
Algorithm for the Therapeutic Approach to Apnea of Prematurity
*Corresponding Author(s):
Lourdes Lemus-Varela PhDDepartamento De Neonatologia, Hospital De Pediatría UMAE, Centro Médico Nacional De Occidente, Instituto Mexicano Del Seguro Social, Guadalajara, Jalisco, México; Avenida Belisario Domínguez, Guadalajara, México; And Council Member, Ibero American Society Of Neonatology (SIBEN), United States
Tel:+52 3334408054,
Email:lulalemus@gmail.com / lourdes.lemus@siben.net
Abstract
Apnea of prematurity is one of the most common and recurrent clinical problems observed in the neonatal intensive care unit; with a higher incidence at a lower gestational age. Survival of premature infants continues to improve; therefore, apnea of prematurity is observed more frequently.
Apneic episodes may prolong the duration of mechanical ventilation, and exposure to additional oxygen, contributing to the pathogenesis of bronchopulmonary dysplasia and retinopathy of prematurity. The long-term implications of apnea are associated with neurodevelopment disturbances. Methylxanthines have been the cornerstone in the pharmacologic treatment for apnea of prematurity. There are controlled clinical trials showing that caffeine is highly effective in treatment of apnea and also reducing the risk for developing bronchopulmonary dysplasia and the need to treat the ductus arteriosus; in addition, it favors extubation success and reduces the risk of neurodevelopmental disorders. However, the protocols of care are variable in different regions and neonatal centers around the world and many remains uncertain regarding optimal dosing, timing of initiation and timing of discontinuation of caffeine therapy, attributed in part to concern because some studies have reported that earlier administration of caffeine increases the risk of mortality. Likewise, solid evidence is needed about the best time to stop caffeine.
Conclusion
Based on currently evidence, we propose an algorithm for the therapeutic approach to apnea of prematurity that allows for orderly decision making for treatment with the greatest efficacy and safety margin.
Keywords
Algorithm; Apnea of prematurity; Caffeine; Methylxanthines; Premature infant
Abbreviations
AOP: Apnea of Prematurity
BPD: Bronchopulmonary Dysplasia
CPAP: Continuous Positive Airway Pressure
DOL: Days of Life
FDA: Food and Drug Administration
GABA: Gamma Aminobutyric Acid
IQR: Interquartile Range
NIPPV: Nasal Intermittent Positive Pressure Ventilation
NICU: Neonatal Intensive Care Unit
PI: Premature Infant
REM: Rapid Eye Movements
ROP: Retinopathy of Prematurity
SpO2: Plasmatic Oxygen Saturation
WGA: Weeks of Gestational Age
Introduction
Nowadays Preterm Infants (PI) not only survives longer but they are extubated and indeed forced to breathe spontaneously earlier than in the past. For this reason, Apnea of Prematurity (AOP) has become one of the most prevalent problems in the Neonatal Intensive Care Units (NICU); it occurs in all PI ≤ 28 Weeks of Gestational Age (WGA) and in 85% of those ≤ 34 WGA [1].
Caffeine has been shown to be effective in treating AOP, and has become one of the most prescribed and cost-effective pharmacotherapy’s in the NICU [1], however, the best time to start it, the useful and safe dose, and when to stop it, still represent a dilemma.
The purpose of this manuscript is to review on pathogenesis of AOP and on its treatment in order to propose an orderly clinical approach together with a simple to follow algorithm based on the available evidence and thus reduce the existing gap between the caffeine recommendation and clinical practice.
Definition and Brief Historical Aspects
The American Academy of Pediatrics, defines AOP as a sudden cessation of breathing for longer than 20 seconds, or respiratory pauses of shorter duration with associated bradycardia or oxygen desideration in PI [2].
For more than 40 years pharmacological treatment with methylxanthines has been shown to be effective in the management of AOP. The initial studies with methylxanthines were with aminophylline and theophylline, published in the seventies [3,4]. The effectiveness of rectal aminophylline was also found to reduce apneic episodes in PI [5,6].
The first report about caffeine was published by Aranda et al in 1977, in the pre-surfactant era, in 18 PI of 27.5±0.6 Weeks of Gestational Age (WGA), mean birth weight of 1065±71.9 g with apneic episodes. The age at the beginning of caffeine citrate was 18.2±4.9 Days of Life (DOL). Seventeen PI showed significant decrease in the frequency of apneic episodes, from 13.6±2.5 episodes/day to 2.1±0.6 episodes/day (p<0.001). Respiratory rate was increased and PaCO2 was decreased [7].
