
Age Related Changes of Trace Element Contents in Intact Thyroid of Females Investigated by Neutron Activation Analysis
*Corresponding Author(s):
Vladimir ZaichickRadionuclide Diagnostics Department, Medical Radiological Research Centre, Russian Federation
Tel:+7 4843960289,
Fax:+7 4959561440
Email:vezai@obninsk.com
Abstract
The prevalence of thyroid dysfunction is higher in the elderly as compared to the younger population. An excess or deficiency of trace element contents in thyroid plays an important role in goitro - and carcinogenesis of gland. The variation with age of the mass fraction of silver (Ag), cobalt (Co), chromium (Cr), iron (Fe), mercury (Hg), rubidium (Rb), antimony (Sb), scandium (Sc), selenium (Se) and zinc (Zn) in intact (normal) thyroid of 33 females (mean age 54.5 years, range 3.5-87) was investigated by instrumental neutron activation analysis with high resolution spectrometry of long-lived radionuclides. Mean values +(or)- standard error of mean for mass fractions (mg/kg, on dry-mass basis) of the trace elements studied were: Ag 0.0140+(or)-0.0020, Co 0.0505+(or)-0.0064, Cr 0.573+(or)-0.049, Fe 232+(or)-22, Hg 0.0329+(or)-0.0051, Rb 6.16+(or)-0.48, Sb 0.116+(or)-0.012, Sc 0.0042+(or)-0.0012, Se 2.22+(or)-0.23 and Zn 85.7+(or)-7.4. This work revealed that there is a significant increase in Co, Rb, Sb and Zn mass fraction in normal female thyroid during a lifespan. Therefore, a goitro- and carcinogenic effect of excessive Co, Fe, Rb, Sb and Zn level in the thyroid of old females should be studied.
Keywords
ABBREVIATIONS
INAA-LLR: Instrumental Neutron Activation Analysis with high resolution spectrometry of Long-Lived Radionuclides
CRM/SRM: Certified/Standard Reference Materials
IAEA: International Atomic Energy Agency
INTRODUCTION
The endocrine organs, including the thyroid gland, undergo important functional changes during aging and a prevalence of thyroid dysfunction is higher in the elderly as compared to the younger population [1,2]. Advancing age is known to influence the formation of adenomatous goiter and thyroid cancer [3]. The prevalence of thyroid nodules is increased in the elderly, reaching a frequency of nearly 50% by the age of 65 [4]. Both prevalence and aggressiveness of thyroid cancer increase with age [2]. Women are affected by thyroid nodule and cancer two to five times more often than men [2-5].
Aging is a complex process involving biochemical and morphologic changes in single cells, in organs and in the whole organism. One of the most generally accepted explanations of how aging occurs at the molecular level is the oxidative stress hypothesis [6]. Reactive Oxygen Species (ROS) are widely considered to be a causal factor not only in aging but in a number of pathological conditions, including carcinogenesis. Aging, considered as an impairment of body functions over time, caused by the accumulation of molecular damage in DNA, proteins and lipids, is also characterized by an increase in intracellular oxidative stress due to the progressive decrease of the intracellular ROS scavenging [7]. Oxidative damage to cellular macromolecules which induce cancer can also arise through overproduction of ROS and faulty antioxidant and/or DNA repair mechanisms [8]. Overproduction of ROS is associated with inflammation, radiation and some other factors, including overload of some trace elements, in both blood and certain tissues, or deficiency of other trace elements with antioxidant properties [9-15]. Studies have shown that the imbalance in the composition of trace elements may cause different types of pathology. The importance of appropriate levels of many trace elements is indisputable, due to their beneficial roles when in specific concentration ranges, while on the other hand they can cause toxic effects with excessively high or low concentrations [12].
In our previous studies [16-24] the high mass fraction of Iodine (I) and some other trace element were observed in intact human thyroid gland when compared with their levels in non-thyroid soft tissues of the human body. However, some questions about the age-dependence of trace element mass fraction in thyroid of adult and particularly, elderly females still remain unanswered. One valuable way to elucidate the situation is to compare the mass fractions of trace elements in young adult (the control group) with those in older adult and geriatric thyroid. The findings of the excess or deficiency of trace element contents in thyroid and the perturbations of their relative proportions in glands of adult and elderly females, may give an indication of their role in a higher prevalence of thyroid dysfunction in the elderly. The reliable data on trace element mass fractions in normal geriatric thyroid is apparently extremely limited. There are many studies regarding trace element content in human thyroid, using chemical techniques and instrumental methods [25-36]. However, the majority of these data are based on measurements of processed tissue and in many studies tissue samples are ashed (are burned in a muffle furnace) before analysis. In other cases, thyroid samples are treated with solvents (distilled water, ethanol etc.,) and then are dried at a high temperature for many hours. There is evidence that certain quantities of trace elements are lost as a result of such treatment [37-39]. Moreover, only a few of these studies employed quality control using Certified/Standard Reference Materials (CRM/SRM) for determination of the trace element mass fractions.
