
Airway Volume Changes after Orthognathic Surgery in Patients with Down Syndrome: A Diagnostic-Therapeutic Algorithm
*Corresponding Author(s):
Giralt-Hernando MClinical Instructor, Department Of Oral And Maxillofacial Surgery, Universitat Internacional De Catalunya, Josep Trueta S/n 08195 - SantCugat Del Vallès (Barcelona), Spain
Tel:+34 935042000,
Email:mariagiralth@gmail.com
Abstract
Background: Down syndrome (DS) patients tend to suffer severe dentofacial and skeletal deformities leading to severe occlusal, speech and respiratory problems. The increase in life expectancy of these subjects opens new horizons for improvement of their oral, dental and facial functions, among others. However, no surgical treatment protocol is described elsewhere in the literature. Then, the present study proposes a protocol for the surgical management of orodental and facial deformities in DS patients based on a series of three cases and a review of the literature.
Methods: The protocol contemplates dentofacial deformity diagnostic work-up, obstructive sleep apnoea diagnosis and follow-up, pre-admission medical and anaesthetic evaluation, treatment plan, and perioperative management.
Results: All patients presented with midface retrusion due to underlying severe maxillary hypoplasia and dental crowding. A mean maxillary advancement of 4.53 mm and a mean maxillary descent of 3.6 mm were obtained. A mean pharyngeal airway volume gain of 10,954.33 mm3 (50%) was recorded at the one-month follow-up visit. Non-relevant skeletal and airway relapses were noted. Stable occlusion was achieved in all cases after postoperative orthodontic treatment, with proper chewing function, and the parents referred decreased snoring.
Conclusion: In selected DS patients with specific dysmorphic orofacial features, orthognathic surgery constitutes the first line treatment to improve the occlusion disorders and associated feeding, respiratory and sleep-disorder problems. Therefore, the use of this algorithm represents the first line surgical treatment for DS patients with dentofacial deformities and allows the clinician to tailor the surgical treatment to each patient’s needs.
Keywords
Down syndrome; Orthognathic surgery; Obstructive sleep apnoea; Pharyngeal airway volume
Abbreviations
DS: Down Syndrome; OSA: obstructive sleep apnea; OS: orthognathic surgery; CBCT: cone-beam computed tomography; PSG: polysomnography; 3D: three-dimensional; PAV: pharyngeal airway volume; SD: standard deviation; EDS: excessive daytime sleepiness
Introduction
Down syndrome (DS) is the most frequent chromosomal disorder, occurring in one out of every 700 births [1]. Since 1866, when the British physician John Langdon Down first described the disorder, trisomy 21 has gained scientific relevance and is currently one of the most extensively studied genetic alterations. Apart from intellectual disabilities of varying degrees, these patients may suffer a broad range of organic defects - the most common being cardiac abnormalities, gastroesophageal reflux, celiac disease, hypothyroidism, hearing and vision problems, leukemia and early Alzheimer’s disease [2]. Dysmorphic cranial and orofacial features have also been widely described in DS patients, particularly a small cranium, a flat nose and flat malar bones with slanted eyes, severe maxillary hypoplasia with a high-arched and constricted palate, and mandibular hypoplasia although seemingly prognathic mandible because of the previous issue, hypodontia, relative macroglossia because of the small maxillo-mandibular framework with the tongue resting inactively between the lips due to muscle hypotonia in the orofacial region and, ultimately, a flattened face with anterior open bite and Class III dental and skeletal relationships [3]. These anatomical features may lead to speech, swallowing and masticatory functional impairments, as well as to an increased predisposition to obstructive sleep apnoea (OSA) and mouth breathing [4]. Medical advances and the multidisciplinary management of DS patient’s have doubled the life expectancy of these patients in the last decade, from 30-40 to 60-70 years, with increased quality of life and more community-involved and productive lives [5]. This in turn opens new horizons for improvement of their oral, dental and facial functions, among others.
The present study describes a protocol for the surgical management of orodental and facial deformities in DS patients based on a series of three cases and a review of the literature.
Patients And Methods
Three consecutive patients with DS were referred to our Department for dentofacial deformity treatment with orthognathic surgery (OS). A retrospective evaluation was made of the treatment applied in all three cases, and a review of the literature was carried out in order to validate the proposed management algorithm (Figure. 1). 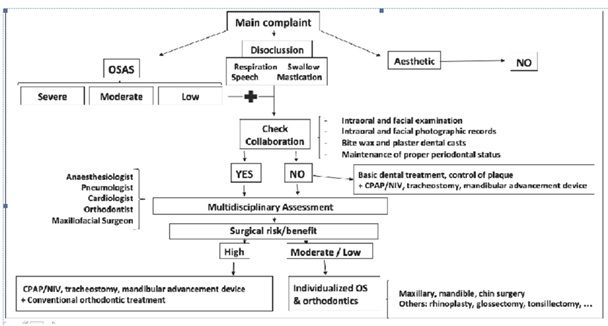
Figure 1: Captions to Illustrations
Decision making algorithm in Down syndrome patients with disocclusion and obstructive sleep apnoea: selection of suitable candidates for orthognathic surgery.