Caffeine has been shown to have greater central activity and fewer peripheral effects than theophylline and aminophylline. The latter one is a composite of theophylline plus ethylenediamine, which provides plasma levels equivalent to 80% of those obtained with the same dose of anhydrous theophylline. In addition, caffeine has a longer half-life, offers excellent enteral bioavailability and a broad therapeutic index [8].
Henderson-Smart and Steer reported that caffeine and theophylline are equally effective in treating AOP, however, caffeine offers advantages over theophylline and aminophylline, both of which present a higher risk of toxicity and adverse effects [9], thus Caffeine is the pharmacological gold standard in the treatment of the AOP; it has also been shown to reduce the occurrence of bronchopulmonary dysplasia.
Pathogenesis Of Apnea Of Prematurity
There is evidence that fetal respiratory movements, phasic smooth muscle contractions of the upper airways and irregular diaphragmatic contractions play an important role in fetal lung development. These respiratory movements have been documented in the third trimester of gestation, are limited to the sleep stage with Rapid Eye Movements (REM) and are interrupted during non-REM sleep. This is due to predominance of the inhibitory pathways descending from the medullary center generating the rhythm. In healthy term infants, once the fetal to neonatal transition has been completed, the postnatal respiratory pattern becomes rhythmic, constant and dependent on neuromuscular interaction and balance [10,11].
The medulla oblongata is the lower half of the brainstem which contains the centers controlling involuntary vital functions, essential for regulating the cardiovascular and respiratory systems. The respiratory rhythm is generated in the central region of the brain stem in a group of excitatory neurons (Pre-Bötzinger complex), that depolarize during the three phases of respiration (inspiration, post inspiration and expiration). The neural impulse is routed to the external intercostals muscles for inspiration and to the diaphragm to initiate exhalation (Figure 1). The conduction reaches the muscles of the upper airway, larynx and pharynx, that are involved in the resistance to air flow [12,13].
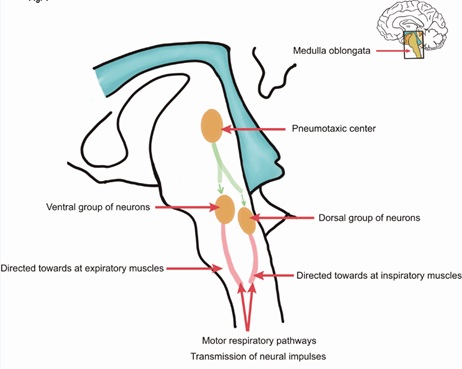 Figure 1: Shows a simplified diagram of respiratory control.
Figure 1: Shows a simplified diagram of respiratory control.
AOP is a developmental disorder resulting from “physiological” immaturity of the respiratory system control in PI that may be exacerbated or triggered by neonatal disease. These include altered ventilatory responses to hypoxia, hypercapnia, altered sleep states, sepsis, patent ductus arteriosus, while the roles of gastroesophageal reflux and anemia remain controversial. AOP may be preceded by breaths with tidal volume of less than 50% of baseline, measured by inductive plethysmography. A loss in lung volume or functional residual capacity [14], high compliance of the rib cage, propensity for airway obstruction and difficulty coordinating breathing with suction and swallowing explain the high incidence of AOP. For these reasons, the lower the gestational age, the higher the incidence of apneic episodes, reflecting the immaturity of the central nervous system, specifically the ventral and dorsal groups of the respiratory neurons (Figure 1). All this is attributed in part to decreased synapses, poor dendrite arborization, and incomplete myelination of these neuron groups [15,16].
A potential consequence of a reduction in lung volume is inhibition of respiration, via activation of the Hering-Breuer deflation reflex that results in a shortening of inspiratory time. This provides the theoretical basis for the effectiveness of strategies that increase lung volume, such as Continuous Pressure Airway Positive (CPAP) [14].
There are also neuromodulators that regulate respiration and their balance is essential to maintain respiratory rhythmogenesis. Among them are adenosine and Gamma Aminobutyric Acid (GABA), that are inhibitors and glutamate that is excitatory. Together they participate in neuronal synchronization and modulate synaptic transmission [13].
The central chemoreceptors located in the medulla oblongata and the peripheral ones in the carotid body, modulate breathing and promote regular and continuous respiration. The activity of these chemoreceptors is influenced by changing levels of pH, PaO2 and PaCO2. However, PI has a decreased response, mostly to PaCO2. Therefore, increased ventilation is not triggered until higher levels of PaCO2 are achieved and the response is not sustained.
