
Acupuncture Combined with Traditional Chinese Medicine for Allergic Rhinitis: A Protocol for Systematic Review and Meta-Analysis
*Corresponding Author(s):
Lintong DaiAffiliated Hospital Of Panzhihua University, Panzhihua, China
Email:15691596@qq.com
Abstract
Background: Allergic Rhinitis (AR) is a prevalent yet underappreciated inflammatory disorder of nasal mucosa, which is characterized by pruritus, sneezing, rhinorrhoea and nasal congestion, some studies have pointed out that if combine Traditional Chinese Medicine (TCM) with acupuncture, which can enhance the effect of acupuncture and moxibustion. However, safety and efficacy of acupuncture combined with traditional Chinese medicine for treating allergic rhinitis remain largely uncertain. In our study, we will perform the first systematic review and meta-analysis to explore the effectiveness and safety of acupuncture combined with TCM for AR.
Methods: We will search the Randomized Controlled (RCT) literatures involving acupuncture combined with TCM for treating AR in seven electric databases, including PubMed, Web of science, EMBASE, the Cochrane library, Chinese National Knowledge Infrastructure (CNKI), Chinese Biomedical Literature Database (CBM), Wan Fang Database. We will define the total effective rate or cure rate, and recurrence rate as the primary outcomes. the Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ) score, symptom score (nasal congestion, snot, sneezing) will be regarded as the secondary outcomes. Quality assessment of included studies will be independently performed according to the Cochrane Risk of Bias tool. Meanwhile, the level of evidence for results will be assessed by using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method. All analysis will be conducted by using the RevMan software V5.4.
Conclusion: The conclusion of this study will confirm safety and efficacy of acupuncture combined with TCM in the treatment of allergic rhinitis, which can provide new evidence to guide appropriate interventions on AR with acupuncture combine with TCM in future.
Systematic Review Registration: PROSPERO CRD42021247621
Keywords
Acupuncture; AR; Meta-analysis; TCM
Abbreviations
AR = Allergic Rhinitis
TCM = Traditional Chinese medicine
CI = Confidence Interval
OR = Odds Ratio
RCTs = Randomized Controlled Trials
Background
Allergic rhinitis, abbreviated AR,is a prevalent yet underappreciated inflammatory disorder of nasal mucosa, which is characterized by pruritus, sneezing, rhinorrhoea, and nasal congestion[1].The impact of AR on quality of life is very significant. Allergic rhinitis is a major contributor to the total cost of health-related absenteeism (eg, missing work) and presenteeism (eg, showing up to work but having reduced productivity). For example, costs of AR and allergic conjunctivitis in the United States have been estimated at more than $6 billion per year[2-4].At present, modern medicine with desensitization therapy, the use of anti-allergic drugs, antihistamines, hormones, and other improved symptoms or immune regulation, but clinical near and long-term efficacy is general, after stopping the symptoms are easy to repeat, long-term drug toxicity side effects are obvious[5].Traditional Chinese medicine has its own unique understanding of allergic rhinitis, which is mainly due to the deficiency of viscera. Traditional Chinese medicine treatment can be divided into internal treatment and external treatment, internal treatment is mostly Chinese patent medicine, external treatment is mostly acupuncture and moxibustion, but its remission of patients’ symptoms does not last long, some studies have pointed out that if the combination of the two can greatly reduce patients’ nasal congestion, runny nose and other symptoms, so that patients’ life can be greatly improved[6-13]. However, there is still a lack of systematic evaluation on the efficacy and safety of acupuncture combine with traditional Chinese medicine for AR in clinical practice. Therefore, the efficacy and safety of acupuncture combine with traditional Chinese medicine for AR will be systematically evaluated and meta-analysis in this paper.
