
Adverse Neonatal outcomes Effects of Demographic and Obstetric factors among Displaced Sudanese Women
*Corresponding Author(s):
Awadalla AbdelwahidObstetrician And Gynecologist Consultant, Bashair Hospital, Faculty Of Medicine, Al Neelain University, Khartoum, Sudan
Tel:+249912921726,
Email:awad336@yahoo.com
Abstract
Background
Adverse neonatal outcomes are affected by maternal and fetal factors, it is the occurrence of Low Birth Weight (LBW), preterm delivery, low Apgar scores at first and fifth minutes after birth, early or late neonatal death, small for gestational age, and/or severe neonatal conditions.
Purpose
This study aimed to determine the association of adverse neonatal outcomes with demographic and obstetric factors among displaced women.
Methodology
This was a descriptive cross-sectional hospital-based study conducted at Algabalin Teaching Hospital, a public hospital in Southern Sudan from January 2023 to June 2023.
Data were collected from patient notes, the maternal information, delivery events, and neonatal outcomes were recorded using an interview questionnaire
Results
A total of 200 women and their neonates were included, adverse neonatal outcomes were 25 (12.5%), intrauterine growth restriction (IUGR) 4(16%), intrauterine fetal death 3(12%), preterm labor 5(20%), early neonatal death 4(16%), need resuccitation1 (4%), respiratory distress syndrome (RDS) 5(20%) and hypoglycemia 2(8%), APGAR score < 7 was 14(7%). Gestational age 32-35 weeks were 6(3%), with adverse neonatal outcomes were 6(24%),35+1-36+6 weeks were 6 (3%) of the total. Neonatal weight less than2.5kg were 18(9%), with adverse neonatal outcomes16(64%), with normal outcomes 2(1.1%), weight >4kg was 6(3%), with adverse neonatal outcomess4(16%), with normal outcomes 2(1.1%). NICU was 22(11%), of 20(80%) of adverse neonatal outcomes and 2(1.1%) in normal neonatal outcomes cases.
Associated with rural unbooked, advanced age, emergency cesarean section, preterm labor, and medical illness. Postpartum hemorrhage (PPH) 3(1.5%), amniotic fluid embolism 1(0.5%), Eclampsia 1(0.5%), maternal death 2(1%).
Conclusion
The study concluded that adverse neonatal outcomes associated with advanced maternal age, rural residence, low education, primigravida, grand multiparous, unbooked women, maternal uncontrolled medical disorders with pregnancy, delivery by emergency cesareans section, early and late preterm fetus, low birth weight, macrosomia.
Keywords
Adverse; Neonatal; Outcomes; Demographic; Obstetric; Factors
Abbreviations
ANC: Antenatal care
CS: Cesarean section
ENND: Early neonatal death
IUFD: Intrauterine fetal death
IUGR: Intrauterine growth restriction
LBW: Low Birth Weight
MSAF: Meconium-stained amniotic fluid
MSF: Meconium aspiration syndrome
NICU: Neonatal intensive care unit
PPH: Postpartum hemorrhage
PPROM: Premature pre-labor ruptured membranes
RDS: Respiratory distress syndrome
SGA: Small for gestational age
WHO: World Health Organization
Introduction
The perinatal period is important and critical; it starts at 22 completed weeks of gravity and lasts for seven days after birth [1]. Perinatal deaths include bearing from 28 weeks of gravity and early (7 days after birth) neonatal deaths, which directly reflect the obstetric outcome and extent of care handed to the pregnant mamas and their neonates during the antepartum, intrapartum, and postpartum periods [2]. Perinatal mortality is a significant public health concern in developing countries. The vast majority of these deaths occur in low-income countries, accounting for 98% of perinatal deaths [3]. Stillbirth is a major adverse pregnancy outcome, with a periodic estimate of 3 million third-trimester bearings worldwide [4]. The neonatal period, from birth to the first 28 days of life, is the most dangerous period of life because of the various conditions faced by neonates. The threat of dying is the highest during this period of life [5]. Adverse neonatal outcomes were defined as the occurrence of Low Birth Weight (LBW), preterm delivery, low Apgar scores at first and fifth minutes after birth, early or late neonatal death, small for gestational age, and/or severe neonatal conditions. [6-8].
A low Apgar score at 5 min was defined as a score lower than 7. [9-10]. Small for gestational age (SGA) fetuses or newborns are smaller than normal in size, most generally defined as a weight below the 10th percentile for the gestational age [11-12]. Neonatal death was defined as death within the first 28 days of life. [10]. Early neonatal death (ENND) is defined as the death of an infant between 0 and 7 days after birth [13]. Worldwide, early neonatal deaths account for 73% of all postnatal deaths. [13].
Preterm birth, defined as birth before a gestational age of 37 weeks, is a major cause of neonatal mortality and morbidity worldwide [14]. The risk of adverse health outcomes correlates equally with gestational age at birth, and neonates born extremely preterm before gravid week 28 are at particularly high risk [15-16].
Regardless of gestational age, low birth weight (LBW) is defined as the birth weight of a live newborn weighing lower than 2.5 kg [17]. Low birth weight, intrauterine growth restriction, and preterm birth are strongly correlated [18]. In developing countries, low birth weight is a public health concern, particularly in Sudan.
