
Activity of Amomum tasao-ko Fruits Essential Oil against Methicillin-Resistant Staphylococcus aureus in vivo
*Corresponding Author(s):
Min DaiSichuan Provincial Engineering Laboratory For Prevention And Control Technology Of Veterinary Drug Residue In Animal-origin Food, Chengdu Medical College, School Of Laboratory Medicine, Chengdu Medical College, Chengdu 610083, China
Tel:+86 18030607566,
Fax:+86 02862739526
Email:daimin1015@cmc.edu.cn
Cheng Peng
State Key Laboratory Of Systematic Research And Exploitation Of Traditional Chinese, College Of Pharmaceuticals, Chengdu University Of Traditional Chinese Medicine, Chengdu 611137, China
Tel:+86 02862739532,
Fax:+86 02862739532
Email:pengchengchengdu@126.com
Abstract
Methicillin-Resistant Staphylococcus Aureus (MRSA) is one of the most serious health concerns bacterial owing to its incidence and the severity of the associated infections. It is necessary to find new chemical compounds with anti-MRSA activity. A model of MRSA infection was prepared by intraperitoneal injection of MRSA. A. tasao-ko essential oil was used for pretreatment and treatment of MRSA-infected animals and the 50% effective dose (ED50) was calculated according to improved Karber’s method respectively. The levels of serum interleukin (IL)-1β, IL-6, Tumor Necrosis Factor alpha (TNF-α), Malondialdehyde (MDA), Glutathione Peroxidase (GSH-Px) and Hydroxyl Radical (·OH) were measured using dedicated assay kits. The statistical analysis of inflammatory factors and oxidative stress factors was tested using one-way ANOVA computed by SPSS software. The ED50 values for the prevention and treatment of MRSA infection in A. tasao-ko essential oil were 0.15 g/kg and 0.18 g/kg, respectively. A. tasao-ko essential oil significantly reduced the inflammatory factors (IL-1β, IL-6, TNF-α), oxidative stress factors (MDA, ·OH) and increased the GSH-Px content in mice infected with MRSA. In the high-dose group, 7 days after treatment, the cytokine contents have no difference with control group (P > 0.05). A. tasao-ko essential oil exhibited anti-MRSA effect in vivo and can also ameliorate the abnormal changes in inflammatory factors and oxidative stress that arise from MRSA.
Keywords
Amomum tasao-ko essential oil; Anti-Inflammatory; ED50; MRSA; Oxidative stress
ABBREVIATIONS
- OH: Hydroxyl radical
MRSA: Methicillin-Resistant Staphylococcus Aureus
SPF: Specific Pathogen-Free
MLD: Minimum Lethal Dose
PH: Preventative High dose
PM: Preventative Medium dose
PL: Preventative Low dose
TH: Treatment High dose
TM: Treatment Medium dose
TL: Treatment Low dose
TNF-α: Tumor Necrosis Factor alpha
IL: Interleukin
GSH-Px: Glutathione Peroxidase
MDA: Malondialdehyde
PVL: Panton-Valentine Leucocidin
ROS: Reactive Oxygen Species
GSH: Glutathione
INTRODUCTION
In the early 1940s, before the introduction of penicillin for the treatment of the disease, blood infection caused by Staphylococcus aureus was usually fatal. The use of penicillin in controlling the infection resulted in the emergence of β-lactamase-producing S. aureus [1,2]. Thus, new drugs were developed against S. aureus infection, such as methicillin and oxacillin. Methicillin-Resistant S. Aureus (MRSA) has appeared as the abuse of methicillin [3]. At present, MRSA is one of the most important bacterial pathogens based on its incidence and the severity of the associated infections [4]. MRSA can transmit between humans and animals [5] and infection occurs mainly through surgical wounds, pneumonia, and trauma [6-9]. Because of the increased susceptibility and drug resistance of MRSA, it is necessary to develop new anti MRSA drugs.