Methods
Study design
The protocol of this review will be conducted and reported in accordance with the preferred Reporting Items for Systematic Reviews and Meta-analysis Protocols (PRISMA-P) statement guidelines[14] and the corresponding checklist can be found in Additional file1. This protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42021247621)
Inclusion Criteria For Study Selection
Type of studies
We will estimate the publications according to the criteria of the review objectives and Participants, Interventions, Comparisons, Outcomes (PICO). Only Randomized Controlled Trials (RCTs) involving acupuncture combine with Traditional Chinese medicine, placebo treatment. Language of literature will be limited to Chinese and English. Studies that mentioned the term of “randomization” will also be considered, while studies using incorrect randomization methods will be excluded. Additionally, other designs such as in vivo, case report and non-RCTs will also be excluded.
Type of participants
Studies that enrolled patients with AR, regardless of gender, age, race, or nationality, who received acupuncture therapy with Traditional Chinese medicine will be included. However, the patients with other serious illnesses, such as cancer, cardiovascular disease, and liver and kidney disease, will be excluded.
Type of interventions
We will consider studies evaluating the following treatments including body acupuncture, scalp acupuncture, warm needle acupuncture, combine with Traditional Chinese medicine.
Type of comparators or control
We will include and classify the comparators in study as follows: (1) TCM, (2) acupuncture, (3) no treatment. Articles comparing different acupoints or different forms of acupuncture will be excluded.
All inclusion and exclusion criteria for considering studies for this review are summarized in supplementary.
Primary outcomes
Clinical efficacy, including total effective rate or cure rate, and recurrence rate will be accepted as the primary outcomes.
Secondary outcomes
The Rhinitis Quality of Life Questionnaire (RQLQ) score, symptom score (nasal congestion, snot, sneezing) will be used as secondary outcomes.
Search strategies
An electronic search will be carried out in the following databases, including PubMed,Web of science, Cochrane Library, Chinese National Knowledge Infrastructure (CNKI), Chinese Biomedical Literature Database (CBM), WanFang Database. Terms of Medical Subjects (MeSH) and keywords will be used individually or in combination during the query. The specific search strategy for PubMed will be taken as an example, which will be shown inTable 1. Nevertheless, the searching strategy for other databases will be minorly modified. In addition, Chinese characters with the same meaning will be used for literature retrieval in the Chinese databases.
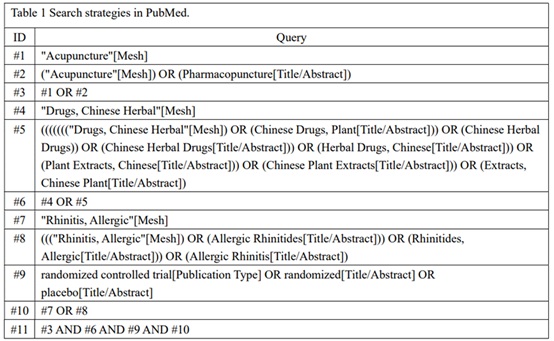 Table 1: Search strategies in PubMed.
Table 1: Search strategies in PubMed.
Moreover, we will filter relevant medical journals and magazines to clarify literature which is not included in the electronic databases. Meanwhile, clinical trials registries, like the WHO International Clinical Trial Registry Platform, Chinese Clinical Registry, and ClinicalTrials.gov, will be searched for ongoing trials with unpublished data. The reference lists of all potential publications, including relevant systematic reviews, will be manually retrieved and reviewed to further locate additional trials. Incomplete data will be obtained via contacting the corresponding author.
Study collection
Two reviewers (YL and GHC) will evaluate the title and abstract of all studies for possible candidates, respectively. Any duplicate studies will be removed. After title and abstract screening, the full-text copies of all eligible studies will be downloaded for re-evaluation. Once the reviewer is uncertain about the eligibility of any study, its full text will be obtained to re-examine. An additional reviewer (DLT) will be consulted in case of disagreement. Excluded studies and the reasons of exclusion will be recorded. The specific process of study screening will be displayed in a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The flow diagram of all study selection procedure is shown in figure 1.