Low birth weight constitutes sixty to eighty percent of the child mortality rate in developing countries. Child mortality due to low birth weight is generally directly unproductive, stemming from other medical complications such as preterm birth, poor motherly nutritive status, lack of antenatal care, maternal sickness during pregnancy, and an unhygienic home environment [19-21].
The application of antenatal care globally recognized by the World Health Organization (WHO) has shown that in North Africa and the Middle East, the use of antenatal care is advanced in 65% of pregnant women. In Sub-Sahara Africa, the uptake of antenatal care (ANC) services is exceptionally low, 68 %of women reported at least one antenatal visit. The number of regions remaining in the world ranges from 82% to 86% [22]. Poor pregnancy outcomes are more egregious in unbooked cases than in booked cases. In low-income countries, fewer than 50% of pregnant women have a minimum of four visits to the ANC [23]. The current infant mortality rate for Sudan in 2023 is 38.041 deaths per 1000 live births, a2.47 decline from 2022 [24]. Cesarean delivery was not associated with improved neonatal outcomes in preterm SGA newborns and was associated with an increased risk of respiratory distress syndrome [25] there are limited studies on neonatal mortality in Sudan; in particular, there have been no studies on Algabalin. Investigating the demographic and obstetric characteristics of neonatal morbidity and mortality is required to generate new data that may be useful in clinical practice. The current study aimed to determine the association between the demographic and obstetric characteristics of pregnant women and neonatal outcomes.
Material and Methods
Study design, setting, and period
This descriptive cross-sectional hospital-based study was conducted at Algabalin Teaching Hospital, a public hospital in Southern Sudan, from January 2023 to June 2023.
Study population, inclusion, and exclusion criteria
The study included all women who delivered at hospitals with babies at a gestational age of ≥32 weeks. Women who delivered at home or in other hospitals and came for management of postpartum complications were excluded.
Sampling techniques
Systematic random sampling was used to select participants.
The principal investigators identified cases daily with the assistance of trained resident registrars for data collection. This was done through daily participation in morning meetings, meeting reports, and daily rounds of labor wards, neonatal intensive care units, operating rooms, and postnatal words. A structured data abstraction form filled in by the data collectors was used for the data collection. Medical staff involved in the management of the included cases were questioned in cases of missing information from the patient records.
Maternal and neonatal Enrollment
Women who met the inclusion criteria were included after obtaining their written consent. Maternal information, delivery events, and neonatal outcomes were recorded using an interview questionnaire, and maternal and neonatal follow-ups were conducted from entry to the hospital until discharge. Neonatal intensive care unit admission was offered at follow-up, and outcomes were recorded until discharge by a pediatrician or early neonatal death.
The participants were interviewed regarding maternal age, education, occupation, residence, parity, booking status, parity, medical illness, mode of delivery, gestational age, antenatal complications, neonatal outcomes, neonatal weight, APGAR score, type of abnormal neonatal outcomes, NICU admission, maternal outcomes, and maternal ICU admission.
Neonates were classified according to the final diagnosis of normal and adverse neonatal outcomes.
Data processing and analysis
The Neonatal outcomes were classified as adverse neonatal outcome and normal neonatal outcome, normal neonatal outcome when delivered at term, alive, normal Apgar score, normal fetal weight, not admitted to the neonatal intensive care unit (NICU). Adverse neonatal outcomes were defined as the occurrence of Low Birth Weight (LBW), preterm delivery, low Apgar scores at first and fifth minutes after birth, early or late neonatal death, small for gestational age, and/or severe neonatal conditions. The proportion of abnormal neonatal outcomes was determined on the basis of the mode of delivery. Vaginal delivery includes spontaneous vaginal delivery and instrumental vaginal delivery, whereas cesarean section (CS) includes emergency and elective CS.
Statistical analysis was performed using the SPSS 27 software (SPSS, Chicago, IL, USA). Continuous variables were compared using Student’s t-test (for paired data) or Mann–Whitney U test for nonparametric data. For categorical data, a comparison was performed using the chi-square test (X2) or Fisher’s exact test, when appropriate. Statistical significance was set at p < 0.05.
Ethical clearance was obtained from the ethical committee of the White Nile University, Council of Obstetrics and Gynecology. An official agreement from the general managers of the Algabalin Teaching Hospital preceded the study. Ethical considerations were taken, and the study was presented to the ethics review committee and approved; permission to conduct the study was requested from the authorities of health care in the study area, data were handled with a high degree of confidentiality throughout the study, and written informed consent was obtained from all participants in the study.
Meconium aspiration syndrome (MAS): is the neonatal respiratory distress that occurs in a newborn in the context of meconium-stained amniotic fluid (MSAF) when respiratory symptoms cannot be attributed to another etiology [26]
Hypoglycemia: Blood glucose concentration <47 mg/dL is the critical threshold associated with adverse neurodevelopmental outcomes [27].
Respiratory distress: Respiratory distress in newborns is recognized by dyspnea, which is a common presenting symptom that usually occurs immediately after exposure to an inciting event. Signs of respiratory distress, tachycardia, tachypnea, and diffuse crackles. In severe cases, patients are somnolent, cyanosed, and diaphoretic diagnosed clinically based on the diagnostic Berlin criteria [28].
