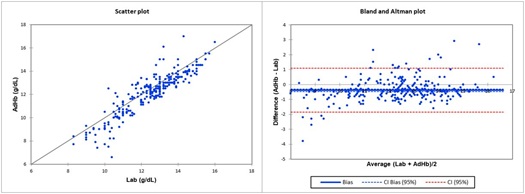
Figure 2: Laboratory total haemoglobin concentration (Lab) and adjustedcontinuous non-invasive Haemoglobin concentration (AdHb): scatter and Bland-Altman plots. Equation regression line is AdHb = -0.5426+1.0125*Lab.
Table 2 shows the percentage of outliers for both SpHb, AdHb and BGA, and a summary of linear regression and Bland-Altman Analysis.
|
|
|
Linear Regression vs Lab |
Bland-Altmann analysis vs Lab |
|||
|
|
Outliers |
Correlation Coefficient |
P-Value |
Mean Bias g/dL |
Precision g/dL |
Limits of agreement g/dL |
|
SpHb |
77.4% |
0.952 |
<0.0001 |
-1.45 |
0.49 |
-2.41 to -0.48 |
|
AdHb |
16.0%* |
0.907 |
<0.0001 |
-0.38 |
0.75 |
-1.86 to 1.09 |
|
BGA |
6.60% |
0.979 |
<0.0001 |
-0.61 |
0.33 |
-1.26 to 0.04 |
Table 2: Summary of correlation analysis and Bland-Altman comparison using laboratory total haemoglobin concentration (Lab, n=288), continuous non-invasive total haemoglobin concentration (SpHb, n=288), adjusted SpHb (AdHb, n=288) and BGA (n=288). Values are proportion or number. *P-value
The adjustment method was associated with a statistically significative reduction in the percentage of outliers compared to SpHb (16.0 % vs 77.4%, p-value <0.001) with a small decrease in correlation coefficient, from 0.952 for SpHb to 0.907 for AdHb, while the percentage of outliers for BGA was 6.6%, with a correlation coefficient of 0.979.
When compare with Lab values, adjustment methoddetermined a decrease inbias from -1.45 g/dL for SpHb to -0.38 g/dL for AdHb (p-value <0.0001). The precision worsened from 0.49 g/dL for SpHb to 0.75 g/dL for AdHb, while precision of BGA was 0.33 g/dL. The limits of agreements for SpHb and AdHbwere wide, respectively, -2.41 to -0.48 g/dL and -1.86 to 1.09 g/dL. Limits of agreement for BGA were -1.26 to 0.04 g/dL.
DISCUSSION
During acute bleeding, conventional haematological method (Coulter counter) or co-oximetry are the tools for measuring Hb value. However, these methods are time-consuming, costly, invasive, intermittent. Instead of ideal Hb monitoring should be non-invasive, rapid/continuous and easy. Our device for non-invasive intraoperative Hb monitoring was Radical 7 device (Masimo Corp, Irvine, CA, USA) pulse CO-Oximetry. The transcutaneous spectrophotometry-based technology allows continuous Hb determination. Light received by the photodetector, after passing through the measurement site, generates electrical signals that, processed by advanced algorithms, provides an estimation of Hb based on its absorbance characteristics. An optical shield that covers the sensor prevents optical interference by other light sources. In two literature metanalyses [1,2] about accuracy and precision of SpHb, the authors concluded with an alert about clinical decision based on continuous non-invasive haemoglobin devices, with the lowest precision and wider 95% limits of agreement for Masimo.To improve the performance of SpHb, some authors have proposed calibration methods based on in-vivo Hb value obtained from other methods [3-6].
In this retrospective analysis, performed on patients scheduled for open abdominal aortic surgery in general anaesthesia, the difference between the initial values for SpHb and BGA was used for the “in-vivo” adjustment, and then the values of SpHb and AdHb were compared with Lab values, considered as the gold standard. The proposed adjustment method was associated with a statistically significative reduction in the percentage of outliers compared to SpHb (16.0 % vs 77.4%, p-value <0.001) with a small decrease in correlation coefficient, from 0.952 for SpHb to 0.907 for AdHb. Adjustment method determined a decrease in bias from -1.45 g/dL for SpHb to -0.38 g/dL for AdHb. However, the precision worsened from 0.49 g/dL for SpHb to 0.75 g/dL for AdHb. For SpHb and AdHb, the limits of agreements were wide, respectively, -2.41 to -0.48 for SpHb and -1.86 to 1.09 g/dL for AdHb. Instead, BGA, when compared with laboratory measurement, presented a low rate of outliers (6.60%), with a correlation coefficient of 0.979, a bias of -0.61 g/dL, precision of 0.33 g/dL, with a limit of agreements -1.26 to 0.04 g/dL.