The ventilatory response to hypoxia is unique in PI, very different to older infants, children and adults. It is characterized by a classic biphasic curve. Immediately after hypoxemia, there is an increase in ventilation lasting 1 to 2 minutes. This is then followed for 5 minutes of hypoxia, due to decrease in ventilation. The breathing pattern becomes irregular and apnea may ensue. This second phase of ventilatory depression is likely related to central release of inhibitory neuromodulators in response to hypoxia. However, this does not mean that hyperoxia reverses apneic episodes [17,18]. Even though experiments in animals and normal humans have suggested that breathing high concentrations of oxygen can cause an increase in ventilation [19]. Actually, hyperoxia increases the risk of oxidative injury with an increased production of reactive oxygen species, directly stimulating brain stem carbon dioxide chemoreceptors. Additionally, in mice offspring, hyperoxia severely depresses breathing, especially when administered repeatedly, with a decrease in minute ventilation and respiratory rate and even total apnea duration [20].
The clinical significance of altered respiratory control and responses of chemoreceptors to changes in pH, PaO2 and PaCO2 secondary to central nervous system immaturity in PI and the hazards of hyperoxia have been previously reviewed. The evidence supports a need for stringent control of oxygen therapy and for avoidance hyperoxia in PI with AOP [21].
Thermoregulation also plays an important role in AOP. Hyperthermia increases the risk for apnea. Exposure to cooler temperatures decreases the duration and frequency of apnea, suggesting that apnea is related to metabolic state and environmental temperature. It is necessary to avoid hyperthermia in PI with severe and/or recurrent apneic episodes [22].
Caffeine and Apnea of Prematurity
In addition to avoidance of hyperthermia, hypoxemia and hyperoxemia, interventions that have shown efficacy in the therapeutic approach to AOP include efforts to reduce work of breathing, like the prone position and the use of CPAP and Nasal Intermittent Positive Pressure Ventilation (NIPPV). Moreover, increasing respiratory drive with caffeine, that is the cornerstone in therapeutic intervention for AOP [23-26].
Caffeine offers several advantages over other methylxanthines, such as longer half-life (102.9±19.9 hours) that allows once-daily administration, earlier onset of action (45 minutes), and a large therapeutic index (8-40 mg/L) with no need to monitor blood levels routinely [27,28].
Caffeine citrate can be administered both intravenously and orally, since regardless of pH, it is rapidly and completely absorbed due to its weakly basic nature and pKa of 14 at 25oC. The route of administration of caffeine does not affect its pharmacokinetics and its peak plasma concentration occur in less than one hour [29,30].
Caffeine has also been used for prophylaxis (early administration) of AOP or to reduce the incidence of extubation failure [31], diminishing events of hypoxemia and bradycardia. Both have been associated with significantly adverse outcomes at 18 months, like motor disturbances, cognitive damage, hearing loss, blindness and mortality [32].
For all of the above, caffeine has become the pharmacological gold standard for AOP, as it represents a significant advance in the care of PI provides both short and long-term benefits and safety.
Mechanism of Action and Metabolic Pathway of Caffeine
Caffeine, 1,3,7 trimethylxanthine, is a purine-like alkaloid, and is a powerful stimulant of respiration. Adenosine on the other hand, inhibits respiration and its metabolism depends on the synthesis, release and breakdown of ATP (Adenosine Triphosphate). Adenosine is involved in many physiological functions in the brain, such as blood flow, energy metabolism, and neuronal excitability, it could be worrying because micromolar concentrations of caffeine are competitive antagonists of adenosine; their physiological functions are mediated by G protein-coupled receptors from the four adenosine receptors (A1, A2a, A2b and A3). Caffeine blocks A1 and A2a receptors and this antagonism inhibits phosphodiesterase and increases levels of cAMP (cyclic Adenosine Monophosphate) and cGMP (Guanosine Cyclic Monophosphate). In addition, it activates K+ channels and blocks Ca++ type N channels. It also antagonizes GABA, acetylcholine, dopamine, serotonin and norepinephrine receptors [33,34].
The effects of caffeine on stimulation of respiration and increased minute volume potentiate the response to hypercarbia largely due to phosphodiesterase antagonism, increased intracellular calcium and GABA receptor antagonism. Furthermore, it has also been reported that it increases the sensitivity of both peripheral and central CO2 chemoreceptors and improves diaphragmatic contractility [35-38].