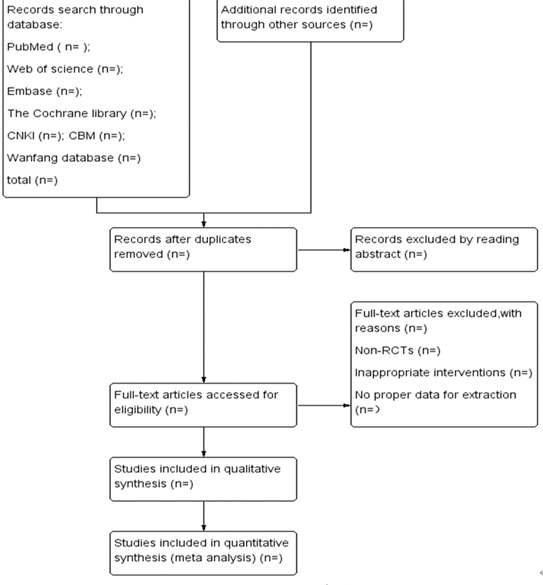 Figure 1: Flowchart of literature selection.
Figure 1: Flowchart of literature selection.
Data extraction
Two investigators (YL and GHC) will independently perform data extraction. The general information, participants, methods, interventions, outcomes, results, adverse events, conflict of interest, ethical approval, and other information will be extracted. Furthermore, we will contact the authors for further information when the reported data is not sufficient. Besides, any disagreement will be resolved by discussion between the two authors (YL and GHC) and further disagreements will be arbitrated by the third author (FJ).
Dealing with missing data
The corresponding authors or relevant authors will be contacted by telephone or e-mail for insufficient or missing data. In case of no reply from the authors or missing data cannot be supplied, the missing data with replacement values will be imputed, treating these as if they were observed. The last observation carried forward imputation method will be used to assume a missing value, and, then, an intention-to-treat analysis will be performed. Moreover, if possible, the sensitive analyses will be performed to assess how sensitive the results are to reasonable changes in the assumptions that are made. The potential impact of missing data on the final outcome of the review will be addressed in the discussion.
Risk of bias assessment
Two authors (YL and GHC) will assess the risk of bias with the Cochrane Collaboration’s tool for risk of bias assessment in all included studies[15]. The following domains for risk of bias will be evaluated; sequence generation, allocation sequence concealment, blinding of participants and personnel and outcome assessors, incomplete outcome data, selective outcome reporting, and other sources of bias. The judgment on these items will be classified into three levels: “low risk of bias,” “high risk of bias,” or “unclear risk of bias.” The conflicts or any discrepancies will be resolved by discussion or will be judged by another reviewer (FJ) to achieve the consensus.
Quality of evidence assessment
According to grading of Recommendations Assessment, Development and Evaluation (GRAADE) method[16],the evidence quality evaluation of key outcomes can be regarded as four levels: high quality, moderate quality, low quality, and very low quality[17]. Evidence quality is generally judged on the bias, inconsistency, indirectness, inaccuracy, and publication bias.
Measures of treatment effect
We will use the Review Manager software 5.4 (V5.4)[18] to carry out statistical analysis. Mean Difference (MD) or Standardized Mean Difference (SMD) will be used for continuous data. In addition, Risk Ratio (RR) or Risk Difference (RD) will be used for analysis of dichotomous data. Furthermore, the corresponding 95% Confidence Interval (CI) for each parameter will be calculated between the treatment group and the control group.
Assessment of heterogeneity
The heterogeneity will be assessed by I2 statistical test. If the I2 test is less than 50%, the fixed-effect model will be used for data synthesis. Otherwise, the random-effects model will be conducted with heterogeneous data, which I2 test between 50 and 75%. If the I2 test is higher than 75%, we will find possible reasons from both clinical and methodological perspectives and provide an explanation or conduct subgroup analysis.
Assessment of reporting bias
A funnel plot will be generated wo assess reporting bias when more than 10 trials included[19].