Results
The demographic distribution of displaced women with adverse neonatal outcomes study found women aged less than 20 years, 4(16%); normal neonatal outcomes, 18(10.3%); adverse neonatal outcomes 20-25years years (28%); normal neonatal outcomes, 52(29.7%), 26-30years adverse outcomes, 4(16%); normal neonatal outcomes42(24%); women age 31-35years years, 7(28%); adverse neonatal outcomes and normal outcomes, 42(24%), 36-40years years, 3(12%); adverse neonatal outcomes and normal neonatal outcomes, 19(10.8%); and more than 40years, 2(1.2%). The educational level of illiterate participants was 6(24%) in women with adverse neonatal outcomes, 16(9.1%) in women with normal neonatal outcomes, 13(52%) in women with adverse neonatal outcomes,74(42.3%) in women with normal neonatal outcomes, 5(20%) in women with adverse neonatal outcomes, 58(33.1%) in women with normal neonatal outcomes, 1(4%) in women with adverse neonatal outcomes, and 27(15.4%) in women with normal neonatal outcomes.
Women with adverse neonatal outcomes were housewives 25(100%), while 172 (98.2 %) were women with normal neonatal outcomes, and 3(1.8%) were workers. Most women with adverse neonatal outcomes resided in rural areas (22 [88 %]) and 3(12%) in urban areas, while women with normal neonatal outcomes resided in rural (101 [57.7 %]) and urban areas (74 [42.3 %]) (Table 1).
|
Demographic
|
Neonatal outcomes |
Total |
|
|
Adverse |
Normal |
||
|
Age |
|
|
|
|
<20 |
4 |
18 |
22 |
|
20-25 |
7 |
52 |
59 |
|
26-30 |
4 |
42 |
46 |
|
31-35 |
7 |
42 |
49 |
|
36-40 |
3 |
19 |
22 |
|
>40 |
0 |
2 |
2 |
|
Education |
|
|
|
|
Illiterate |
6 |
16 |
22 |
|
Primary |
13 |
74 |
87 |
|
Secondary |
5 |
58 |
63 |
|
University |
1 |
27 |
28 |
|
Occupation |
|
|
|
|
Housewife |
25 |
172 |
197 |
|
Worker |
0 |
3 |
3 |
|
Residence |
|
|
|
|
Rural |
22 |
101 |
123 |
|
Urban |
3 |
74 |
77 |
|
Total |
25 |
175 |
200 |
Table 1: Demographic characteristics and neonatal outcomes of displaced women (n=200)
Neonatal outcomes and obstetrics characteristics of displaced women with adverse neonatal outcomes, booked women were 19(76%), while unbooked 6 (14%), booked women with normal neonatal outcomes were 158(90.3%) and unbooked 17(9.3%). Primigravida women with adverse neonatal outcomes were 6(24%), multiparous were 16(64%), grand multiparous were 3(12%), primigravida women with normal neonatal outcomes were 29(16.6%), multiparous were 129(73.7%), grand multiparous were 17(9.7%), women with adverse neonatal outcomes without medical illness were 22(88%), Bronchial Asthma one(4%), hypertension two(8%), most women with normal neonatal outcomes not had medical illness 170(97%), heart disease one(0.6%), Asthma one(0.6%), Diabetes Mellitus one(0.6%), Hepatitis B+ one (0.6%), and Psychiatric illness one(0.6%). Most women with adverse neonatal outcomes delivered by emergency cesarean section 13(52%), vaginal delivery 5(20%), elective cesarean section 3(12%), instrumental delivery 3(12%), and successful vaginal birth after cesarean section (4%). Most women with normal neonatal outcomes were delivered by elective cesarean section 96 (54.9%), vaginal delivery 57(32.6%), emergency cesarean section 20(11.4%), and successful vaginal birth after cesarean section 2 (1.1%) (Table 2).
|
Obstetrics
|
Neonatal outcomes |
Total |
|
|
Adverse |
Normal |
||
|
Booking status |
|
|
|
|
Booked |
19 |
158 |
177 |
|
Unbooked |
6 |
17 |
23 |
|
Parity |
|
|
|
|
Primigravida |
6 |
29 |
35 |
|
Multipara |
16 |
129 |
145 |
|
Grand multipara |
3 |
17 |
20 |
|
Medical illness |
|
|
|
|
Heart disease |
0 |
1 |
1 |
|
Asthma |
1 |
1 |
2 |
|
Diabetes Mellitus |
0 |
1 |
1 |
|
Hypertension |
2 |
0 |
2 |
|
Hepatitis B+ |
0 |
1 |
1 |
|
Psychiatric illness |
0 |
1 |
1 |
|
No Medical Illness |
22 |
170 |
192 |
|
Mode of delivery |
|
|
|
|
Elective CS |
3 |
96 |
99 |
|
Emergency CS |
13 |
20 |
33 |
|
Vaginal delivery |
5 |
57 |
62 |
|
Instrumental delivery |
3 |
0 |
3 |
|
VBAC |
1 |
2 |
3 |
|
Total |
25 |
175 |
200 |
Table 2: Obstetrics characteristics and neonatal outcomes of displaced women (n=200)
Gestational age 32-35 weeks was 6(3%), with adverse neonatal outcomes in 6(24%),35+1-36+6 weeks in 6 (3%), adverse outcomes in 4(16%), normal neonatal outcomes in 2(1.1%), women gestational age 37-38+6 weeks in 103(51.5%), adverse neonatal outcomes5(20%), normal neonatal outcomes in 98(56%),39-40+6 weeks in 68(34%), adverse neonatal outcomes5(20%), normal neonatal outcomes63(36%),≥ 41weeks were 17(8.5%), adverse neonatal outcomes in 5(20%), and normal neonatal outcomes in 12(6.8%) (Table 3).