OSA: Obstructive sleep apnea; OS: orthognathic surgery; CPAP: continuous positive airway pressure; NIV: non-invasive ventilation.
The guidelines of the Declaration of Helsinki were followed in all the treatment phases. Consent was requested from the legal guardians of the patients. As this was a retrospective analysis, Institutional Review Board approval of the study was not considered necessary.
Diagnostic work-up
The diagnostic work-up comprised three phases, which were also used to concomitantly evaluate patient and parent collaboration: a) physical intraoral and facial examination, with intraoral and facial photographic records and the study of plaster dental casts and wax bites; b) periodontal evaluation and a follow-up visit to check patient and parent capacity to maintain proper periodontal health; and c)cone-beam computed tomography (CBCT) (i-CAT, Imaging Sciences International, Inc., Hatfield, USA) study to complete the facial analysis.
Obstructive sleep apnoea diagnosis and follow-up
A thorough anamnesis is required, with quality of life evaluation in children with OSA [6,7]. When OSA is suspected, polysomnography (PSG) should be requested. In addition, three-dimensional (3D) pharyngeal airway volume (PAV) should be measured with CBCT (T0).
The three tests (quality of life evaluation, PSG and CBCT) should be repeated after one month and one year of follow-up in order to assess the clinical and radiological changes and their long-term stability in relation to the OS procedure.
Pre-admission medical and anaesthetic evaluation
The anaesthetic management of patients with DS constitutes a challenge for the anesthetist, due to the difficulty of the airway, the possible associated comorbidities they may present, and behavioral and communication problems [8]. The airway may prove difficult due to the following DS-related issues: a) small airway size secondary to maxillary hypoplasia, micrognathia, macroglossia, tonsillar hypertrophy, short neck, glottic and subglottic stenosis and tracheomalacia; b) atlanto-axial/atlanto-occipital instability with a high risk of spinal cord injury; and c) OSA secondary to central apnoea, low muscle tone in the mouth and upper airway, poor coordination of airway movements, and the abovementioned small airway size [4,9,10]. On the other hand, associated comorbidities may develop in any body system11. The most frequent and relevant conditions are congenital cardiac alterations (40-50% of all patients), such as interatrial / interventricular communications, transposition of large vessels and tetralogy of Fallot, which are generally diagnosed in advance. However, there are also other cardiac diseases such as valve defects or arrhythmias (with increased susceptibility to bradycardia) that typically are not diagnosed and should be evaluated preoperatively. Other alterations that are also often seen in this population comprise gastroesophageal reflux disease (with an increased risk of bronchoaspiration), obesity, diabetes, hypotonia, immune suppression with susceptibility to pulmonary infections, autoimmune alterations such as hypothyroidism, and moderate to severe mental retardation. Thus, potential disease conditions require in depth evaluation, as well as the management of potential complications planned beforehand, in order to ensure a safe anaesthetic procedure [8,11].
Treatment plan
If both patient and parent collaboration proved good enough in all phases, the orthodontic-surgical treatment plan was established and orthodontic treatment was started. Once again, provided cooperation with the orthodontic treatment was adequate, surgery was virtually planned using specific software (Dolphin® 3D Orthognathic Surgery Planning Software Version 11.8) [12,13].
Perioperative management
In order to increase comfort and relaxation of the patients, a caregiver accompanied them to the anaesthetic induction area, and to the recovery room. The patients were operated upon under general anesthesia and with endotracheal intubation, as in conventional orthognathic surgery procedures. However, a smaller tube than expected for the age of the patient was chosen in all cases in order to reduce the incidence of subglottic oedema and post-intubation stridor. As mentioned above, airway management of DS patients is considered to be highly complex, so trained anaesthesia personnel and a difficult airway cart were ready for both induction and eduction procedures [8].
There are no perioperative drug contraindications or medications specifically recommended for patients with DS; nevertheless, perioperative treatment was patient-tailored according to the comorbidity present in each case.
Surgery
A bilateral mandibular sagittal split osteotomy was performed using the Dal Pont-Obwegeser technique, with a maxillary LeFort I osteotomy using the “twist technique” [14]. All patients were extubated in the operating room, and all wore a closed-circuit cold mask (17ºC) during hospital admission. Standard antibiotic and anti-inflammatory medication for OS was prescribed. Functional training with light guiding elastics was followed for one month, together with a soft diet during the same period of time.