This work had three aims. The primary purpose of this study was to determine reliable values for the silver (Ag), cobalt (Co), chromium (Cr), iron (Fe), mercury (Hg), rubidium (Rb), antimony (Sb), scandium (Sc), selenium (Se) and zinc (Zn) mass fractions in the normal (intact) thyroid of subjects ranging from children to elderly females using non-destructive Instrumental Neutron Activation Analysis with high resolution spectrometry of Long-Lived Radionuclides (INAA-LLR). The second aim was to compare the Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se and Zn mass fractions in thyroid gland of age group 2 (adults and elderly persons aged 41 to 87 years), with those of group 1 (from 3.5 to 40 years) as well as to find the correlations between age and trace element contents and the final aim was to estimate the inter-correlations of trace elements in normal thyroid of females. All studies were approved by the Ethical Committee of the Medical Radiological Research Center, Obninsk, Russia (the reference no. 115050610007).
MATERIALS AND METHODS
Samples of the human thyroid were obtained from randomly selected autopsy specimens of 33 females (European-Caucasian) aged 3.5 to 87 years within 48 hours after a sudden death. All the deceased were citizens of Obninsk and had undergone routine autopsy at the Forensic Medicine Department of City Hospital, Obninsk. Age ranges for subjects were divided into two age groups, with group 1, 3.5-40 years (30.9±3.1 years, M±SEM, n=11) and group 2, 41-87 years (66.3±2.7 years, M±SEM, n=22). These groups were selected to reflect the condition of thyroid tissue in the children, teenagers, young adults and first period of adult life (group 1) and in the second period of adult life as well as in old age (group 2). The available clinical data were reviewed for each subject. None of the subjects had a history of an intersex condition, endocrine disorder, or other chronic disease that could affect the normal development of the thyroid. None of the subjects were receiving medications or used any supplements known to affect thyroid trace element contents. The typical causes of sudden death of most of these subjects included trauma or suicide and also acute illness (cardiac insufficiency, stroke, embolism of pulmonary artery, alcohol poisoning). All right lobes of thyroid glands were divided into two portions using a titanium scalpel [40]. One tissue portion was reviewed by an anatomical pathologist while the other was used for the trace element content determination. A histological examination was used to control the age norm conformity as well as the unavailability of microadenomatosis and latent cancer.
After the samples intended for chemical element analysis were weighed, they were transferred to -20°C and stored until the day of transportation in the Medical Radiological Research Center, Obninsk, where all samples were freeze-dried and homogenized [41]. The pounded sample weighing about 50mg was used for trace element measurement by INAA-LLR. The samples for INAA-LLR were wrapped separately in a high-purity aluminum foil washed with rectified alcohol beforehand and placed in a nitric acid-washed quartz ampoule.
To determine contents of the elements by comparison with a known standard, Biological Synthetic Standards (BSS) prepared from phenol-formaldehyde resins were used [42]. In addition to BSS, aliquots of commercial, chemically pure compounds were also used as standards. Ten certified reference material IAEA H-4 (animal muscle) and IAEA HH-1 (human hair) sub-samples weighing about 50mg were treated and analyzed in the same conditions that thyroid samples to estimate the precision and accuracy of results. The vertical channel of nuclear reactor was applied to determine the content of Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se and Zn by INAA-LLR. The quartz ampoule with thyroid samples, standards and certified reference material was soldered, positioned in a transport aluminum container and exposed to a 24-hour neutron irradiation in a vertical channel with a neutron flux of 1.3 1013 ncm-2s-1. Ten days after irradiation samples were reweighed and repacked. The samples were measured for period from 10 to 30 days after irradiation. The duration of measurements was from 20 min to 10 hours subject to pulse counting rate. The gamma spectrometer included the 100 cm3Ge (Li) detector and on-line computer-based MCA system. The spectrometer provided a resolution of 1.9 keV on the 60 Co 1332 keV line. Details of used nuclear reactions, radionuclides and gamma-energies were presented in our earlier publications concerning the INAA chemical element contents in human prostate and scalp hair [43,44].
A dedicated computer program for INAA mode optimization was used [45]. All thyroid samples were prepared in duplicate and mean values of trace element contents were used in final calculation. Using Microsoft Office Excel, a summary of the statistics, including, arithmetic mean, standard deviation, standard error of mean, minimum and maximum values, median, percentiles with 0.025 and 0.975 levels was calculated for trace element contents. The reliability of difference in the results between two age groups was evaluated by the parametric Student’s t-test and non-parametric Wilcoxon-Mann-Whitney U-test. For the construction of “age - trace element mass fraction” diagrams and the estimation of the Pearson correlation coefficient between age and trace element mass fraction as well as between different trace elements the Microsoft Office Excel programs were also used.