|
Gestational Age /weeks
|
Neonatal outcomes |
Total |
|
|
Adverse |
Normal |
||
|
(32-35) |
6 |
0 |
6 |
|
(35+1 -36+6) |
4 |
2 |
6 |
|
(37-38+6) |
5 |
98 |
103 |
|
(39-40+6) |
5 |
63 |
68 |
|
≥ 41 |
5 |
12 |
17 |
|
Total |
25 |
175 |
200 |
Table 3: Gestational Age and neonatal outcomes of displaced women (n=200)
Antenatal complications included 14( 7%), Gestational Diabetes 2( 1%), 1(4%) with adverse neonatal outcomes, 1(0.6%) with normal neonatal outcomes, antepartum hemorrhage 2(1%), 1(4%) with adverse neonatal outcomes, 1(0.6%) with normal neonatal outcomes, 6(3%), one(4%) with adverse neonatal outcomes, 5(2.8%) with normal outcomes, preeclampsia (4%) with adverse neonatal outcomes, PPROM one(0.6%) with normal outcomes, severe preeclampsia in 2(8%) with adverse outcomes, and 186(86%),19(76%) adverse, and 167(95.4%) normal outcomes (Table 4).
|
Antenatal complications |
Neonatal outcomes |
Total |
|
|
Adverse |
Normal |
||
|
Gestational Diabetes |
1 |
1 |
2 |
|
Antepartum haemorrhage |
1 |
1 |
2 |
|
Pregnancy-induced hypertension |
1 |
5 |
6 |
|
Preeclampsia |
1 |
0 |
1 |
|
PPROM |
0 |
1 |
1 |
|
Sever preeclampsia |
2 |
0 |
2 |
|
No complications |
19 |
167 |
186 |
|
Total |
25 |
175 |
200 |
Table 4: Antenatal complications of displaced women and neonatal outcomes (n=200)
Neonatal weight less than2.5 kg were 18(9%), with adverse neonatal outcomes16(64%), with normal outcomes 2(1.1%), weight 2.5-3.5 kg was 79(39.5%), with adverse outcomess2(8%), with normal outcomes77(44%), weight>3.5-4 kg was 97(48.5%), with adverse neonatal outcomes 3(12%), with normal outcomes 94(53.7%), weight >4 kg was 6(3%), with adverse neonatal outcomess4(16%), with normal outcomes 2(1.1%) (Table 5).
|
Neonatal weight /kg
|
Neonatal outcomes |
Total |
|
|
Adverse |
Normal |
||
|
<2.5 |
16 |
2 |
18 |
|
2.5-3.5 |
2 |
77 |
79 |
|
>3.5-4 |
3 |
94 |
97 |
|
>4 |
4 |
2 |
6 |
|
Total |
25 |
175 |
200 |
Table 5: Neonatal weight and neonatal outcomes of displaced women (n=200)
The types of adverse neonatal outcomes among displaced women were intrauterine growth restriction (IUGR) in 4(16%), intrauterine fetal death in 3(12%), preterm labor in 5(20%), early neonatal death in 4(16%), need resuccitation 1(4%), respiratory distress syndrome (RDS) in 5(20%), and hypoglycemia in 2(8%) (Table 6).
|
Adverse neonatal outcomes |
Frequency |
Percent |
|
Intrauterine fetal death |
3 |
12 |
|
Preterm Labor |
5 |
20 |
|
Early Neonatal death |
4 |
16 |
|
Meconium spiration |
1 |
4 |
|
Fresh stillbirth |
1 |
4 |
|
RDS |
5 |
20 |
|
Intrauterine growth restriction |
4 |
16 |
|
Hypoglycemia |
2 |
8 |
|
Total |
25 |
100 |
Table 6: Type of Adverse neonatal outcomes among displaced women (n=200)
An APGAR score ≥9 was observed among 182(91%) neonates, 7(28%) had adverse neonatal outcomes, 175(100%) had normal neonatal outcomes, 14(7%), 14(56%) had adverse neonatal outcomes, and 4(16%) had adverse neonatal outcomes due to stillbirth (Figure 1).
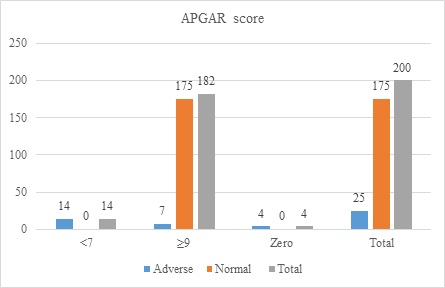 Figure 1: APGAR score and neonatal outcomes of displaced women (n=200)
Figure 1: APGAR score and neonatal outcomes of displaced women (n=200)
Admission to NICU was 22(11%), of 20(80%) of adverse neonatal outcomes and 2(1.1%) in normal neonatal outcomes cases (Figure 2).