Botanical medicines were widely used in the prevention and treatment of various diseases for a long time [10,11]. Amomum tsao-ko, which is widely distributed in the south-west regions of China and Korea, is used for treatment of hemorrhoids, flatulence, vomiting, diarrhea, malaria, throat infections, stomach disorders, dyspepsia, nausea, and abdominal pain in China. It also is used in many culinary dishes, such as Chinese medicinal soup dishes, herbal beef dishes, and hotpots [12-15]. Several researchers have conducted pharmacological studies on A. tasao-ko oil and identified that it has anti-inflammatory, antioxidant, antitumor, antihepatitis B, antimicrobial, anti-apoptosis activities [16-21]. Our previous studies found that A. tasao-ko essential oil had strong anti-MRSA activity in vitro [22], so this study focused on the anti-MRSA activity of A. tasao-ko essential oil in vivo. We observed the protective effect of A. tasao-ko essential oil on mice by intraperitoneal injection of MLD MRSA. The in vivo anti-MRSA mechanism due to its bacterial inhibitory activity and decrease the inflammatory level and oxidative stress in mice. The ED50 also calculated and these data make a foundation for further development of A. tasao ko essential oil.
MATERIALS AND METHODS
Materials
Vancomycin hydrochloride for injection was obtained from VIANEX S.A. (PLANT C) (Greece). Nutrient agar was purchased from Beijing Aobo Star Bio-technology Co., Ltd (China). Gastric mucin was obtained from Sigma-Aldrich (United States). Sodium chloride injection (0.9%) was purchased from Sichuan Kelun Pharmaceutical Co., Ltd (China). The inject able soybean oil was obtained from Zhejiang Tian Yu Shan Pharmaceutical Oil Co., Ltd (China) and MRSA was obtained from the American Type Culture Collection (ATCC 43300).
The extraction of Amomum tsao-ko essential oil
A. tsao-ko (1000 g) was purchased from Tong Ren Tang Co., Ltd (Beijing, China) and identified by Prof. Min Li at the Chengdu University of Traditional Chinese Medicine. The extract and GC-MS analysis method of A. tsao-ko essential oil were described in our previous study, and a total of 32 compounds, representing 81.89% of the essential oil, were identified, the major compounds of essential oil were 1,8-cineole (25.92%) and geraniol (13.69%) [23]. Air-dried A. tsao-ko (200 g) was subjected to hydro distillation for 4 h in a modified Clevenger-type apparatus with a water-coupled oil receiver to obtain the essential oil (approximately 4 mL). The oils were dried over sodium sulfate and stored in air-tight glass bottles in a refrigerator prior to experiments.
Animals
All animal experiments were performed in accordance with the guidelines and approved by Chengdu Medical College Animal Care and Use Committee. A total of 160 Specific Pathogen-Free (SPF) strains of both male and female KM mice (weight: 18-22 g) were purchased from Chengdu Institute of Biological Products Co., Ltd (Chengdu, China) and acclimatized for 3 days Zprior to the start of the experiment. All animals were housed in cages (five animals of the same sex per cage) maintained at a constant temperature (25 ± 2 °C) and humidity (50 ± 10%) and given free to access food and water throughout the study.
Methods
The experiments on antibacterial activity were conducted according to a previously described method, with a little modification [24]. Briefly, the mice were randomly divided into 16 groups (n = 10). The blank (control) group and the model group were infected with 6×104 CFU/10gMRSA , two positive control groups (the mice were injected with vancomycin hydrochloride (0.30g/kg) before or after infection), gastric mucin control group (in which the mice were injected with the same amount of 5% gastric mucin), the oil solvent control group (in which the mice were injected with the same vehicle oil for injection), and the groups treated with A. tsao-ko essential oil. The A. tsao-ko essential oil was diluted with inject able soybean oil at different concentrations, with five pretreatment groups and five treatment groups (Figure 1). For the five experimental pretreatment groups, all the experimental doses design is based on our preliminary experiment and the ineffective and the full cure dose were 0.05, 0.07, 0.16, 0.24 and 0.37g/kg respectively. For the five experimental treatment groups, the dosage of reagents was 0.08, 0.13, 0.20, 0.30, 0.47g/kg. After the end of the experiment, blood samples were collected from the surviving mice, and labeled.
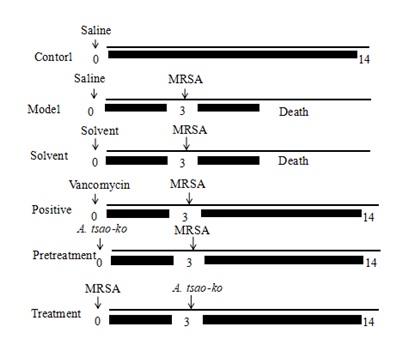 Figure 1: Schematic diagram of administration scheme.