RESULTS
Table 1 depicts our data for Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se and Zn mass fractions in ten sub-samples of IAEA H-4 (animal muscle) and IAEA HH-1 (human hair) certified reference material and the certified values of this material. Table 2 represents certain statistical parameters (arithmetic mean, standard deviation, standard error of mean, minimal and maximal values, median, and percentiles with 0.025 and 0.975 levels) of the Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se and Zn mass fractions in intact (normal) thyroid of females. The comparison of our results with published data for the Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se and Zn contents in the human thyroid is shown in table 3. To estimate the effect of age on the trace element contents we examined two age groups, described above (Table 4). In addition, the Pearson correlation coefficient between age and trace element mass fraction was calculated (Table 5). Figure 1 shows the individual data sets for the Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se and Zn mass fraction in all samples of thyroid and also lines of trend with age. The data of inter-correlation calculations (values of r-coefficient of correlation) including all trace elements identified by us are presented in table 6.
| Element | IAEA H-4 Animal Muscle | This Work Results | IAEA HH-1 Human Hair | This Work Results |
| 95% Confidence Interval | M±SD | 95% Confidence Interval | M±SD | |
| Ag | - | 0.033±0.008 | 0.19b | 0.18±0.05 |
| Co | 0.0027b | 0.0034±0.0008 | 5.97±0.42a | 5.4±1.1 |
| Cr | 0.06b | 0.071±0.010 | 0.27b | ≤0.3 |
| Fe | 49.1±6.5a | 47.0±1.0 | 23.7±3.1a | 25.1±4.3 |
| Hg | 0.014b | 0.015±0.004 | 1.70±0.09a | 1.54±0.14 |
| Rb | 18.7±3.5a | 23.7±3.7 | 0.94b | 0.89±0.17 |
| Sb | 0.0056b | 0.0061±0.0021 | 0.031b | 0.033±0.009 |
| Sc | 0.0059b | 0.0015±0.0009 | - | - |
| Se | 0.28±0.08a | 0.281±0.014 | 0.35±0.02a | 0.37±0.08 |
| Zn | 86.3±11.5a | 91±2 | 174±9a | 173±17 |
| Gender | Element | M | SD | SEM | Min | Max | Median | P 0.025 | P 0.975 |
| Ag | 0.0140 | 0.0093 | 0.0020 | 0.0012 | 0.0331 | 0.0130 | 0.0021 | 0.0321 | |
| Co | 0.0505 | 0.0322 | 0.0064 | 0.0170 | 0.140 | 0.0405 | 0.0183 | 0.130 | |
| Cr | 0.573 | 0.246 | 0.049 | 0.290 | 1.22 | 0.488 | 0.303 | 1.11 | |
| Fe | 232 | 112 | 22 | 63 | 512 | 199 | 64.8 | 480 | |
| Females n=33 | Hg | 0.0329 | 0.0246 | 0.0051 | 0.0065 | 0.100 | 0.0263 | 0.0079 | 0.100 |
| Rb | 6.16 | 2.42 | 0.48 | 1.11 | 12.8 | 6.3 | 2.38 | 10.8 | |
| Sb | 0.116 | 0.063 | 0.012 | 0.0115 | 0.248 | 0.108 | 0.0183 | 0.247 | |
| Sc | 0.0042 | 0.0040 | 0.0012 | 0.0002 | 0.0143 | 0.0032 | 0.0003 | 0.0124 | |
| Se | 2.22 | 1.19 | 0.23 | 0.439 | 5.32 | 2.07 | 0.773 | 4.85 | |
| Zn | 85.7 | 38 | 7.44 | 8.10 | 166 | 83 | 22.9 | 156 |
| Element | Published Data [Reference] | This work | ||
| Median of Means | Minimum of Means | Maximum of Means | ||
| (n)* | M or M±SD, (n)** | M or M±SD, (n)** | M±SD | |
| Ag | 0.25 (12) | 0.000784 (16) [25] | 1.20±1.24(105) [26] | 0.0140±0.0093 |
| Co | 0.336 (17) | 0.026±0.031 (46) [27] | 70.4±40.8 (14) [28] | 0.0505±0.0322 |
| Cr | 0.69 (17) | 0.105 (18) [29] | 24.8±2.4 (4) [30] | 0.573±0.246 |
| Fe | 252 (21) | 56 (120) [31] | 2444±700 (14) [28] | 232±112 |
| Hg | 0.08(13) | 0.0008±0.0002(10) [32] | 396±40 (4) [30] | 0.0329±0.0246 |
| Rb | 12.3 (9) | ≤0.85 (29) [32] | 294±191 (14) [28] | 6.16±2.42 |