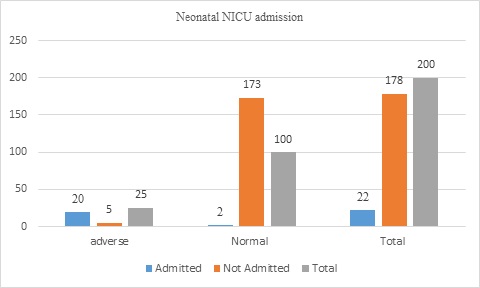 Figure 2: Neonatal NICU admission of displaced women (n=200)
Figure 2: Neonatal NICU admission of displaced women (n=200)
The maternal outcomes of the displaced women were postpartum hemorrhage (PPH) in 3(1.5%), amniotic fluid embolism in 1(0.5%), eclampsia in 1(0.5%), maternal death in 2(1%), and no complications in 193(96.5%). Of all women, four (2%) were admitted to the intensive care unit (ICU) (Figure 3&4).
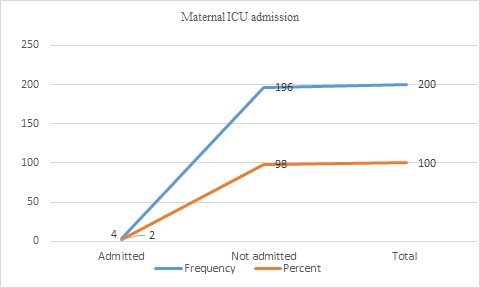 Figure 3: Maternal ICU admission of displaced women (n=200)
Figure 3: Maternal ICU admission of displaced women (n=200)
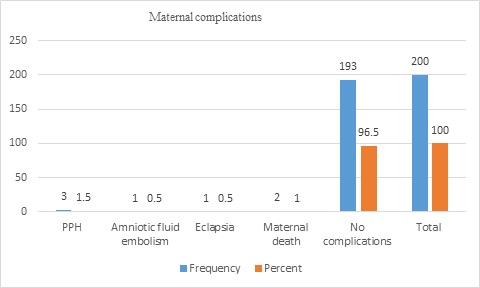 Figure 4: Maternal outcomes of displaced women (n=200)
Figure 4: Maternal outcomes of displaced women (n=200)
Discussion
This study was conducted among Sudanese women displaced from the hell of war to determine the effects of demographic and obstetric factors on adverse neonatal outcomes. The study found that the occurrence of several adverse neonatal outcomes was associated with specific demographic, maternal antenatal, and intrapartum characteristics. Maternal and fetal factors have determinant effects on neonatal outcomes.
Maternal age, residence, education, unbooked, medical illness, primigravida, grand multiparity, fetal gestational age, delivery by emergency cesarean section, neonatal birth weight, APGAR score, and maturity significantly adversely affect neonatal outcomes.
Advanced maternal age 40 years had 13.65% adverse neonatal outcomes, which is similar to a study in Swedish [29]. Occupation did not affect neonatal outcomes, but primary education (52%) was associated with adverse neonatal outcomes.
Rural residence was associated with adverse outcomes in 17.9%, and 3.9% of women residing in urban areas had adverse neonatal outcomes. This is similar to an Ethiopian study that found that neonates with adverse outcomes born to mothers who originally reside in rural areas [30] are also similar to South Ethiopia and Australian studies [31-32]. Most women in rural areas work during pregnancy, so they are unable to join antenatal care services easily due to distant locations, which leads to an increased risk of maternal complications during pregnancy and adverse neonatal outcomes.
Among the unbooked cases, 26.1% delivered babies with adverse outcomes, while 10.7% of booked women had adverse fetal outcomes, which is similar to a Nigerian study [33]. Booked women delivered by elective cesarean section in contrast to unbooked women who delivered an emergency cesarean section, as they did not plan the mode of delivery. Emergency caesarian sections, though a life-saving procedure also it is associated with maternal morbidity and neonatal morbidities which similar to Chourasia et al study [34].
In this study, we found that preterm labor and low birth weight were higher among unbooked women than among booked women.
In this study, adverse neonatal outcomes were most common in primigravida (17.1 %), less common in grand multiparous women (15%), and less common in multiparous women (11%), similar to the study by Schimmel et al. [35], which found that primiparous women had more adverse maternal and neonatal outcomes, similar to Lilin et al., who found that multiparity had a reduced risk of preterm labor and low birth weight in comparison with primigravida [36].
Maternal medical illnesses, especially Bronchial Asthma and Hypertension, significantly contributed to adverse neonatal outcomes. Among the eight women with medical illnesses in this study, 37.5% delivered adverse neonatal outcomes. A previous study of neonatal health with mother Asthma [37] found that bronchial asthma was associated with the risk of small for gestational age, prematurity, and adverse neonatal outcomes. Also, hypertension is associated with adverse outcomes similar to the study of chronic hypertension and adverse maternal and neonatal outcomes [38].