Postoperative evaluation
Eventual surgical complications were recorded at one week and 1, 6 and 12 months of follow-up. In addition, two control CBCT scans were performed at one month (T1) and one year of follow-up (T2) in order to assess both airway and bony surgical enlargement (T1-baseline [T0]) and its long-term stability after surgery(T2-T1). CBCT scans were obtained in DICOM (Dental Imaging Communication) format and processed with specific third-party software (Dolphin® 3D Orthognathic Surgery Planning Software Version 11.8). A 3D volume was created with hard tissue reconstruction for the T0, T1 and T2 databases. Three-dimensional superimposition and dimensional comparisons were performed by means of surface matching between different datasets [15].
In order to evaluate surgical bony enlargement and stability, the following linear measurements were obtained at the maxillary midline in all three spatial planes:
- Sagittal plane: projected distance from A-point to nasion perpendicular (A-Nper) for the maxilla; and projected distance from B-point to nasion perpendicular (B-Nper) for the mandible.
- Transverse plane: distance between both greater palatine foramina (PFR-PFL) for the maxilla; and distance between both gonions (GoR-GoL) for the mandible.
- Vertical plane: perpendicular distance from A-point to the Frankfort horizontal plane through the nasion (A-FHN) for the maxilla; and distance from B-point to the Frankfort horizontal plane through the nasion (B-FHN) for the mandible.
Lastly, PAV enlargement and its stability were assessed by measuring enlargement three-dimensionally (3D) at three different levels with respect to the limits of the pharyngeal airway sub regions: nasopharynx, oropharynx and hypopharynx, following a previously validated protocol described in detail elsewhere [16].
Statistical Analysis
A descriptive analysis was made of the study variables, with calculation of the mean, standard deviation (SD), minimum and maximum values, and median for continuous variables. Absolute and relative frequencies (percentages) were reported in the case of qualitative variables. The statistical analysis was carried out using the SPSS version 15.0.1 statistical package (SPSSInc., Chicago, IL, USA). Descriptive statistics were used for quantitative analysis. Percentage variation referred to maxillary or mandibular surgical movements (relapse) for each patient was calculated as follows: one-year postoperative A/B-point position • 100 / one-month postoperative A/B-point position. Similarly, percentage variation referred to PAV (relapse) for each patient was calculated as follows: one-year postoperative PAV • 100 / one-month postoperative PAV.
Results
The clinical cases are summarized in (Table 1). The study sample comprised two men and a woman with a median age of 26.7 years (range 20-37). The main reason for consultation was the presence of patient chewing difficulties, though thorough anamnesis also evidenced snoring and excessive daytime sleepiness (EDS) in all patients. None of them were diagnosed of OSA or used night time continuous positive airway pressure (CPAP). No multi organ alterations were observed at the pre-admission medical evaluation.
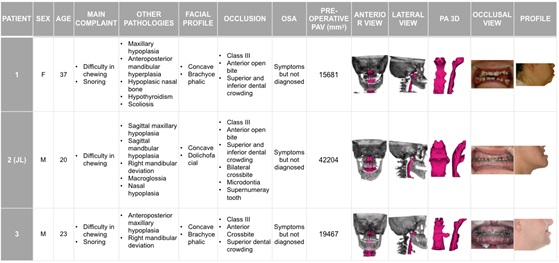 Table 1: Preoperative facial, occlusal and airway features and images.
Table 1: Preoperative facial, occlusal and airway features and images.
a F: female; M: male; OSA: obstructive sleep apnea; PAV: pharyngeal airway volume; PA: pharyngeal airway
The salient feature in the facial analysis was midface retrusion due to underlying severe maxillary hypoplasia, and dental crowding in all cases. These findings are consistent with the characteristic orofacial dysmorphic features of DS that contribute to OSA. Basal PAV was quantified (3D) based on the preoperative CBTC study, with a mean value of 25.784 mm3 (range 15.681-42.204). Cases 1 and 3 presented an underlying constricted upper airway (15.681 mm3 and 19.467 mm3, respectively). Specifically, narrowing was observed in all airway sub regions: naso-, oro- and hypopharynx (Table 1). On the other hand, case 2 presented a normal initial PAV (42.204 mm3).