Data synthesis
Clinical data will be imported into the RevMan software (V5.4) to perform data synthesis, and the significance threshold will be P
Sensitivity analysis
Sensitivity analysis will be conducted to monitor the robustness of primary decision made in the review process. Several decision nodes, such as sample size, methodological weakness, and missing data, will be considered. The results of the sensitivity analysis will be presented in summary tables. The risk of bias in the review process as indicated by the results of the sensitivity analysis will be discussed.
Subgroup analysis
If data is available, a subgroup analysis will be conducted according to variations in the characteristics of the trial participants and acupuncture combine with TCM treatment. When considerable heterogeneity is detected in previous analysis, a subgroup analysis will be performed if necessary.
Ethics and dissemination
Ethics approval will not be necessary, because the included publications in our study do not involve patients’ individual privacy. The main data will be extracted from published literature.This systematic review will be published in peer-reviewed journal or conference report to provide a reference for safety and efficacy of acupuncture combined with traditional Chinese medicine in the treatment of allergic rhinitis.
Discussion
According to TCM the body’s immunity is the function of what is termed the defensive Qi. Allergy in the viewpoint of TCM is a “wind” disease that penetrates from externally together with “cold” or “heat” and impedes the defensive Qi flow in the lung, the skin and the mucous membranes[20]. Treatment of AR including acupuncture and western medicine, but a lot of the side effects of western medicine may affect the therapeutic effects. Therefore, it is necessary to find alternative therapy with higher effective. The effects of acupuncture with respect to the reduction of allergic symptoms and an improvement of the quality of life have been examined in several studies. Here acupuncture was reported to be effective particularly in the reduction of nasal and conjunctival signs and symptoms with improvement in the quality of life[21,22].
Acupuncture combined with TCM is a reliable method to relieve the suffering of the patient. Unfortunately, there is no comprehensive systematic review to provide enough evidence for acupuncture combined with TCM treatment for AR. Hence, carrying out a systematic review and meta-analysis from available literature to evaluate the effectiveness and safety of the acupuncture combined with TCM treatment AR is warranted. The protocol will include and integrate the latest and most comprehensive literature in this field, hoping to provide an objective treatment method for patients with AR and inspire more peer experts to implement more relevant clinical trials in the future.
However, there are some limitations in this study. Firstly, there are not enough publications on the treatment AR by using acupuncture combined with TCM. Secondly, we will only acquire the studies that were reported in Chinese or English; databases in other language such as Japanese or Korean will not be involved, which may lead to language bias.
In summary, based on rigorous study design and accurate evaluation of the literature, we expect that the review will provide evidence of acupuncture combined with TCM treatment on AR.
Acknowledgement
The authors would like to thank the editors and reviewers for their kindness and enlightening comments on this manuscript.
Author’s Contribution
YL, GHL and DLT originally conceptualized the study. All the authors contributed to the development of the protocol. YL drafted the original manuscript. GHC brought expertise in prediction model building. YL brought expertise in clinical managements of osteoarthritis. YL and GHC did the preliminary searches, piloted the study selection process, and developed the data analysis strategy, with assistance from YL, GHC, JH, and DLT. FJ polished the English language of the manuscript. DLT and FJ supervised the protocol developing process. YL acquired the funding and is the guarantor of this manuscript. All authors critically reviewed and approved the final version of the manuscript.
Funding
This research was supported by Sichuan Provincial Department of Education Fund Key Project (18ZA0203), Nanchong science and technology and intellectual property office of the project(16YFZJ0021)
Availability of data and materials:Not applicable.
Declarations
Ethics approval and consent to participate:Not applicable
Consent for publication:Not applicable
Competing interests: The authors declare that they have no competing interests.
References
- Greiner AN, Hellings PW, Rotiroti G, Scadding GK (2011) Allergic rhinitis. Lancet 378: 2112-222.
- Meltzer EO (2016) Allergic Rhinitis: Burden of Illness, Quality of Life, Comorbidities, and Control. Immunol Allergy Clin North Am 36: 235-248.