Figure 1: Schematic diagram of administration scheme.
Determination of cytokines
Blood obtained from the mice was centrifuged and the supernatant was separated to measure the levels of three cytokines Tumor Necrosis Factor alpha (TNF-α), Interleukin-6 (IL-6), and Interleukin-1 beta (IL-1β) by using ELISA kits (Elabscience Biotechnology Co., Ltd, China). The quantification of cytokines and the experiments were conducted in accordance with the manufacturer’s instructions. And the data were recorded by Thermo Scientific™ Varioskan™ LUX multimode microplate reader. In the pretreatment groups, the 0.37 g/kg, 0.24 g/kg and 0.16 g/kg dose groups were labeled as Preventative High dose (PH), medium dose (PM), low dose (PL), respectively. In the treatment groups, the 0.46 g/kg, 0.30 g/kg and 0.20 g/kg dose groups were labeled as high dose (CH), medium dose (CM) and low dose (CL), respectively.
Determination of oxidative factors
The activity of Glutathione Peroxidase (GSH-Px) and the contents of Malondialdehyde (MDA), Hydroxyl radicals (·OH) in serum was determined according to the instructions of kits. Bloods were collected and its content was measured by GSH kits, MDA kits, and ·OH kits (Naijing Jiancheng Bioengineering Institute). And the data were recorded by Thermo Scientific™ Varioskan™ LUX multimode microplate reader.
Statistical analysis
The ED50 of A. tsao-ko essential oil against MRSA was calculated by Karber’s method. Other statistical analyses were analyzed by using SPSS. One-way ANOVA was used to evaluate the effects of each variable and to determine the statistical significance. Values of P < 0.05 and P < 0.01 were considered significant and very significant, respectively.
RESULTS
Prophylactic activity of Amomum tsao-ko oil
In a pilot study, we discussed the effect of pretreatment time of A. tsao-ko essential oil on the survival rate of mice; we found that there was no significant difference in injection with A tsao-ko oil for 5 days or 3 days. Thus, we selected the pretreatment time was 3 days. A. tsao-ko essential oil showed potent in vivo activity against the injections of MRSA (Figure 2) as both a preventive and a treatment. The survival rates of mice raised along with the incensement of the injected doses, and the calculated ED50 was 0.15 mg/kg. When the injected dose of A. tsao-ko essential oil was 0.37mg/kg, it can protect the entire mouse from MRSA infection.
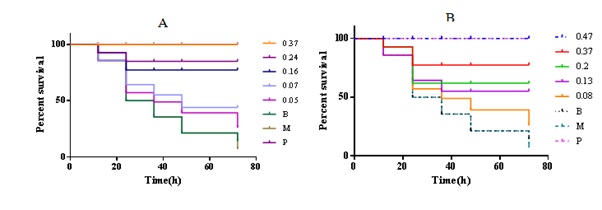 Figure 2: Survival rate of mice.
Figure 2: Survival rate of mice.
Notes: A: In vivo anti-MRSA activity of Amomum tsao-ko essential oil pretreatment administered by intramuscular injection; B: In vivo anti-MRSA activity of Amomum tsao-ko essential oil treatment administered by intramuscular injection. B, blank group; M, model group; P, positive group.
Treatment activity of tsao-ko oil
For the treatment of MRSA infection, this oil demonstrated a good therapeutic effect and showed a significant dose-dependent relationship (Figure 2B). At different injected doses of 0.47 g/kg, 0.30 g/kg, 0.20 g/kg, 0.13 g/kg, and 0.08 g/kg for injection, the survival rates of mice infected with MRSA were 100, 70, 50, 40 and 10%. By using Karber’s method, the calculated ED50 was 0.18 g/kg.