All patients received pre- and postoperative orthodontic treatment and were operated upon under general anesthesia by the same surgeon (FHA). All underwent maxillary surgery using the minimally invasive ”twist technique”14, but only case 2was subjected to mandibular surgery for backward movement (Table 2). 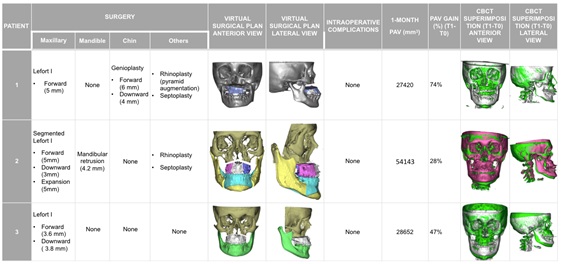
Table 2: Surgical virtual plan, procedures and complications. Postoperative PAV gain and skeletal movement through CBCT superimposition after one month of follow-up.
a PAV: pharyngeal airway volume; CBCT: cone-beam computerized tomography.
There were no surgical complications in the form of dental, nerve or vascular injuries, or poor split osteotomy. All patients were extubated in the operating room, and anaesthetic perioperative management proved uneventful. All patients wore a closed-circuit cold mask (17ºC) during hospital admission and were discharged 24 hours after surgery, with pain control using common analgesics. Standard antibiotic and anti-inflammatory medication for OS was prescribed. Functional training with light guiding elastics was followed for one month, together with a soft diet during the same period of time. The immediate and long-term (one year of follow-up) postoperative courses were uneventful. Surgical skeletal movement and PAV gain was assessed through CBCT superimposition (comparison between T0 and T1). A mean maxillary advancement of 4.53 mm and a mean maxillary descent of 3.6 mm were obtained. Consequently, a mean PAV gain of 10.954.33 mm3 (50%) was recorded at the one-month follow-up visit (Table 2). Stable occlusion was achieved in all cases after postoperative orthodontic treatment, with proper chewing function and decreased snoring as reported by the parents. The stability of both bony and PAV gain was assessed based on two postoperative CBCT evaluations (T1 and T2). Regarding skeletal stability, a non-relevant relapse was observed in maxillary bone: a mean relapse of 1.2 mm in the sagittal dimension, 0.6 mm vertically, and none in the transverse dimension. On the other hand, in relation to PAV stability, we recorded a mild mean relapse at one year of follow-up of -2.712 mm3 (5%), though the final mean PAV gain was notorious 35.834 mm3 (45%) (Table 3).
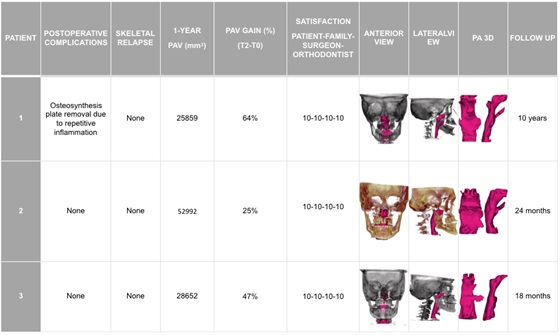 Table 3: Final outcome after one year of follow-up: skeletal and airway stability and degree of satisfaction with the overall procedure.
Table 3: Final outcome after one year of follow-up: skeletal and airway stability and degree of satisfaction with the overall procedure.
a PAV: pharyngeal airway volume; PA: pharyngeal airway
Lastly, the degree of satisfaction with the functional outcome and quality according to the patients, family and surgeon was excellent, with a very good professional-patient relationship.
Discussion
The main reasons why patients seek OS and related surgical orthodontic treatments are occlusal and aesthetic problems, and OSA. Although DS is associated to altered facial dimensions, and some authors advocate cosmetic facial surgery to avoid stigmatization and ensure better social acceptance [17], in our opinion aesthetics should not be the sole indication of OS in DS patients, in view of its unfavorable benefit/risk balance. This means that when orthodontic treatment suffices to correct the malocclusion, or when OSA is well controlled with CPAP in DS patients with balanced occlusion, surgery should be ruled out even though cosmetic skeletal disharmony may persist. Nevertheless, OS may be the best management option in other carefully selected patients (Figure 1). On one hand, occlusal disharmonies with anterior open bite, dental Class III and a lack of inter-arch contacts are common in patients with DS, due to their abovementioned skeletal cranial and orofacial dysmorphic features. Moreover, other dental anomalies such as oligodontia, periodontal disease, tooth agenesis, taurodontism, microdontia and altered eruption of primary and permanent dentition are often present in this population [3,18]. In the attempt to create more dental contacts these individuals protrude the mandible, which in the end can jeopardize temporomandibular joint function [19]. Besides, such malocclusion involves speech and feeding problems, which are aggravated by their inherent neuro-motor disability for articulation and chewing/swallowing, respectively. Moreover, this severe malocclusion may have respiratory consequences such as OSA or the aspiration of food or fluids into the lungs [20]. Thus, it is evident that DS patients are in need of treatment for their malocclusions. On the other hand, persons with DS are prone to develop OSA due to a series of associated anatomical and physiological features [21]: a) a small airway size because of underlying maxillary hypoplasia, micrognathia, relative macroglossia, adeno- and lingual- tonsillar hypertrophy, fat deposits in the lateral wall of the pharynx, glottic and subglottic stenosis and tracheomalacia; and b) low muscle tone in the mouth and upper airway, poor coordination of airway movements, and gastroesophageal reflux disease that leads to inflammation and obstruction of the upper airway4,9. Some studies suggest that