In the literature, authors proposed different in-vivo calibration methods to improve performance of SpHb, using different reference methods as laboratory or co-oxymeter. Isosu et al., [5] examined the effects of adjustment methods for SpHb based on satellite co-oximeter in 20 Japanese surgical patients, for a total of 92 blood samples collected. Bland-Altman analysis showed a bias of 0.2±1.5 g/dL, with limits of agreement of -2.8 to 3.1 g/dL before in-vivo adjustment and -0.7±1.1 g/dL with limits of agreement of -2.8 to 1.4 g/dL after in vivo adjustment. Linear regression analysis resulted in a correlation coefficient of 0.76 prior to in-vivo adjustment and 0.90 after in-vivo adjustment. This study showed that in-vivo adjustment improved the agreement of SpHb withsatellite co-oximeter, considered as reference methods in these patients. In vivo adjustment may represent a signi?cant advance in noninvasive monitoring of haemoglobin as it improved the bias, precision, limit of agreement and correlation coef?cient compared to satellite co-oximeter measurements.
Miyashita et al., [3] performed a study to evaluate a simple method for adjusting in-vivo SpHb values measured in patients undergoing elective abdominal surgical procedures (19 patients) based on calibrated blood gas analyser. The author used two SpHb sensors: R1-25 and R2-25a. Seventy-three haemoglobin measurements were obtained by R2-25a sensor. When compared to calibrated blood gas analyser, in-vivo adjustment method significantly reduced the percentages of outliers in AdHb values (13.6%, 32.8% for SpHb), improved the correlation coefficient (0.93, 0.83 for SpHb), mean bias (0.16 g/dL vs 0.68 g/dL for SpHb), precision (0.77 g/dL vs 1.02 g/dL for SpHb) and limits of agreement (AdHb -1.35 to 1.67 g/dL, SpHb -1.31 to 2.67 g/dL).
Frasca et al., [6] performed a prospective, observational study in patients undergoing elective major surgery with significant blood loss expected (41 patients, 173 haemoglobin measurements) to compare the accuracy of SpHb adjusted in-vivo with the mean of three arterial HemoCue measurements and with laboratory values. In-vivo adjustment by HemoCue measurements improved precision (1.4 for SpHb, 1.1 for adjusted Hb), without significant impact on SpHb bias, which remained close to 0 g/dL. In-vivo adjustment by laboratory values provided the same results.
About the use of laboratory values for in-vivo adjustment, Patino et al., [4] performed a prospective observational study in pediatric population scheduled for major surgical procedures associated with substantial blood loss (76 subjects, 156 SpHb-Hb data pairs). A retrospective analysis of bias and precision of SpHb to Hb obtained from laboratory measurement after in vivo adjustment of the SpHb values allowed the user to adjust the SpHb value manually to match an initial laboratory value so that all subsequent SpHb values were offset by the bias entered by the user. Absolute bias ± precision of SpHb to Hb was 0.4±1.3 g/dL. The retrospective in-vivo adjustment resulted in a bias ± precisionof 0.1±1.2 g/dL. Bland-Altman analysis displayed limits of agreement of -2.0 to 3.2 g/dL before and -2.4 to 2.2 g/dL after in-vivo adjustment. The study concluded that an adjustment of SpHb values by the first bias to Hb slightly improved the agreement of SpHb with laboratory values, yet one-third of the values can still reach a 2g/dL difference with Hb over time.
In these studies, calibration and reference methods were the same. Instead, we used the first haemoglobin value provided by BGA to calibrate SpHb, and then we compared the results with laboratory values, considered as the reference methods. This aspect could explain the different results obtained in the present study, particularly about precision.
This study presents some limitations. First, this study is a retrospective analysis, so it is not possible to know whether the proposed in vivo adjustment method is associated with a change in the clinical decision-making about blood transfusions. Second, we did not evaluate whether this method is related to a change in the trending ability of SpHb. However, in our previous study [12], SpHb showed to be useful to assess the trending of the Hb concentration when compared with laboratory measurement in patients scheduled for elective abdominal aortic surgery. Finally, this study did not evaluate the interaction between Perfusion Index (PI) and SpHb accuracy. The PI is an indirect indicator of peripheral circulation and is obtained by calculating the ratio of the amplitude of the arterial pulse detected by the sensor to the amplitude of non-pulsatile factors such as the veins, bones, or subcutaneous fats [13]. In the present study, two factors could alter the PI and, then, the SpHb accuracy: inhalation anaesthetic agents and noradrenaline bolus. The use of inhalation anaesthetic agentscauses arteriolar dilation with alteration in PI value. However, Park et al., [14] demonstrated that the useof sevoflurane for general anaesthesiaincreasedthe PI with an improvement in SpHb accuracy. According to our GDFTP, if MAP was < 65 mmHg, despite fluid challenge, anesthesiologist administered noradrenaline bolus (0.01-0.02 mg) to maintain adequate organ perfusion [8]. Saito et al., [15], in a randomised controlled trial, demonstrated that the increase in PI during induction of anaesthesia by maintaining arterial pressure through administration of a vasoconstrictor improved the agreement between laboratory haemoglobin values and SpHb. Based on these evidences, we believe that the aspects mentioned above could minimally interfere with our analysis. However we advocate the need of further studies investigating the interaction between PI, SpHb accuracy and GDFTP application.