| Sb | 0.105 (10) | 0.040±0.003(-) [33] | 4.0 (-) [34] | 0.116±0.063 |
| Sc | 0.009 (4) | 0.0018±0.0003(17) [35] | 0.0135±0.0045 (10) [32] | 0.0042±0.0040 |
| Se | 2.61 (17) | 0.95±0.08(29) [32] | 756±680 (14) [28] | 2.22±1.19 |
| Zn | 118 (51) | 32(120) [31] | 820±204 (14) [28] | 85.7±38.0 |
| Element | Female Thyroid Tissue | Ratio | |||
| AG1 | AG2 | t-test | U-test | AG2 to AG1 | |
| 3.5-40 years | 41-87 years | p≤ | p | ||
| n=11 | n=22 | ||||
| Ag | 0.0143±0.0032 | 0.0138±0.0027 | 0.909 | >0.05 | 0.97 |
| Co | 0.0328±0.0042 | 0.0644±0.0096 | 0.0076 | £1. | 1.96 |
| Cr | 0.567±0.065 | 0.578±0.073 | 0.913 | >0.05 | 1.02 |
| Fe | 172±22 | 279±31 | 0.011 | £1. | 1.62 |
| Hg | 0.0275±0.0046 | 0.0370±0.0084 | 0.333 | >0.05 | 1.35 |
| Rb | 4.95±0.58 | 7.05±0.63 | 0.021 | £.01 | 1.42 |
| Sb | 0.0880±0.0096 | 0.136±0.019 | 0.034 | £.1 | 1.55 |
| Sc | 0.0026±0.0017 | 0.0045±0.0014 | 0.438 | >0.05 | 1.73 |
| Se | 1.86±0.27 | 2.48±0.34 | 0.169 | >0.05 | 1.33 |
| Zn | 59.8±8.7 | 104.7±8.4 | 0.0011 | £.1 | 1.75 |
| Element | Ag | Co | Cr | Fe | Hg | Rb | Sb | Sc | Se | Zn |
| Age | 0.089 | 0.565b | 0.199 | 0.262 | 0.038 | 0.516b | 0.472a | 0.291 | 0.468a | 0.609c |
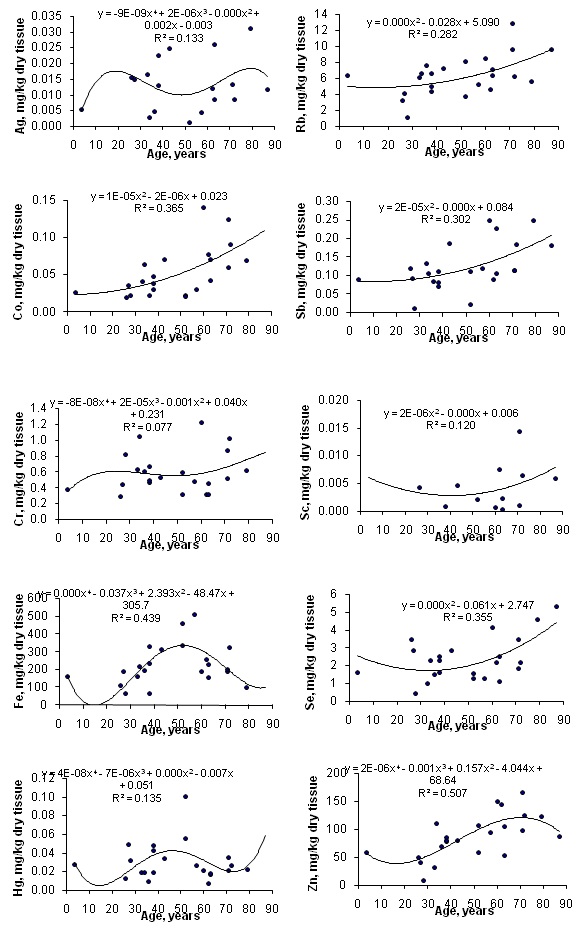 Figure 1: Data sets of individual Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se and Zn mass fraction values in intact thyroid of females and their trend lines.
Figure 1: Data sets of individual Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se and Zn mass fraction values in intact thyroid of females and their trend lines.
| Element | Co | Cr | Fe | Hg | Rb | Sb | Sc | Se | Zn |
| Ag | 0.182 | -0.083 | -0.468a | -0.340 | -0.231 | 0.411a | -0.158 | 0.253 | 0.012 |
| Co | 1 | 0.499a | -0.043 | -0.260 | 0.572b | 0.644c | 0.408a | 0.606b | 0.804c |
| Cr | 0.499a | 1 | -0.172 | -0.239 | 0.257 | 0.319 | -0.133 | 0.213 | 0.312 |
| Fe | -0.043 | -0.172 | 1 | 0.550b | 0.108 | -0.070 | -0.102 | -0.240 | 0.214 |
| Hg | -0.260 | -0.239 | 0.550b | 1 | -0.048 | -0.351a | -0.083 | -0.150 | -0.174 |
| Rb | 0.572b | 0.257 | 0.108 | -0.048 | 1 | 0.276 | 0.417a | 0.401a | 0.581b |
| Sb | 0.644c | 0.319 | -0.070 | -0.351a | 0.276 | 1 | -0.205 | 0.674c | 0.441a |
| Sc | 0.408a | -0.133 | -0.102 | -0.083 | 0.417a | -0.205 | 1 | 0.312 | 0.520a |
| Se | 0.606b | 0.213 | -0.240 | -0.15 | 0.401a | 0.674c | 0.312 | 1 | 0.470a |
| Zn | 0.804c | 0.312 | 0.214 | -0.174 | 0.581b | 0.441a | 0.520a | 0.470a | 1 |
DISCUSSION
Good agreement of the Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se and Zn contents analyzed by INAA-LLR with the certified data of CRM IAEA H-4 and IAEA HH-1 (Table 1) indicates an acceptable accuracy of the results obtained in the study of trace elements of the thyroid presented in tables 2-5. The obtained values for Cr, Fe, Hg, Rb, Sb, Sc, Se and Zn contents, as shown in table 3, agree well with median of means cited by other researches for the human thyroid, including samples received from persons who died from different diseases. However, the means for Ag and Co are an order of magnitude lower than the median of previously reported data. A number of values for trace element mass fractions were not expressed on a dry mass basis by the authors of the cited references. However, we calculated these values using published data for water (75%) and ash (4.16% on dry mass basis) contents in thyroid of adults [27,46].