the prevalence of OSA in children with DS is 30-50%, and that approximately 90% of the adults will develop OSA, which in such cases moreover tends to be severe [9,11,22]. Apart from the typical comorbidities associated to OSA, such as arterial hypertension, altered blood glucose homeostasis, cardiovascular and cerebrovascular diseases, pulmonary hypertension, cognitive deficits and even death, individuals with DS specifically suffer worsening of overall cognitive function - starting with weakening of neurocognitive development in early ages, and followed later on by deteriorated communication ability, behavior, functional outcomes and quality of life [23].In this regard, several management strategies have been described: a) positive airway support in the form of noninvasive ventilation (NIV) or CPAP, though this is associated to high dropout and non-adherence rates [24]; b) weight loss, which does not cure OSA, but is recommended in addition to other therapies in patients who are overweight [25]; c) airway soft tissue surgery, such as adeno- and lingual-tonsillectomy, which are associated to high OSA persistence rates4 (adeno- and lingual-tonsillectomy therefore should be indicated only when hypertrophy is clearly evidenced) [4,26]; d) partial glossectomy, which should only be indicated when true excessive enlargement of the tongue results in insufficient space for the organ [27]; e) hypoglossal nerve stimulation, which is a promising and minimally invasive technique, though further studies are needed to optimize patient selection and better assess the long-term efficacy of the technique [28]; and f) tracheostomy, which is linked to severe short- and long-term complications, including decannulation, bleeding or infection among others, and may be required only in cases of severe OSA not amenable to other forms of treatment.
These poor outcomes point to OS as the first line treatment option, considering the characteristic orofacial dysmorphic features of individuals with DS and that contribute to airway narrowing, such as retrusion or shortening of the mandible and maxillary hypoplasia [29]. Although recommending OS in this population is controversial, it has been demonstrated that OS procedures can be carried out with success rates (predictability, complications during and after the operation, and overall treatment stability) as high as in mentally healthy individuals [30]. Nonetheless, the overall complexity of patients with DS calls for a multidisciplinary team comprising primary care physicians, maxillofacial surgeons, orthodontists and other dental professionals, anesthetists, medical rehabilitators, physiotherapists, speech therapists, pulmonologists and neurophysiologists, among others. Once the patient reports for maxillofacial consultation due to occlusal problems or OSA, a number of aspects must be taken into account. Firstly, regarding the craniofacial features, patients with DS have reduced head and facial dimensions with a brachiocephalic cranium, a shorter and flatter cranial base, reduced or absent frontal sinus and nasal bone, small ears, and hypertelorism with slanted eyes. Thus, cephalometric landmarks such as the nasion or porion for facial analysis and head orientation purposes may be altered, making it difficult to properly classify the underlying dentofacial anomaly [31]. For this reason, it is highly advisable to use 3D CBCT for facial analysis instead of 2D X-rays. On the other hand, the diagnosis of OSA in children is based on an association of PSG parameters when the apnoea-hypopnea index is > 5 episodes/hour, though the SpO2 and PtcCO2 levels are also taken into account [32,33] and on clinical symptoms based on a specific OSA quality of life test for children (the OSA-18 survey) [6,7,34]. Apart from the cardinal manifestations of OSA, such as snoring, fatigue and restless sleep, children with DS specifically may also present with failure to thrive, hyperactivity, behavioral disruptions and poor school performance, whereas adults with DS may present with mood dysregulation and depression [22]. Although the cases in our study did not undergo PSG, because their main complaint was malocclusion, it is advisable to systematically perform PSG in all patients presenting OSA symptoms. Besides, diagnostic CBCT for facial analysis may also be used to detect upper airway constrictions. In our study, cases 1 and 3 showed basal upper airway constriction (15.681 mm3 and 19.467 mm3), respectively (Table 1), compared to reference normal PAV values of 23.400mm [35,36]. Specifically, a narrowed airway was observed in all upper airway sub regions: naso- oro- and hypopharynx. Conversely, mandibular advancement devices, apart from being an option for treating mild to moderate OSA with better patient compliance than when CPAP is used22, are also useful in deciding which patients may benefit from surgical mandibular advancement in the context of OSA. Unfortunately, similar maxillary devices for predicting the impact of maxillary advancement upon OSA are not available. On the other hand, it is essential to detect as far as possible central origin OSA cases through PSG, since OS would not be worthwhile in such situations.