When we look at the impact of mode of delivery on neonatal outcome, elective cesarean section delivery was 3%, emergency cesarean section 39.4%, vaginal delivery 8%, VBAC 4%, and instrumental delivery. This study found that cesarean delivery was associated with more adverse neonatal outcomes than vaginal delivery, which can be explained by respiratory distress syndrome, IUGR, and fetal distress leading to emergency cesarean section. The macrosomic baby, IUGR, and SGA benefit from elective cesarean delivery, and emergency cesarean section increase the risk of adverse neonatal outcomes, which is similar to the study [39-40] which studied the mode of delivery and it is neonatal adverse effects. Women who underwent emergency cesarean section with different maternal or fetal indications had increased adverse neonatal outcomes, which agreed with a South African study and an Australian study [41-42], also cesarean delivery compared with vaginal delivery did not differ in the outcomes when related to preterm and small for gestational age.
Preterm birth is the delivery of a neonate less than 37 weeks of gestational, it causes neonatal mortality and morbidity, the adverse neonatal outcomes correlate inversely with the fetus's gestational age at delivery, all women presented at gestational age (28+0–33+6) weeks has adverse neonatal outcomes, gestational age (34+0–36+6) weeks 66.6% had adverse outcomes, gestational age ≥ 41asociated with adverse outcomes in29.4%, gestational age 37-38+6 associated with normal neonatal outcomes in 95%and gestational age 39-40+6 associated with adverse outcomes in 7.4%, which is similar to Bastek et al study [43] which found late preterm gestation associated with increased risk of adverse neonatal outcomes in compared with term pregnancy infant and 23% reduction in adverse neonatal outcomes with each week of progressive gestation from 32weeks to 39 weeks.
Antenatal complications associated with adverse neonatal outcomes include Gestational Diabetes. Antepartum hemorrhage, and pregnancy-induced hypertension by 50%, 50%, and 16.7 % respectively, and all cases of preeclampsia and severe preeclampsia were associated significantly with adverse fetal outcomes.
Neonatal weight ( < 2.5 kg) was significantly associated with adverse neonatal outcomes in 88.9%, weight >4 kg was associated with 66.7% of adverse outcomes, and neonatal weight > 3 kg was associated with 96.9% of normal fetal outcomes which could be explained that low birth weight at risk of prematurity, fetal death which macrosomia at risk of neonatal hypoglycemia.
An APGAR score < 7 was significantly associated with adverse neonatal outcomes in 100%, which is similar to [44] when an APGAR score less than 7 was significantly associated with adverse neonatal outcomes as well as infant mortality, while an APGAR score >9 had 4% adverse neonatal outcomes and four babies were stillbirths.
Types of adverse neonatal outcomes intrauterine fetal death 3(12%), preterm Labor5 (20%)
Early neonatal death at 16% and fresh stillbirth at 4%, which is similar to the study conducted in Eastern Sudan which found a neonatal mortality rate of 21.9% [45], and the Ethiopian study found 21.3% [46]. The neonatal death in this study is explained by very preterm, late preterm, low birth weight, and low APGAR score, which is similar to the Nigerian study [47]. In our study, most stillbirths were early neonatal deaths due to complications of prematurity and low birth weight fresh stillbirths occurred during labor events, and hospital delivery was described as safe due to early intervention.
Adverse neonatal outcomes were meconium spiration at 4%, respiratory distress syndrome (RDS) at 20%, IUGR at 16%, and hypoglycemia at 8%, which is similar to A. Thevarajah [48] found that 7.8% of infants developed hypoglycemia, and the risk increased 1.8-fold per gestational week at GDM diagnosis and 6.2-fold with a maternal history of macrosomia. In the neonatal intensive care unit admission, 90.9% had adverse outcomes, and only five neonates with adverse outcomes were not admitted, including intrauterine fetal death (IUFD) and fresh stillbirth. Severe preeclampsia and preeclampsia contribute to 12% of NICU admissions, which is similar to that reported in [49]. Maternal outcomes included PPH (1.5 %), amniotic fluid embolism (0.5 %), eclampsia (0.5 %), ICU admission (four women), and maternal death (two women).
This study had some limitations: the relatively small number of study participants may have negatively significant factors; neonates involved from one hospital may not indicate the situations of neonates delivered in other hospitals or private centers; therefore, the true situation of the community was not known, and it was difficult to generalize the results and make a comparison.
Conclusion
The study concluded that adverse neonatal outcomes were associated with advanced maternal age, rural residence, low education, primigravida, grand multiparous, unbooked women, and uncontrolled maternal medical disorders with pregnancy, delivery by emergency cesarean sections, early and late preterm fetuses, low birth weight, and macrosomia.
Adverse neonatal outcomes can be avoided with the introduction of protocols for the management of preterm labor, intrauterine growth restrictions, ultrasound detection, booking, and planned delivery.
Recommendations
Advise women about the importance of antenatal care visits and inform them of their conditions and needs. Preconception control of medical illnesses can improve neonatal outcomes.
Fetal surveillance for IUGR, SGA, and macrosomia. Local labor management protocols
Acknowledgments
The authors would like to express their sincere thanks and gratitude to all the women who participated in this study.
Consent
All participants provided informed consent.
Approval
Ethical clearance and supportive letters were obtained from White Nile University and Algabalin Teaching Hospital.
Author contribution
All authors contributed to the manuscript writing of the manuscript.
Disclosure statement
No potential conflicts of interest were reported by the authors.
Funding
Not funded
References
- International Statistical Classification of diseases and related health problems. (2011) Encyclopedia of Clinical Neuropsychology: 1347.
- Zupan J (2005) Perinatal Mortality in Developing Countries. New England Journal of Medicine 352: 2047-2048.