A significant age-related increase in Co, Fe, Rb, Sb and Zn mass fraction was observed in female thyroid (Table 4). In second group of females with mean age 66.3 years the mean mass fractions of these trace elements in thyroids were 1.42-1.96 times higher than in thyroids of the first age group (mean age 30.9 years). There were no statistically significant differences between the Ag, Cr, Hg, Sc and Se mass fractions within two different age-groups. Age-dependence of some trace element mass fractions found using the comparison between results for two age groups was confirmed for Co, Rb, Sb and Zn, while was not confirmed for Fe, when the Pearson correlation coefficient was calculated (Table 5). Moreover, the Pearson correlation coefficient showed a significant increase in Se mass fraction in female thyroid with age (Table 5).
The mass fractions of Co, Rb and Sb began to increase from the third decade and reached the highest values in the thyroid of elderly persons (Figure 1). The mass fraction of Fe increased in the third to sixth decades and reached a maximum at about the age of 50-60 years. After age 60 years, level of Fe began to decrease (Figure 1). This is the reason why the Pearson correlation did not show the age-dependence for Fe. The mass fraction of Zn increased in the third to seven decades and reached a maximum at about the age of 70 years. After age 70 years, content of Zn was maintained at more or less steady level (Figure 1). Age-dependence of Co, Fe and Zn mass fractions showed in present study did not agree with earlier findings. No changes with age in Fe mass fraction was demonstrated in the study of Ataulchanov [31]. In accord with results of Ataulchanov, the mass fractions of Co and Zn have maximum at age 26-30 and 31-35 years, respectively [31]. Vlasova, in contrast, found that Zn content in the human thyroid decrease with age [26]. No other published data referring to age-related changes of Co, Fe, Rb, Sb and Zn mass fractions in human thyroid were found.
A significant direct correlation between the Co-Cr, Co-Rb, Co-Sc, Co-Se, Co-Zn, Fe-Hg, Rb-Sc, Rb-Se, Rb-Zn, Sb-Se, Sb-Zn, Sc-Zn and Se-Zn mass fractions as well as an inverse correlation between Ag-Fe and Hg-Sb mass fractions was seen in female thyroid. No correlation was demonstrated between any other chemical elements (Table 6). If some correlations between the elements were predictable (e.g., Fe-Co), the interpretation of other observed relationships would require further study. No published data referring to inter-correlations Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se and Zn mass fractions in thyroid of females was found.
An age-related increase and excess in Co, Fe, Rb, Sb and Zn mass fractions in thyroid tissue may contribute to harmful effects on the gland. There are good reasons for such speculations since many reviews and numerous papers raise the concern about toxicity and tumorigenesis of the metals [10,43,47-79]. Each of the metals is distinct in its primary mode of action. Moreover, there are several forms of synergistic action of the metals as a part of intracellular metabolism, during which several reactive intermediates and byproducts are created [47,48,53]. These reactive species are capable of potent and surprisingly selective activation of stress-signaling pathways, inhibition of DNA metabolism, repair and formation of DNA cross links, which are known to contribute to the development of human cancers [48,80,81]. In addition to genetic damage via both oxidative and non oxidative (DNA adducts) mechanisms, metals can also cause significant changes in DNA methylation and histone modifications, leading to alterations in gene expression [49,51,80]. Invitro and animal tumorigenic studies provided strong support for the idea that metals can also act as co-carcinogens in combination with nonmetal carcinogens [80].
All the deceased were citizens of Obninsk. Obninsk is the small nonindustrial city not far from Moscow in unpolluted area. None of those who died a sudden death had suffered from any systematic or chronic disorders before. The normal state of thyroid was confirmed by morphological study. Thus, our data for Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se and Zn mass fractions in intact thyroid may serve as indicative normal values for females of urban population of the Russian Central European region. This study has several limitations. Firstly, analytical techniques employed in this study measure only 10 trace elements (Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se and Zn) mass fractions. Future studies should be directed toward using other analytical methods which allow extend the list of chemical elements investigated in thyroid tissue. Secondly, the sample size of our study was relatively small. Nonetheless, our data showed the pronounced tendency of increase in Co, Rb, Sb and Zn mass fraction in the normal thyroid of female during a lifespan. This tendency was found by two ways of statistical data manipulation. Age-dependence of Fe and Se was confirmed by only one from two ways of statistical data manipulation used in the study. Therefore, large studies are needed in the future to confirm the effect of age on Fe and Se contents in normal thyroid of females. Lastly, generalization of our results may be limited to Russian women. In spite of these limitations, this is the first study that evaluated the relationship between age and trace element levels in normal thyroid of females.
CONCLUSION
The instrumental neutron activation analysis with high resolution spectrometry of long-lived radio nuclides is a useful analytical tool for the non-destructive determination of trace element content in the thyroid tissue samples. This method allows determine means for Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se and Zn (10 trace elements). Our data reveal that there is a significant increase in Co, Rb, Sb and Zn mass fraction in the normal thyroid of female during a lifespan. Therefore, a goitro- and carcinogenic effect of excessive Co, Fe, Rb, Sb and Zn level in the thyroid of old females should be studied.
ACKNOWLEDGEMENT
We are grateful to Dr. Yu Choporov, Head of the Forensic Medicine Department of City Hospital, Obninsk, for supplying thyroid samples.