The effect of tsao-ko essential oil on the inflammatory factors in mouse serum
The effect of A. tsao-ko essential oil on the levels of the inflammatory cytokines of TNF-α, IL-6, and IL-1β was measured in the serum of survived mice by using enzyme-linked immunosorbent assays. As shown in Figure 3, after MRSA infection, the level of TNF-α, IL-6 and IL-1β increased significantly. After the treatment of A. tsao-ko essential oil the contents of TNF-α, IL-6, and IL-1β was reduced significantly. We also found that the contents of TNF-α, IL-6 and IL-1β decreased along with the increasement of the injection dose of A. tsao-ko essential oil. There was no significant difference in the contents of TNF-α, IL-6 and IL-1β between the high dose group and the control group regardless of the methods of administration. These results indicated that A. tsao-ko essential oil showed obvious anti-inflammatory properties except for the antimicrobial activities.
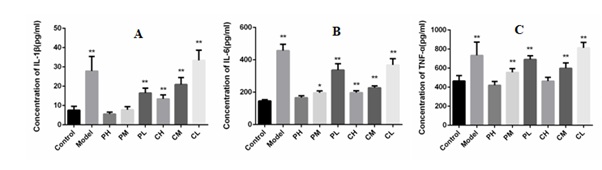 Figure 3: Effect of Amomum tsao-ko essential oil on inflammatory factors in MRSA-infected blood.
Figure 3: Effect of Amomum tsao-ko essential oil on inflammatory factors in MRSA-infected blood.
Notes: A: IL-1β factor blood concentration; B: IL-6 factor blood concentration; C: TNF-α factor blood concentration. Compared with the blank control group, * <0.05, ** <0.01.
Effects of Amomum tsao-ko essential oil on oxidative stress
Oxidative stress refers to the exposure of cells to high concentrations of reactive oxygen species caused by cell damage. As shown in figure 4, A. tsao-ko essential oil increased the activity of GSH-Px, reduced the levels of MDA and hydroxyl radicals, especially the activity of GSH-Px after high dose of A. tsao-ko essential oil treatment, was increased significantly when compared to the control group, which indicates that the A. tsao-ko essential oil showed antioxidant activity except for the antimicrobial activity. The activity of GSH-Px and the contents of MDA and hydroxyl radicals showed significant dose-dependent effect. High doses restored oxidative stress markers to normal levels.
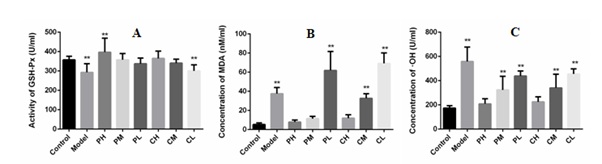 Figure 4: Effect of Amomum tsao-ko essential oil on oxidative stress factors.
Figure 4: Effect of Amomum tsao-ko essential oil on oxidative stress factors.
Notes: A: concentration of GSH-Px in the blood; B: concentration of MDA in the blood; C: concentration of ·OH in the blood. Compared with the blank control group, * <0.05, ** <0.01.
DISCUSSION
S. aureus causes a wide range of infections, such as skin and soft tissue infections, implant infections, pneumonia, septicemia, and toxic shock syndrome, but can also be the source of more serious bacteremia [25,26]. In recent years, several researchers are looking for natural products as potential antibacterial agents in the treatment of MRSA infection. Several essential oils which was extracted from several plants has got researchers attention due to its excellent antimicrobial activity, such as patchouli, tea tree, geranium, lavender essential oils, citricidal and wilhelmsii essential oil [10,27]. These essential oils have good anti-MRSA activity in vitro but have no antibacterial activity in vivo. Compared with in vitro bioactivity study, in vivo bioactivity study is more instructive for clinical use, which is also the significance of this study. In this study, we explored the exact efficacy of A. tsao-ko essential oil against MRSA infection by establishing a model of systemic infection in mice. The result showed that both the prevention and treatment modes of administration resulted in a 100% survival rate. Studies have shown that the antibacterial activities of various plant extracts are related to their monomer components, for example, quinolone derivatives [28]. The main components in the essential oil of A. tsao-ko were 1,8-cineole (25.92%) and geraniol (13.69%) [23] and some research showed that geraniol was the main active ingredient [29-31]. In the follow-up studies, we will continue to focus on the anti-MRSA activity of the monomers in the essential oil of straw fruit and hopefully find more effective anti-MRSA drugs.