- Ray NF, Baraniuk JN, Thamer M, Rinehart CS, Gergen PJ, et al. (1999) Direct expenditures for the treatment of allergic rhinoconjunctivitis in 1996, including the contributions of related airway illnesses. J Allergy Clin Immunol 103: 401-407.
- Thompson AK, Juniper E, Meltzer EO (2000) Quality of life in patients with allergic rhinitis. Ann Allergy Asthma Immunol 85: 338-347.
- Okubo K, Kurono Y, Ichimura K, Enomoto T, Okamoto Y, et al. (2017) Japanese guidelines for allergic rhinitis 2017. Allergol Int 66: 205-219.
- Chen YC, Lin YH, Hu S, Chen HY (2016) Characteristics of traditional Chinese medicine users and prescription analysis for pediatric atopic dermatitis: a population-based study. BMC Complement Altern Med 16: 173.
- Li XM (2009) Complementary and alternative medicine in pediatric allergic disorders. Curr Opin Allergy Clin Immunol 9: 161-167.
- Liang X, Wang Q, Jiang Z, Li Z, Zhang M, et al. (2020) Clinical research linking Traditional Chinese Medicine constitution types with diseases: a literature review of 1639 observational studies. J Tradit Chin Med 40: 690-702.
- Liao HH, Yen HR, Muo CH, Lee YC, Wu MY, et al. (2017) Complementary traditional Chinese medicine use in Children with cerebral palsy: a nationwide retrospective cohort study in Taiwan. BMC Complement Altern Med 17: 155.
- Lin PY, Chu CH, Chang FY, Huang YW, Tsai HJ, et al. (2019) Trends and prescription patterns of traditional Chinese medicine use among subjects with allergic diseases: A nationwide population-based study. World Allergy Organ J 12: 100001.
- Lu YC, Yang CW, Lin YH, Hsueh JY, Chen JL, et al. (2020) Identifying the Chinese Herbal Medicine Network and Core Formula for Allergic Rhinitis on a Real-World Database. Evid Based Complement Alternat Med 2020: 5979708.
- Yang J, Xu H (2008) External application of herbal medicine to acupoints. J Tradit Chin Med 28: 21-23.
- Yen HR, Lai WY, Muo CH, Sun MF (2017) Characteristics of Traditional Chinese Medicine Use in Pediatric Cancer Patients: A Nationwide, Retrospective, Taiwanese-Registry, Population-Based Study. Integr Cancer Ther 16: 147-155.
- Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, et al. (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Bmj 350: 7647.
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, et al. (2011) The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj 343: 5928.
- Puhan MA, Schünemann HJ, Murad MH, Li T, Petersen RB, et al. (2015) A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. Bmj 350: 3326.
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, et al. (2011) GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64: 383-394.
- Schmidt L, Shokraneh F, Steinhausen K, Adams CE (2019) Introducing RAPTOR: RevMan Parsing Tool for Reviewers. Syst Rev 8: 151.
- Cumpston M, Li T, Page MJ, Chandler J, Welch VA, et al. (2019) Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 10: 000142.
- Hauswald B, Yarin YM (2014) Acupuncture in allergic rhinitis: A Mini-Review. Allergo J Int 23: 115-119.
- Adam D, Grabenhenrich L, Ortiz M, Binting S, Reinhold T, et al. (2018) Impact of acupuncture on antihistamine use in patients suffering seasonal allergic rhinitis: secondary analysis of results from a randomised controlled trial. Acupunct Med 36: 139-145.
- Moustafa Y, Nady HGE, Saber MM, Dabbous OA, Kamel TB, et al. (2019) Assessment of Allergic Rhinitis among Children after Low-Level Laser Therapy. Open Access Maced J Med Sci 7: 1968-1973.
Citation: Yan L, Gou H, Feng J, Dai L (2022) Acupuncture Combined with Traditional Chinese Medicine for Allergic Rhinitis: A Protocol for Systematic Review and Meta-Analysis. J Altern Complement Integr Med 8: 219.
Copyright: © 2022 Le Yan, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