Thus, correct screening referred to patient eligibility for orthodontic-surgical treatment is essential. We thus propose the above described diagnostic work-up in order to evaluate patient and parent collaboration (examination - periodontal status and maintenance - CBCT) regardless of the patient intelligence quotient (IQ) and thus to refine the selection of suitable candidates for OS (Figure 1). Equally important is the establishment of a good and trusting professional-patient relationship. In this regard it is useful to explain the planned procedures in depth and indicate the expected results and eventual complications to the patient and his/her relatives. Keeping close contact through telephone support and more frequent follow-up visits is also useful.
Surgical planning differs from the regular scenario where aesthetics constitute a key element, and instead priority is placed on minimal surgery in terms of mono- rather than bi maxillary operations, with reduction of the amount of skeletal movements, while always ensuring proper occlusion and sufficient PAV enlargement. Thus, in general, in the presence of a typical midface deficiency with high palate, reduction of its length, together with a narrowed oropharynx, usually implies advancement, widening and antero-posterior leveling/upward maxillary movements. Then, the mandible may be adjusted to maxillary positioning. Regarding the specific surgical management of OSA, a maxilla-mandibular advancement of 1cm is considered the gold standard in OS [37]. Nevertheless, there is not enough evidence to establish the magnitude and direction of maxillary and/or mandibular movements required in order to cure OSA, which additionally should be individualized for each patient. Our sample of patients underwent a mean maxillary advancement of 4.53 mm, which was enough to correct both occlusal and narrowed airway problems. Although one patient required mandibular setback for occlusal purposes, it did not adversely affect overall PAV enlargement. Occasionally, where required, procedures concomitant to OS are recommended to optimize airway permeability and prevent open bite relapse, such as tongue reduction [27] or adeno- and lingual-tonsillectomy [4,26]. Although it has been widely demonstrated that OS is the most consistent and predictable surgical treatment option for adult patients diagnosed with moderate to severe OSA [38,39], its outcomes are less predictable in the DS population because, as previously mentioned, the causes of OSA in these patients are multiple and additive. Thus, a systematic sleep study based on PSG is strongly recommended prior to and after OS in order to check surgical effectiveness and determine whether further treatments are necessary. In cases where OSA persists after upper airway surgery, CPAP or NIV in the case of alveolar hypoventilation are indicated [4].
Postoperative discomfort should be reduced as far as possible, adopting minimally invasive approaches such as the “twist technique”14, the shortening of surgery time, the use of apiezoelectric saw when possible [40], the prescription of standard anti-inflammatory medication, manual lymphatic drainage for OS [41], and the wearing of a closed-circuit cold mask during the postoperative period [42]. Furthermore, whenever possible, light guiding elastics for functional training should be used instead of rigid intermaxillary fixation [30].
Regarding patient age at surgery, the standards advise waiting until cessation of mandibular growth. In the meantime, a two-phase or multiphase orthodontic treatment program is beneficial to assist correction of misalignment and maxillary transverse deficiency by means of palatal expansion or surgically assisted rapid palatal expansion, before and after closure of the palatal suture, respectively [19,43]. Similarly, myofunctional therapy or orofacial rehabilitation should be started during the growth period in order to favorn proper maxilla-mandibular growth, establish an adequate resting position of the tongue behind the upper incisors, reinforce orofacial tonicity, encourage nasal respiration and improve swallowing and speech functionality [44]. Besides, it also may reduce the inherent muscular imbalance that predisposes to an increased prevalence of relapse after OS [30]. Likewise, dentofacial harmonization by means of orthodontic treatment and OS have shown significant improvement in oral motor function, including mouth closure, inactive protrusion and positioning of the tongue in DS patients.
Conclusion
In selected patients with DS presenting specific orofacial dysmorphic features, orthognathic surgery is an effective and secure treatment to address both occlusion (and its consequent feeding- respiratory- and communication-related problems) and OSA. The implementation of this algorithm, together with minimally invasive surgery and cutting-edge technologies, allows OS to be safely performed in patients with DS. This algorithm allows clinicians to tailor the surgical treatment to each patient’s needs, consider in gage, comorbidities and barriers to treatment adherence. However, further clinical studies are needed to determine whether OS in DS patients with OSA is able to reduce cardiovascular risks, mortality and long-term outcomes after surgery.
References
- Sherman SL, Allen EG, Bean LH, Freeman SB (2007) Epidemiology of Down syndrome. Ment Retard Dev Disabil Res Rev 13: 221-227.
- Potter H (2016) Beyond Trisomy 21: Phenotypic Variability in People with Down Syndrome Explained by Further Chromosome Mis-segregation and Mosaic Aneuploidy. Journal of Down Syndrome & Chromosome Abnormalities 2: 1-4.