REFERENCES
- Gesing A (2015) The thyroid gland and the process of aging. Thyroid Res 8: 8.
- Mitrou P, Raptis SA, Dimitriadis G (2011) Thyroid disease in older people. Maturitas 70: 5-9.
- Kwong N, Medici M, Angell TE, Liu X, Marqusee E, et al. (2015) The influence of patient age on thyroid nodule formation, multinodularity, and thyroid cancer risk. J Clin Endocrinol Metab 100: 4434-4440.
- Mazzaferri EL (1993) Management of a solitary thyroid nodule. NEJM 328: 553-559.
- Smailyte G, Miseikyte-Kaubriene E, Kurtinaitis J (2006) Increasing thyroid cancer incidence in Lithuania in 1978-2003. BMC Cancer 11: 284.
- Olinski R, Siomek A, Rozalski R, Gackowski D, Foksinski M, et al. (2007) Oxidative damage to DNA and antioxidant status in aging and age-related diseases. Acta Biochim Pol 54: 11-26.
- Minelli A, Bellezza I, Conte C, Culig Z (2009) Oxidative stress-related aging: A role for prostate cancer? Biochim Biophys Acta 1795: 83-91.
- Klaunig JE, Kamendulis LM, Hocevar BA (2010) Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol 38: 96-109.
- Järup L (2003) Hazards of heavy metal contamination. Br Med Bull 68: 167-182.
- Zaichick V, Zaichick S (1999) Role of zinc in prostate cancerogenesis. In: Anke M (ed.). Mengen- und Spurenelemente. Friedrich-Schiller-Universitat, Jena, Germany. Pg No: 104-115
- Zaichick V (2004) INAA and EDXRF applications in the age dynamics assessment of Zn content and distribution in the normal human prostate. J Radioanal Nucl Chem 262: 229-234.
- Zaichick V (2006) Medical elementology as a new scientific discipline. J Radioanal Nucl Chem 269: 303-309.
- Toyokuni S (2008) Molecular mechanisms of oxidative stress-induced carcinogenesis: from epidemiology to oxygenomics. IUBMB Life 60: 441-447.
- Gupte A, Mumper RJ (2009) Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat Rev 35: 32-46.
- Lee JD, Wu SM, Lu LY, Yang YT, Jeng SY (2009) Cadmium concentration and metallothionein expression in prostate cancer and benign prostatic hyperplasia of humans. J Formos Med Assoc 108: 554-559.
- Zaichick VYe, Tsyb AF, Vtyurin BM (1995) Trace elements and thyroid cancer. Analyst 120: 817-821.
- Zaichick VYe, Choporov Yu Ya (1996) Determination of the natural level of human intra-thyroid iodine by instrumental neutron activation analysis. J Radioanal Nucl Chem 207: 153-161.
- Zaichick V, Zaichick S (1997) Normal human intrathyroidal iodine. Sci Total Environ 206: 39-56.
- Zaichick V (1998) Iodine excess and thyroid cancer. J Trace Elements in Experimental Medidicne 11: 508-509.
- Zaichick V (1998) In vivo and in vitro application of energy-dispersive XRF in clinical investigations: experience and the future. J Trace Elements in Experimental Medidicne 11: 509-510.
- Zaichick V, Iljina T (1998) Dietary iodine supplementation effect on the rat thyroid 131I blastomogenic action. In: Anke M (ed.). Die Bedentung der Mengen- und Spurenelemente. 18. Arbeitstangung. Friedrich-Schiller-Universität, Jena, Germany. Pg No: 294-306.
- Zaichick V, Zaichick S (1999) Energy-dispersive X-ray fluorescence analysis of iodine in thyroid puncture biopsy specimens. J Trace and Microprobe Techniques 17: 219-232.
- Zaichick V (1999) Human intrathyroidal iodine in health and non-thyroidal disease. In: Abdulla M (ed.). New aspects of trace element research. Pg No: 114-119.
- Zaichick V (2000) Relevance of, and Potentiality for, in Vivo Intrathyroidal Iodine Determination. In: Yasumura S, Harrison JE (eds.). In Vivo Body Composition Studies. Annals of the New York Academy of Sciences, New York. 904: 630-632.
- Zhu H, Wang N, Zhang Y, Wu Q, Chen R, et al. (2010) Element contents in organs and tissues of Chinese adult men. Health Phys 98: 61-73.
- Vlasova ZA (1969) Dynamics of trace element contents in thyroid gland in connection with age and atherosclerosis. Proceedings of the Leningrad Institute of Doctor Advanced Training 80: 135-144.
- Katoh Y, Sato T, Yamamoto Y (2002) Determination of multielement concentrations in normal human organs from the Japanese. Biol Trace Elem Res 90: 57-70.
- Salimi J, Moosavi K, Vatankhah S, Yaghoobi A (2004) Investigation of heavy trace elements in neoplastic and non-neoplastic human thyroid tissue: A study by proton-induced X-ray emissions. Iran J Radiat Res 1: 211-216.
- Tipton IH, Cook MJ (1963) Trace elements in human tissue. II. Adult subjects from the United States. Health Phys 9: 103-145.