CONCLUSION
In conclusion, the proposed adjustment method for SpHb, when compared with laboratory values, reduced the numbers of the outlier and reduced the bias. However, it was associated with a deterioration of precision with wide limits of agreement. During an acute bleeding episode, the data derived by this device should be interpreted with caution and compared with other quickly available methods such asco-oximeter. Further well-designed studies are needed.
ACKNOWLEDGEMENTS
None
AUTHOR CONTRIBUTIONS
Conception and Design, R.C. De Rosa; Data Collection, R.C. De Rosa; Statistical analysis and interpretation, R.C. De Rosa, A. Romanelli; Writing-Original draft preparation, R.C. De Rosa, A. Romanelli; Writing-Review & editing, R.C. De Rosa, A. Corcione; Supervision R.C. De Rosa; Project Administration, R.C. De Rosa.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
FUNDING STATEMENT
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
REFERENCE
- Kim SH, Lilot M, Murphy LS, Sidhu KS, Yu Z, et al. (2014) Accuracy of continuous noninvasive hemoglobin monitoring: a systematic review and meta-analysis. Anesth Analg 119: 332-346.
- Hiscock R, Kumar D, Simmons SW (2015) Systematic review and meta-analysis of method comparison studies of Masimo pulse co-oximeters (Radical-7™ or Pronto-7™) and HemoCue® absorption spectrometers (B-Hemoglobin or 201+) with laboratory haemoglobin estimation. Anaesth Intensive Care 43: 341-350.
- Miyashita R, Hirata N, Sugino S, Mimura M, Yamakage M (2014) Improved non-invasive total haemoglobin measurements after in-vivo Anaesthesia 69: 752-756.
- Patino M, Schultz L, Hossain M, Moeller J, Mahmoud M, et al. (2014) Trending and accuracy of noninvasive hemoglobin monitoring in pediatric perioperative patients. Anesth Analg 119: 920-925.
- Isosu T, Obara S, Hosono A, Ohashi S, Nakano Y, et al. (2013) Validation of continuous and noninvasive hemoglobin monitoring by pulse CO-oximetry in Japanese surgical patients. J Clin Monit Comput 27: 55-60.
- Frasca D, Mounios H, Giraud B, Boisson M, Debaene B, et al. (2015) Continuous monitoring of haemoglobin concentration after in-vivo adjustment in patients undergoing surgery with blood loss. Anaesthesia 70: 803-809.
- Johnson A, Mohajer-Esfahani M (2014) Exploring hemodynamics: a review of current and emerging noninvasive monitoring techniques. Crit Care Nurs Clin North Am 26: 357-375.
- Salmasi V, Maheshwari K, Yang D, Mascha EJ, Singh A, et al. (2017) Relationship between Intraoperative Hypotension, Defined by Either Reduction from Baseline or Absolute Thresholds, and Acute Kidney and Myocardial Injury after Noncardiac Surgery: A Retrospective Cohort Analysis. Anesthesiology 126: 47-65.
- Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1: 307-310.
- Bland JM, Altman DG (2007) Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat 17: 571-582.
- Bland JM, Altman DG (2012) Agreed statistics: measurement method comparison. Anesthesiology 116: 182-185.
- De Rosa RC, Romanelli A, Calabria M, Abbate R, Montesano R, et al. (2019) Continuous Noninvasive Haemoglobin Monitoring in Vascular Surgery within the Goal-Directed Therapy Protocol. CEACR 1: 1-15.
- Nguyen BV, Vincent JL, Nowak E, Coat M, Paleiron N, et al. (2011) The accuracy of noninvasive hemoglobin measurement by multiwavelength pulse oximetry after cardiac surgery. Anesth Analg 113: 1052-1057.
- Park SG, Lee OH, Park YH, Shin HY, Kang H, et al. (2015) The changes of non-invasive hemoglobin and perfusion index of Pulse CO-Oximetry during induction of general anesthesia. Korean J Anesthesiol 68: 352-357.
- Saito J, Kitayama M, Amanai E, Toyooka K, Hirota K (2017) Impact of acute changes in perfusion index and blood pressure on the accuracy of non-invasive continuous hemoglobin concentration measurements during induction of anesthesia. J Anesth 31: 193-197.
Citation: De Rosa RC, Romanelli A, Corcione A (2020) Accuracy of Continuous Monitoring of Haemoglobin Concentration after In-Vivo Adjustment. J Angiol Vasc Surg 5: 040.
Copyright: © 2020 Rosanna Carmela De Rosa, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