- World Health Organization, Department of essential drugs and medicines policy. (2005, February). Bundesgesundheitsblatt Gesundheitsforsch.Gesundheitsschutz, 48: 221-231.
- Goldenberg RL, McClure EM, Bhutta ZA, Belizán JM, Reddy UM, et al. (2011) Stillbirths: the vision for 2020. The Lancet 377: 1798-1805.
- Roberts I (2017) Nelson’s textbook of pediatrics (20th edn.), by R. Kliegman B Stanton J St. Geme N Schor (eds). Pediatric Radiology 47: 1364-1365.
- Kassa GM, Arowojolu AO, Odukogbe AA, Yalew AW (2019) Adverse neonatal outcomes of adolescent pregnancy in Northwest Ethiopia. PLOS ONE 14: e0218259.
- Bastek JA, Sammel MD, Paré E, Srinivas SK, Posencheg MA, et al. (2008) Adverse neonatal outcomes: examining the risks between preterm, late preterm, and term infants. American Journal of Obstetrics and Gynecology 199: 367.e1-367.e8.
- Wu Y, Chen Y, Shen M, Guo Y, Wen SW, e tal. (2019) Adverse maternal and neonatal outcomes among singleton pregnancies in women of very advanced maternal age: a retrospective cohort study. BMC Pregnancy and Childbirth 19.
- Gutbir Y, Wainstock T, Sheiner E, Segal I, Sergienko R, et al. (2020) Low Apgar score in term newborns and long-term infectious morbidity: A population-based cohort study with up to 18 years of follow-up. European Journal of Pediatrics 179: 959-971.
- Cnattingius S, Johansson S, Razaz N (2020) Apgar Score and Risk of Neonatal Death Among Preterm Infants. Obstetric Anesthesia Digest 40: 194-195.
- Finken MJJ, van der Steen M, Smeets CCJ, Walenkamp MJE, de Bruin C, ett al. (2018) Children Born Small for Gestational Age: Differential Diagnosis, Molecular Genetic Evaluation, and Implications. Endocrine Reviews 39: 851-894.
- Schlaudecker EP, Munoz FM, Bardají A, Boghossian NS, Khalil A, et al. (2017) Small for gestational age: Case definition & guidelines for data collection, analysis, and presentation of maternal immunisation safety data. Vaccine 35: 6518-6528.
- Lehtonen L, Gimeno A, Parra-Llorca A, Vento M (2017) Early neonatal death: A challenge worldwide. Seminars in Fetal and Neonatal Medicine 22: 153-160.
- Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, et al. (2019) Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. The Lancet Global Health 7: e37-e46.
- Platt M (2014) Outcomes in preterm infants. Public Health 128: 399-403.
- Saigal S, Doyle LW (2008) An overview of mortality and sequelae of preterm birth from infancy to adulthood. The Lancet 371: 261-269.
- Regional consultation on the WHO-UNICEF global report on assistive technology (2022) Eastern Mediterranean Health Journal 28: 847-848.
- Garg R (2023) DC Dutta\’s Textbook of Obstetrics. Journal of South Asian Federation of Obstetrics and Gynaecology 15: 375-375.
- Liu L, Oza S, Hogan D, Perin J, Rudan I, et al. (2015) Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. The Lancet 385: 430-440.
- Kumar N, Yadav A (2020) Perinatal Outcome in Women with Hypertensive Disorders of Pregnancy in Rural Tertiary Center of Northern India: A Retrospective Cohort Study”. Current Pediatric Reviews, 16: 71-78.
- Gebremariam A (2006) Factors predisposing to low birth weight in Jimma Hospital South Western Ethiopia. East African Medical Journal 82: 554-558.
- Træen B, Fischer N, Grøndahl P (2022) Norwegian Data on Prevalence, Sexual Risk Behaviors, Sexual Problems, and Sexual Satisfaction in Women Who Have Sex Exclusively with Women, Women Who Have Sex Exclusively with Men, and Women Who Have Sex with Men and Women. International Journal of Sexual Health 35: 152-166.
- Rayis D (2013) An Epidemic of Cesarean Deliveries at Khartoum Hospital in Sudan with Over Two-Fifths of Neonates Delivered through the Abdomen. Journal of Women’s Health Issues and Care.
- Infant-mortality-rate (2023) Sudan Infant Mortality Rate 1950-2023
- Werner E, Savitz D, Janevic T, Ehsanipoor R, Thung S, et al. (2013) Mode of Delivery and Neonatal Outcomes in Preterm, Small-for-Gestational-Age Newborns. Obstetric Anesthesia Digest 33: 213-214.
- Sayad E, Silva-Carmona M (2023) Meconium Aspiration. In: StatPearls Treasure Island (FL): StatPearls Publishing.
- Adamkin DH (2017) Neonatal hypoglycemia. Seminars in Fetal and Neonatal Medicine 22: 36-41.
- Rubenfeld GD, Slutsky AS, Thompson BT, (2012)Definition of Acute Respiratory Distress Syndrome-Reply. JAMA 13: 1321.
- Sydsjö G, Lindell Pettersson M, Bladh M, Skoog Svanberg A, Lampic C., (2019) Evaluation of risk factors’ importance on adverse pregnancy and neonatal outcomes in women aged 40 years or older. BMC Pregnancy and Childbirth 19.