- Reddy SB, Charles MJ, Kumar MR, Reddy BS, Anjaneyulu Ch, et al. (2002) Trace elemental analysis of adenoma and carcinoma thyroid by PIXE method. Nucl Instrum Meth B 196: 333-339.
- Ataulchanov IA (1969) Age-related changes of manganese, cobalt, coper, zinc, and iron contents in the endocrine glands of females. Problemy Endocrinologii 15: 98-102.
- Boulyga S, Zhuk I, Lomonosova E, Kievetz M, Denschlag H, et al. (2005) Determination of microelements in thyroids of the inhabitants of Belarus by neutron activation analysis using the k 0-method. J Radioanal Nucl Chem 222.
- Boulyga SF, Becker JS, Malenchenko AF, Dietze H-J (2000) Application of ICP-MS for Multielement Analysis in Small Sample Amoun ts of Pathological Thyroid Tissue. Microchimica Acta 134: 215-222.
- Fuzailov Yu. M (1981) Reaction of human and animal thyroids in the conditions of antimony sub-region of the Fergana valley. In: Kovalsky VV (ed.). IX All-Union Conference on Trace Elements in Biology. Kishinev, Moldova. Pg No: 58-62.
- Kví?ala J, Havelka J, Zeman J, N?mec J (2005) Determination of some trace elements in the thyroid gland by INAA. J Radioanal Nucl Chem 149.
- Zabala J, Carrión N, Murillo M, Quintana M, Chirinos J, et al. (2009) Determination of normal human intrathyroidal iodine in Caracas population. J Trace Elem Med Biol 23: 9-14.
- Zaichick V (1997) Sampling, sample storage and preparation of biomaterials for INAA in clinical medicine, occupational and environmental health. In: IAEA (ed.). Harmonization of Health-Related Environmental Measurements Using Nuclear and Isotopic Techniques. International Atomic Energy Agency, Vienna, Austria. Pg No: 123-133.
- Zaichick V (2004) Losses of chemical elements in biological samples under the dry ashing process. Trace Elements in Medicine 5: 17-22.
- Zaichick V, Zaichick S (2000) INAA applied to halogen (Br and I) stability in long-term storage of lyophilized biological materials. J Radioanal Nucl Chem 244: 279-281.
- Zaichick VE, Zaichick SV (1996) Instrumental effect on contamination of biomedical samples in the process of sampling. J Anal Chem 51: 1322-1327.
- Zaichick V, Zaichick S (1997) A search for losses of chemical elements during freeze-drying of biological materials. J Radioanal Nucl Chem 218: 249-253.
- Zaichick V (1995) Applications of synthetic reference materials in the medical Radiological Research Centre. Fresenius J Anal Chem 352: 219-223.
- Zaichick S, Zaichick V (2011) The effect of age on Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se, and Zn contents in intact human prostate investigated by neutron activation analysis. Appl Radiat Isot 69: 827-833.
- Zaichick S, Zaichick V (2010) The effect of age and gender on 37 chemical element contents in scalp hair of healthy humans. Biol Trace Elem Res 134: 41-54.
- Korelo AM, Zaichick V (1993) Software to optimize the multielement INAA of medical and environmental samples. In: Nazarov VM (ed.). Joint Institute for Nuclear Research, Dubna, Russia, Pg No: 326-332.
- Schroeder HA, Tipton IH, Nason AP (1972) Trace metals in man: strontium and barium. J Chronic Dis 25: 491-517.
- Sunderman FW (1979) Mechanisms of metal carcinogenesis. Biol Trace Elem Res 1: 63.
- Snow ET (1992) Metal carcinogenesis: mechanistic implications. Pharmacol Ther 53: 31-65.
- Toyokuni S (2009) Role of iron in carcinogenesis: cancer as a ferrotoxic disease. Cancer Sci 100: 9-16.
- Martinez-Zamudio R, Ha HC (2011) Environmental epigenetics in metal exposure. Epigenetics 6: 820-827.
- Tokar EJ, Benbrahim-Tallaa L, Waalkes MP (2011) Metal ions in human cancer development. Met Ions Life Sci 8: 375-401.
- Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy Metals Toxicity and the Environment. EXS 101: 133-164.
- Koedrith P, Kim H, Weon JI, Seo YR (2013) Toxicogenomic approaches for understanding molecular mechanisms of heavy metal mutagenicity and carcinogenicity. Int J Hyg Environ Health 216: 587-598.
- Tabrez S, Priyadarshini M, Priyamvada S, Khan MS, Na A, et al. (2014) Gene-environment interactions in heavy metal and pesticide carcinogenesis. Mutat Res Genet Toxicol Environ Mutagen 760: 1-9.
- Zaichick V (2004) INAA and EDXRF applications in the age dynamics assessment of Zn content and distribution in the normal human prostate. J Radioanal Nucl Chem 262: 229-234.
- Zaichick S, Zaichick V (2011) INAA application in the age dynamics assessment of Br, Ca, Cl, K, Mg, Mn, and Na content in the normal human prostate. J Radioanal Nucl Chem 288: 197-202.
- Zaichick V, Nosenko S, Moskvina I (2012) The effect of age on 12 chemical element contents in the intact prostate of adult men investigated by inductively coupled plasma atomic emission spectrometry. Biol Trace Elem Res 147: 49-58.