- Bauer D, Evans CA, Begole EA, Salzmann L (2012) Severity of occlusal disharmonies in down syndrome. Int J Dent 872367: 1-7.
- Dudoignon B, Amaddeo A, Frapin A, Thierry B, Sanctis LD, et al. (2017) Obstructive sleep apnea in Down syndrome: Benefits of surgery and noninvasive respiratory support. Am J Med Genet A 173: 2074-2080.
- Gupta NA, Kabra M (2014) Diagnosis and Management of Down Syndrome. Indian J Pediatr 81: 560-567.
- Chiner E, Landete P, Sancho-Chust JN, Perez Ferrer P, Pastoret E, et al. (2016) Adaptation and Validation of the Spanish Version of OSA-18, a Quality of Life Questionnaire for Evaluation of Children with Sleep Apnea-Hypopnea Syndrome. Arch Bronconeumol 52: 553-559.
- Franco RA, Rosenfeld RM, Rao M (2000) Quality of life for children with obstructive sleep apnea. Otolaryngol Head Neck Surg 123: 9-16.
- Borland LM, Colligan J, Brandom BW (2004) Frequency of anesthesia-related complications in children with Down syndrome under general anesthesia for noncardiac procedures. Paediatr Anaesth. 14: 733-738.
- Bassell JL, Phan H, Leu R, Kronk R, Visootsak J, et al. (2015) Sleep profiles in children with Down syndrome. Am J Med Genet A 167A: 1830-1835.
- Waage NS, Baker S, Sedano HO (2009) Pediatric conditions associated with compromised airway. Int J Paediatr Dent 31: 236-248.
- Alexander M, Petri H, Ding Y, Wandel C, Khwaja O, et al. (2016) Morbidity and medication in a large population of individuals with Down syndrome compared to the general population. Dev Med Child Neurol 58: 246-254.
- Centenero SAH, Alfaro FH (2012) 3D planning in orthognathic surgery: CAD/CAM surgical splints and prediction of the soft and hard tissues results - our experience in 16 cases. J Craniomaxillofac Surg. 40: 162-168.
- Alfaro FH, Martinez RG (2013) New protocol for three-dimensional surgical planning and CAD/CAM splint generation in orthognathic surgery: an in vitro and in vivo study. Int J Oral Maxillofac Surg 42: 1547-1556.
- Alfaro FH, Martinez RG (2013) “Twist Technique” for Pterygomaxillary Dysjunction in Minimally Invasive Le Fort I Osteotomy. J Oral Maxillofac Surg 71: 389-392.
- Junior OL, Martinez RG, Gil AP, Meirelles LS, Oliveira RB, et al. (2017) Stability and surgical complications in segmental Le Fort I osteotomy: a systematic review. Int J Oral Maxillofac Surg 46: 1071-1087.
- Martinez RG, Swennen GRJ (2013) Three-dimensional cone beam computed tomography definition of the anatomical subregions of the upper airway: a validation study. Int J Oral Maxillofac Surg 42: 1140-1149.
- Olbrisch RR (1982) Plastic surgical management of children with Down’s syndrome: indications and results. Br J Plast Surg 35: 195-200.
- Trias MA, Perez JL, Perez AP (2016) Comparative study of dental anomalies assessed with panoramic radiographs of Down syndrome and non-Down syndrome patients. Eur J Paediatr Dent. 17: 65-69.
- Javed F, Akram Z, Barillas AP, Kellesarian SV, Ahmed HB (2018) Outcome of orthodontic palatal plate therapy for orofacial dysfunction in children with Down syndrome: A systematic review. Orthodontics & Craniofacial Research 21: 20-26.
- Hennequin M, Faulks D, Veyrune JL, Bourdiol P (2007) Significance of oral health in persons with Down syndrome: a literature review. Dev Med Child Neurol 41: 275-283.
- Fung E, Witmans M, Ghosh M, Cave D, El-Hakim H, et al. (2012) Upper airway findings in children with Down syndrome on sleep nasopharyngoscopy: case-control study. J Otolaryngol Head Neck Surg 41: 138-144.
- Lal C, White DR, Joseph JE, Bakergem KV, LaRosa A, et al. (2015) Sleep-disordered breathing in Down syndrome. Chest 147: 570-579.
- Skotko BG, Macklin EA, Muselli M, Voelz L, McDonough ME, et al. (2017) A predictive model for obstructive sleep apnea and Down syndrome. Am J Med Genet A 173: 889-896.
- Brooks LJ, Olsen MN, Bacevice AM, Beebe A, Konstantinopoulou S, et al. (2015) Relationship between sleep, sleep apnea, and neuropsychological function in children with Down syndrome. Sleep Breath 19: 197-204.