- Workineh YA, Workie HM (2022) Adverse Neonatal Outcomes and Associated Risk Factors: A Case-Control Study. Global Pediatric Health 9: 2333794X2210840.
- Mingude AB, Gebretsadik W, Misker D, Woldeamanuel GG (2020) Determinants of low birth weight among live birth newborns delivered at public hospitals in Gamo Gofa Zone, South Ethiopia: Unmatched case control study. SAGE Open Medicine 8: 205031212094054.
- Abdel-Latif ME, Bajuk B, Oei J, Vincent T, Sutton L (2006) Does rural or urban residence make a difference to neonatal outcome in premature birth? A regional study in Australia. Archives of Disease in Childhood - Fetal and Neonatal Edition 91: F251-F256.
- Okojie O, Ogboghodo E, Omoijuanfo E (2022) Maternal and Neonatal Outcomes of Booked and Un-booked Pregnancies in Benin City, Southern Nigeria: A Comparative Study. Journal of Community Medicine and Primary Health Care 34: 109-127.
- Chourasia S, Yadav K (2016) Analytical study to assess fetal and perinatal outcome in booked and unbooked obstetric cases. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 6: 203.
- Schimmel MS, Bromiker R, Hammerman C, Chertman L, Ioscovich A, et al. (2014) The effects of maternal age and parity on maternal and neonatal outcome. Archives of Gynecology and Obstetrics 291: 793-798.
- Lin L, Lu C, Chen W, Li C, Guo VY (2021) Parity and the risks of adverse birth outcomes: a retrospective study among Chinese. BMC Pregnancy and Childbirth 21.
- Mendola P, Männistö TI, Leishear K, Reddy UM, Chen Z, et al. (2014) Neonatal health of infants born to mothers with asthma. Journal of Allergy and Clinical Immunology 133: 85-90.
- Broekhuijsen K, Ravelli AC, Langenveld J, van Pampus MG, van den Berg PP (2015) Maternal and neonatal outcomes of pregnancy in women with chronic hypertension: a retrospective analysis of a national register. Acta Obstetricia Et Gynecologica Scandinavica 94: 1337-1345.
- Riskin A, Riskin-Mashiah S, Bader D, Kugelman A, Lerner-Geva L, et al. (2008)Delivery Mode and Severe Intraventricular Hemorrhage in Single, Very Low Birth Weight, Vertex Infants. Obstetrics & Gynecology 112: 21–28.
- Riskin A, Riskin-Mashiah S, Lusky A, Reichman B (2004) The relationship between delivery mode and mortality in very low birthweight singleton vertex?presenting infants. BJOG: An International Journal of Obstetrics & Gynaecology 111: 1365-1371.
- Bishop D, Dyer RA, Maswime S, Rodseth RN, van Dyk D, et al. (2019) Maternal and neonatal outcomesafter caesarean delivery in the African Surgical OutcomesStudy: a 7-day prospective observational cohort study. Lancet Glob Health 7: e513-e22.
- Soong S, Greer RM, Gardener G, Flenady V, Kumar S (2015) Impact of mode of delivery after 32 weeks’ gestation on neonatal outcome in dichorionic diamniotic twins. Journal of Obstetrics and Gynaecology Research 42: 392-398.
- Bastek JA, Sammel MD, Paré E, Srinivas SK, Posencheg MA, Elovitz MA (2008) Adverse neonatal outcomes: examining the risks between preterm, late preterm, and term infants. American Journal of Obstetrics and Gynecology 199: 367.e1-367.e8.
- Chen HY, Blackwell SC, Chauhan SP (2020) Association between apgar score at 5 minutes and adverse outcomes among Low-Risk pregnancies. The Journal of Maternal-Fetal & Neonatal Medicine, 35(7), 1344–1351.
- Ahmed MAA, Mahgoub HM, Al-Nafeesah A, Al-Wutayd O, Adam I (2022) Neonatal Mortality and Associated Factors in the Neonatal Intensive Care Unit of Gadarif Hospital, Eastern Sudan. Children 9: 1725.
- Nabwera HM, Wang D, Tongo OO, Andang'o PEA, Abdulkadir I, et al. (2021) Burden of disease and risk factors for mortality amongst hospitalized newborns in Nigeria and Kenya. PLOS ONE 16: e0244109.
- https://www.who.int/initiatives/every-newborn-action-plan
- Thevarajah A, Simmons D (2019) Risk factors and outcomes for neonatal hypoglycaemia and neonatal hyperbilirubinaemia in pregnancies complicated by gestational diabetes mellitus: a single centre retrospective 3?year review. Diabetic Medicine 36: 1109-1117.
- Habli M, Levine RJ, Qian C, Sibai B (2007) Neonatal outcomes in pregnancies with preeclampsia or gestational hypertension and in normotensive pregnancies that delivered at 35, 36, or 37 weeks of gestation. American Journal of Obstetrics and Gynecology, 197: 406.e1-406.e7.
Citation: Abdelwahid A, Mohammed K, Eltigania A, Siralkatim I, Suliman H (2023) Adverse Neonatal outcomes Effects of Demographic and Obstetric factors among Displaced Sudanese Women. J Neonatol Clin Pediatr 10: 117.
Copyright: © 2023 Awadalla Abdelwahid, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