In previous studies, MRSA infection was reported on the surface of the body and can cause inflammation [32,33]. MRSA can express Panton-Valentine Leukocidin (PVL), which is an additional cause for concern [25], the infection often causes an inflammatory response. During the process of infection in diseases, the concentration of cytokines and disease severity and prognosis are related [34]. Among them, IL-6 is considered a sign of inflammatory response [35]. Compared with the blank group, the inflammatory factors of the model group were significantly increased (P < 0.01). MRSA infection was found to cause this inflammatory reaction in mice and increased the inflammatory factors. In the group administered the higher dose, the factors were restored to the level of the control group (P > 0.05); the high dose resulted in significantly more reduction than the lower dose, which showed a significant dose-effect relationship. Recent studies have shown that two novel components isolated from the dried fruits of A. tsao-ko displayed significant in vitro anti-inflammatory activity in a dose-dependent manner [36]. This finding was also observed in this in vivo study.
Under normal physiological conditions, cells have antioxidant systems that protect against the harmful effects of Reactive Oxygen Species (ROS) [37]. Studies have shown that oxidative stress is associated with a variety of diseases [38]. When the body is subjected to oxidative stress, the main changes in the endogenous oxidation system are an increase in MDA and decreases in SOD, GSH-Px, CAT activity of T-AOC and the endogenous antioxidant system; they were also important markers of oxidative stress [39-41]. In this study, when the model group was compared with the control group, GSH decreased but MDA and ·OH increased, which indicated that the oxidation reactions of the mice in the model group were outside of the normal range (i.e., oxidative stress). In the absence of any drug in the model group, the oxidative stress response was not improved, which lead to the death of the mice. In the experimental group, the oxidative stress factor improved and the oxidative response improved compared with the model group. The high dose oil restored oxidative stress to a normal level (P > 0.05). Therefore, A. tsao-ko essential oil has an ameliorative role on antioxidant stress in vivo. This may be related to geraniol, it has the effect of regulating oxidative stress [42].
CONCLUSION
The present study found that A. tsao-ko essential oil has strong anti-MRSA activity in vivo and exerts anti-inflammatory activity through the reduction of inflammatory factors. Based on these results, A. tsao-ko essential oil appears to show strong potential as an anti-MRSA drug for the treatment of various human and animal diseases.
ACKNOWLEDGEMENT
The study was supported by a grant from the Fund of the National Natural Science Foundation of China (no. 31970137), the Benefit People Project of Science and Technology of Chengdu Science and Technology Bureau (no.2016-HM01-00362-SF), the Province Training Program of Innovation and Entrepreneurship for Undergraduate, China (no.201813705049), and Supported by Sichuan Science and Technology Program (no.2020JDRC0071), the Open-Study Funds of State Key Laboratory Breeding Base of Systematic Research, Development and Utilization of Chinese Medicine, Chengdu University of Traditional Chinese Medicine.
REFERENCES
- Rammelkamp CH, Maxon T (1942) Resistance of Staphylococcus aureus to the action of penicillin. Proc Royal Soc Exper Biol Med 51: 86-389.
- Jessen O, Rosendal K, Bülow P, Faber V, Eriksen KR (1969) Changing staphylococci and staphylococcal infections. A ten-year study of bacteria and cases of bacteremia. N Engl J Med 281: 627-635.
- Jevons MP (1961) “Celbenin” - resistant Staphylococci. Br Med J 1: 124-125.
- Gleghorn K, Grimshaw E, Kelly EK (2015) New antibiotics in the management of acute bacterial skin and skin structure infections. Skin Ther Lett 20: 7-9.
- Huijsdens XW, Dijke BJV, Spalburg E, Marga, GV, Santen-verheuvel MG (2006) Community-acquired MRSA and pig-farming. Annals of Clinical Microbiology & Antimicrobials 5: 26.
- Ikeda H, Kurisu K, Kihira K (2004) Vancomycin ointment for MRSA infection at a cranioplasty site. Annals of Pharmacotherapy 38: 70-72.
- Cowie SE, Ma L, Lee SK, Smith RM, Hsiang YN (2005) Nosocomial MRSA infection in vascular surgery patients: impact on patient outcome. Vasc Endovascular Surg 39: 327-334.