- Corral AR, Caples SM, Jimenez FL, Somers VK (2010) Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest 137: 711-719.
- Prosser JD, Shott SR, Rodriguez O, Simakajornboon N, Meinzen-Derr J, et al. (2017) Polysomnographic outcomes following lingual tonsillectomy for persistent obstructive sleep apnea in down syndrome: PSG Outcomes of Lingual Tonsillectomy in DS. The Laryngoscope 127: 520-524.
- Salmen F, Dedivitis R (2013) Partial glossectomy as an auxiliary method to orthodontic treatment of dentofacial deformity. International Archives of Otorhinolaryngology 16: 414-417.
- Diercks GR, Wentland C, Keamy D (2018) Hypoglossal Nerve Stimulation in Adolescents With Down Syndrome and Obstructive Sleep Apnea. JAMA Otolaryngol Head Neck Surg 144: 37-42.
- Lee RWW, Chan ASL, Grunstein RR, Cistulli PA (2009) Craniofacial phenotyping in obstructive sleep apnea a novel quantitative photographic approach. Sleep 32: 37-45.
- Bock JJ, Maurer P, Otto C, Fuhrmann RAW, Schubert J, et al. (2006) Complications of orthodontic orthognathic surgery treatment in mentally handicapped patients. Journal of Cranio-Maxillofacial Surgery 34: 156-161.
- Suri S, Tompson BD, Cornfoot L (2010) Cranial base, maxillary and mandibular morphology in Down syndrome. The Angle Orthodontist 80: 861-869.
- Bull MJ (2011) Health supervision for children with Down syndrome. Pediatrics 128: 393-406.
- Amaddeo A, Frapin A, Fauroux B (2016) Long-term non-invasive ventilation in children. Lancet Respir Med 4: 999-1008.
- Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, et al (2012) Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 130: e714-755.
- Shahidi S, Danaie SM, Omidi M (2016) Comparison of the Pharyngeal Airway Volume between Non-Syndromic Unilateral Cleft Palate and Normal Individuals Using Cone Beam Computed Tomography. J Dent (Shiraz) 17: 268-275.
- Chousangsuntorn K, Bhongmakapat T, Apirakkittikul N, Sungkarat W, Supakul N, et al. (2018) Upper Airway Areas, Volumes, and Linear Measurements Determined on Computed Tomography During Different Phases of Respiration Predict the Presence of Severe Obstructive Sleep Apnea. J Oral Maxillofac Surg 76: 1524-1531.
- Bartolucci ML, Bortolotti F, Raffaelli E, Anto VD, Michelotti A, et al. (2016) The effectiveness of different mandibular advancement amounts in OSA patients: a systematic review and meta-regression analysis. Sleep Breath 20: 911-919.
- Zaghi S, Holty JEC, Certal V, Abdullatif J, Guilleminault C, et al (2016) Maxillomandibular Advancement for Treatment of Obstructive Sleep Apnea: A Meta-analysis. JAMA Otolaryngol Head Neck Surg 142: 58-66.
- Caples SM, Rowley JA, Prinsell JR, Pallanch JF, Elamin MB (2010) Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep 33: 1396-1407.
- Bussolaro CT, Galvan JG, Pereira CP, Mir CF (2019) Maxillary osteotomy complications in piezoelectric surgery compared to conventional surgical techniques: a systematic review. Int J Oral Maxillofac Surg 48: 720-731.
- Yaedu RYF, Mello MDAB, Tucunduva RA, Silveira JSZ, Takahashi MPMS, et al. (2017) Postoperative Orthognathic Surgery Edema Assessment With and Without Manual Lymphatic Drainage. J Craniofac Surg 28: 1816-1820.
- Lateef TA, Al-Anee AM, Agha MTF (2018) Evaluation the Efficacy of Hilotherm Cooling System in Reducing Postoperative Pain and Edema in Maxillofacial Traumatized Patients and Orthognathic Surgeries. J Craniofac Surg 29: e697-e706.
- Musich DR (2006) Orthodontic intervention and patients with Down syndrome. Angle Orthod 76: 734-735.
- Faulks D, Mazille MN, Collado V, Veyrune JL, Hennequin M, et al. (2008) Masticatory dysfunction in persons with Down’s syndrome. Part 2: management. J Oral Rehabil 35: 863-869.
Citation: Hernández-Alfaro F, Montes-Fernández-Micheltorena P, Molins-Ballabriga G, Giralt-Hernando M, Mayoral-Trias A, et al. (2021) Airway Volume Changes after Orthognathic Surgery in Patients with Down Syndrome: A Diagnostic-Therapeutic Algorithm. J Otolaryng Head Neck Surg 7: 54
Copyright: © 2021 Hernández-Alfaro F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