- Zaichick S, Zaichick V, Nosenko S, Moskvina I (2012) Mass fractions of 52 trace elements and zinc/trace element content ratios in intact human prostates investigated by inductively coupled plasma mass spectrometry. Biol Trace Elem Res 149: 171-183.
- Zaichick V, Zaichick S (2014) INAA application in the assessment of chemical element mass fractions in adult and geriatric prostate glands. Appl Radiat Isot 90: 62-73.
- Zaichick V, Zaichick S (2014) Determination of trace elements in adults and geriatric prostate combining neutron activation with inductively coupled plasma atomic emission spectrometry. Open Journal of Biochemistry 1: 16-33.
- Zaichick V, Zaichick S (2014) Use of INAA and ICP-MS for the assessment of trace element mass fractions in adult and geriatric prostate. J Radioanal Nucl Chem 301: 383-397.
- Zaichick V, Zaichick S (2014) Age-related histological and zinc content changes in adult nonhyperplastic prostate glands. Age 36: 167-181.
- Zaichick V, Zaichick S (2015) Differences and Relationships between Morphometric Parameters and Zinc Content in Nonhyperplastic and Hyperplastic Prostate Glands. BJMMR 8: 692-706.
- Zaichick V, Zaichick S, Davydov G (2015) Differences between chemical element contents in hyperplastic and nonhyperplastic prostate glands investigated by neutron activation analysis. Biol Trace Elem Res 164: 25-35.
- Zaichick S, Zaichick V (2015) Prostatic Tissue Level of some Androgen Dependent and Independent Trace Elements in Patients with Benign Prostatic Hyperplasia. Androl Gynecol: Curr Res 3: 3.
- Zaichick V, Zaichick S (2016) The Bromine, Calcium, Potassium, Magnesium, Manganese, and Sodium Contents in Adenocarcinoma of Human Prostate Gland. J Hematology and Oncology Research 2: 1-12.
- Zaichick V, Zaichick S (2016) Trace Element Contents in Adenocarcinoma of Human Prostate Investigated by Energy Dispersive X-Ray Fluorescent Analysis. Journal of Adenocarcinoma 1: 1-7.
- Zaichick V, Zaichick S (2016) Trace element contents in adenocarcinoma of the human prostate gland investigated by neutron activation analysis. Cancer Research and Oncology 1: 1-10.
- Zaichick V, Zaichick S (2016) Variations in concentration and distribution of several androgen-dependent and -independent trace elements in nonhyperplastic prostate gland tissue throughout adulthood. J Androl Gynaecol 4: 1-10.
- Zaichick V, Zaichick S (2016) Prostatic tissue levels of 43 trace elements in patients with prostate adenocarcinoma. Cancer and Clinical Oncology 5: 79.
- Zaichick V, Zaichick S (2016) Levels of 43 Trace Elements in Hyperplastic Human Prostate. BJMMR 15: 1-12.
- Zaichick V, Zaichick S (2016) Prostatic tissue level of some major and trace elements in patients with BPH. JJ NephroUrol 3: 026.
- Zaichick V, Zaichick S (2016) Age-related changes in concentration and histological distribution of Br, Ca, Cl, K, Mg, Mn, and Na in nonhyperplastic prostate of adults. European Journal of Biology and Medical Science Research 4: 31-48.
- Zaichick V, Zaichick S (2016) Variations in concentration and histological distribution of Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se, and Zn in nonhyperplastic prostate gland throughout adulthood. J J Cell Mol Bio 2: 011.
- Zaichick V, Zaichick S (2016) Age-related Changes in Concentration and Histological Distribution of 18 Chemical Elements in Nonhyperplastic Prostate of Adults. World Journal of Pharmaceutical and Medical Research 2(4): 5-18.
- Zaichick V, Zaichick S (2016) Age-related changes in concentration and histological distribution of 54 trace elements in nonhyperplastic prostate of adults. Int Arch UrolComplic 2: 019.
- Zaichick V, Zaichick S (2016) The Comparison between the contents and interrelationships of 17 chemical elements in normal and cancerous prostate gland. Journal of Prostate Cancer 1: 105.
- Zaichick V, Zaichick S, Wynchank S (2016) Intracellular zinc excess as one of the main factors in the etiology of prostate cancer. Journal of Analytical Oncology 5: 124-131.
- Zaichick V, Zaichick S, Rossmann M (2016) Intracellular calcium excess as one of the main factors in the etiology of prostate cancer. AIMS Molecular Science 3: 635-647.
- Salnikow K, Zhitkovich A (2008) Genetic and epigenetic mechanisms in metal carcinogenesis and cocarcinogenesis: nickel, arsenic, and chromium. Chem Res Toxicol 21: 28-44.
- Chervona Y, Arita A, Costa M (2012) Carcinogenic metals and the epigenome: understanding the effect of nickel, arsenic, and chromium. Metallomics 4: 619-627.
Citation: Zaichick V, Zaichick S (2017) Age-Related Changes of Trace Element Contents in Intact Thyroid of Females Investigated by Neutron Activation Analysis. J Gerontol Geriatr Med 3: 015.
Copyright: © 2017 Vladimir Zaichick, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