- Parish D, Scheinfeld N (2008) Ceftaroline fosamil, a cephalosporin derivative for the potential treatment of MRSA infection. Curr Opin Investig Drugs 9: 201-209.
- Yates C, May K, Hale T, Allard B, Rowlings N, et al. (2009) Wound chronicity, inpatient care, and chronic kidney disease predispose to MRSA infection in diabetic foot ulcers. Diabetes Care 32: 1907.
- Alfatemi SMH, Rad JS, Rad MS, Mohsenzadeh S, Silva JATD (2014) Chemical composition, antioxidant activity and in vitro antibacterial activity of Achillea wilhelmsii, C. Koch essential oil on methicillin-susceptible and methicillin-resistant Staphylococcus aureus, spp. Biotech 5: 39-44.
- Sharifi-Rad J, Salehi B, Varoni EM, Sharopov F, Yousaf Z, et al. (2017) Plants of the Melaleuca Genus as Antimicrobial Agents: From Farm to Pharmacy. Phytotherapy Research 31: 1475-1494.
- Rahman RT, Lou Z, Yu F, Wang P, Wang HX (2017) Anti-quorum sensing and anti-biofilm activity of Amomum tsaoko (Amommum tsao-ko Crevost et Lemarie) on foodborne pathogens. Saudi Journal of Biological Sciences 24: 324-330.
- Feng X, Jiang ZT, Wang Y, Rong L (2010) Composition compar-ison of essential oils extracted by hydrodistillation and microwave-assisted hydrodistillation from Amomum tsao-ko in China. Journal of Essential Oil Bearing Plants 13: 286-291.
- Wu M, Guo P, Tsui SW, Chen H, Zhao Z (2012) An ethnobotanical survey of medicinal spices used in Chinese hotpot. Food Res Int 48: 226-232.
- Lim TK (2013) Amomum tsao-ko. Handbook of Herbs and Spices. 813-817.
- Shin JS, Ryu S, Jang DS, Cho YW, Chung EK, et al. (2016) Amomum tsao-ko fruit extract suppresses lipopolysaccharide-induced inducible nitric oxide synthase by inducing heme oxygenase-1 in macrophages and in septic mice. Int J Exp Pathol 96: 395-405.
- Yang Y, Yan RW, Cai XQ, Zheng ZL, Zou GL (2008) Chemical composition and antimicrobial activity of the essential oil of Amomum tsao-ko. J Sci Food Agric 88: 2111-2116.
- Li W, Zou ZY, Zha G, Zheng MS (1999) Experimental research on anti-HBV activity of 170 kinds of Chinese herbs. World Chinese J Digestol 7: 89-90.
- Yang Y, Yue Y, Runwei Y, Guolin Z (2010) Cytotoxic, apoptotic and antioxidant activity of the essential oil of Amomum tsao-ko. Bioresour Technol 101: 4205-4211.
- Martin TS, Kikuzaki H, Hisamoto M, Nakatani N (2000) Constituents of Amomum tsao-ko and their radical scavenging and antioxidant activities. J Am Oil Chem Soc 77: 667-673.
- Lee KY, Kim SH, Sung SH, Kim YC (2008) Inhibitory constituents of lipopolysaccharide-induced nitric oxide production in BV2 microglia isolated from Amomum tsao-ko. Planta Med 74: 867-869.
- Xu H, Long NN, Lin L, Li JL, Dai M, et al. (2017) .In vitro Antibacterial Activity of Amomum Tsao-ko Essential Oil Against Methicillin-resistant Staphylococcus Aureus. Journal of Chengdu Medical College 12: 241-246.
- Dai M, Peng C, Sun F (2016) Anti-infectious efficacy of essential oil from Caoguo (Fructus Tsaoko). J Tradit Chin Med 36: 799-804.
- Cong-Ran Li, Li Y, Li GQ, Yang XY, Zhang WX, et al. (2010) In vivo antibacterial activity of nemonoxacin,a novel non-fluorinated quinolone. Journal of Antimicrobial Chemotherapy 65: 2411-2415.
- Monecke S, Coombs G, Shore AC, Coombs DC, Akpaka P (2011) A Field Guide to Pandemic, Epidemic and Sporadic Clones of Methicillin-Resistant Staphylococcus aureus. PLoS One 6: 17936.
- Bassetti M, Carnelutti A, Righi E (2017) The role of methicillin-resistant staphylococcus aureus in skin and soft tissue infections. Current Opinion in Infectious Diseases 30: 150-157.
- Edwardsjones V, Buck R, Shawcross S, Dawson MM, Dunn K (2004) The effect of essential oils on methicillin-resistant Staphylococcus aureus using a dressing model. Burns 30: 772-777.
- Gao C, Fan YL, Zhao F, Ren QC, Wu X, et al. (2018) Quinolone derivatives and their activities against methicillin-resistant Staphylococcus aureus (MRSA). Eur J Med Chem 157: 1081-1095.
- Meng DW, Wei L, Wang PJ, Shigematsu M, Ren HF, et al. (2013) Identification of compositions of Amomum tsao-ko essential Oil Using GC-MS and Its Antibacterial Activity. J Food Sci and Tech 31: 24-30.
- LI XQ, Hui HY, Luo ZC (2016) Study on activities of geraniol, β-Citronellol and eugenol in vitro. J Mod Lab Med 31: 87-89.
- Leite MCA, De, BBAP, De Sousa JP, Lima EDO (2015) Investigating the antifungal activity and mechanism(s) of geraniol against Candida albicans strains. Medical Mycology 53: 275-284.
- Thangamani S, Nepal M, Chmielewski J, Seleem M (2015) Antibacterial activity and therapeutic efficacy of Fl-P R P R P L -5, a cationic amphiphilic polyproline helix, in a mouse model of staphylococcal skin infection. Drug Des Devel Ther 9: 5749-5754.
- Thangamani S, Mohammad H, Abushahba MFN,Sobreira TJP, Hedrick VE, (2015) Exploring simvastatin, an antihyperlipidemic drug, as a potential topical antibacterial agent. Scientific Reports 5: 16407.
- Díaz PV, Gaggero AA, Pinto RA,Mamani R, Uasapud PA, et al. (2013) Levels of inflammatory cytokines and plasma cortisol in respiratory syncytial virus bronchiolitis. Rev Med Chile 141: 574-581.
- Knaus WA, Draper EA, Wagner DP, Zimmerman J (1985) APACHE II: a severity of disease classification system. Crit Care Med 13: 818-829.
- Zhang TT, Lu CL, Jiang JG (2016) Neuroprotective and anti-inflammatory effects of diphenylheptanes from the fruits of Amomum tsaoko, a chinese spice. Plant Foods Hum Nutr 71: 450-453.
- Feilletcoudray C, Sutra T, Fouret G, Ramos J, Wrutniakcabello C, et al. (2009) Oxidative stress in rats fed a high-fat high-sucrose diet and preventive effect of polyphenols: Involvement of mitochondrial and NAD(P) H oxidase systems. Free Radic Biol Med 46: 624-632.
- Tramutolaa A, Lanzillotta C, Perluigi M, Butterfield DA (2017) Oxidative stress, protein modification, alzheimer disease. Brain Research Bulletin 133: 88-96.
- Jin Y, Wang J, Pan X, Wang L, Fu Z (2013) Cis-Bifenthrin enantioselectively induces hepatic oxidative stress in mice. Pestic Biochem Physiol 107: 61.
- Moselhy HF, Reid RG, Yousef S, Boyle SP (2013) A specific, accurate, and sensitive measure of total plasma malondialdehyde by hplc. Journal of Lipid Research 54: 85.
- Zhou J, Li P, Cheng N, Gao H, Wang B, et al. (2012) Protective effects of buckwheat honey on DNA damage induced by hydroxyl radicals. Food Chem Toxicol 50: 2766-2773.
- EBassossy HM, Ghaleb H, Elberry AA, Balamash KS, Ghareib SA, et al. (2017) Geraniol alleviates diabetic cardiac complications: Effect on cardiac ischemia and oxidative stress. Biomedicine & Pharmacotherapy 88: 1025-1030.
Citation: Long N, Tang H, Lin L, Sun F, Peng C, et al. (2020) Activity of Amomum tasao-ko Fruits Essential Oil against Methicillin-Resistant Staphylococcus aureus in vivo. J Altern Complement Integr Med 6: 126.
Copyright: © 2020 Nana Long, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