The metabolic pathway for caffeine is the oxidative N-demethylation by the isoenzyme oxidase 1A2 in the liver. It is mainly broken down into four forms of dimethylxanthines: Paraxanthin or 1,7 dimethylxanthine is the most abundant (80 to 84%), theobromine or 3,7 dimethylxanthine (10 to 12%), theophylline or 1,3 dimethylxanthine (5%) and trimethyluretic acid, 1%. All are pharmacologically active. These metabolites are completely demethylated primarily via cytochrome 1A2 and acetylated by N-acetyl transferase 2 and xanthine oxidase pathway, to give rise to the metabolite that is excreted by renal route [39].
Caffeine is one of the most researched drugs in neonatology, accepted by the FDA since 1999 for the treatment of AOP in PI. It is the only methylxanthine approved for this use, at the standard dose of caffeine citrate of 20 mg/kg as an impregnation dose and 5mg/kg for maintenance dose, administered every 24 hours, in infants between 28-33 WGA. The short-term benefits appeared significant and this drug started to be used before clinical trials and safety studies were completed [40].
There is currently no doubt that caffeine is effective in reducing AOP and the need for mechanical ventilation, improves success in extubation, reduces the risk of developing BPD and for treatment of the patent ductus arteriosus, and is associated with better neurocognitive development at two years of age and lung development at 11 years of age. However, there is still debate about the optimal dose and the best time to start it and when to stop [41].
The multinational study CAP (Caffeine for Apnea of Prematurity), included 2006 PI, randomized to caffeine group (n=1006) or placebo (n=1000), started on average at 3 DOL, with Interquartile Range (IQR) 2 to 5 days and the duration of caffeine administration was 37 days, IQR: 24 to 46 days (34.4 corrected WGA). The main outcome initially proposed were mortality and neurological developmental disturbances at 18 to 21 months old. The caffeine group required one week less ventilatory support and additional oxygen in contrast to the placebo group. Consequently, a lower incidence of BPD was found: 36.3% versus 46.9% respectively (p=0.001) [42]. In PI with early treatment (1-3 DOL) there was a 52% reduction in the rate of BPD in contrast with a reduction of only 23% if started after day 3 [43], for that reason that it seems to star caffeine early, however more randomized clinical trials are needed to recommend it.
In addition, PI who received caffeine required less pharmacological treatment for ductus arteriosus; 29%versus 38% (p=0.001), and less need for surgical ductal closure: 4.5%versus12.6% (p=0.001), attributed in part to caffeine antagonizing the activity of prostaglandins and increasing urine flow. Likewise, in the caffeine group there was a decrease in the need for postnatal corticosteroids [42]. The caffeine group presented a lower likelihood of death and/or neurological developmental disturbances at 18 to 21 months old (40.2% versus 46.2%; p=0.008), reduced incidence of cerebral palsy (4.4% versus 7.3%; p=0.009) and of delay in cognitive development (33.8% versus 38.3%; p=0.04) [44]. However, the 5-year follow-up study, did not show significant differences in the combined outcome of death or disability (21.1% versus 24.8%; p=0.09), but there was significant improvement in gross motor function in the caffeine group (p=0.006) [45].
In addition to the above, in the follow-up at 11 years of age, the caffeine group performed better than the placebo group in fine motor coordination (p=0.01), with significant improvement in visuomotor integration, visual perception and visuospatial organization skills and no long-term adverse effects on intelligence, attention and behavior. Therefore, long-term safety of caffeine for AOP in very low birth weight neonates was demonstrated [46]. The longer-term consequences of caffeine in adults born preterm are being studied and may depend on the ability of caffeine to modulate both the expression and the maturation of adenosine receptors in infants treated with caffeine [47].
Lastly in both experimental models and humans, caffeine has been shown to possess antioxidant properties. Therefore, it modulates the oxidative damage produced by additional oxygen and would at least partially explain the decrease in the incidence of BPD and ROP [48].
Clinical Approach And Proposed Algorithm
We designed an algorithm for clinical approach to AOP which we present in figure 2. It aims to map out the appropriate therapeutic strategies in order to increase safety and reduce the variations between different neonatal centers, to try to reach homogeneity of care.
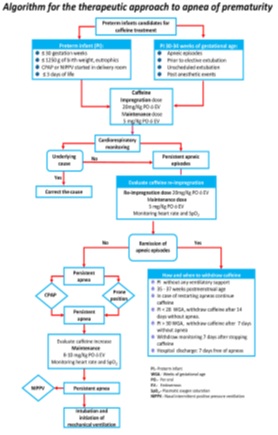 Figure 2: Algorithm for the therapeutic approach to apnea of prematurity.
Figure 2: Algorithm for the therapeutic approach to apnea of prematurity.
Clinically, the relationship between AOP, bradycardia and oxygen desaturation are complex. Most often apnea is the initiating phenomenon causing oxygen desaturation, which leads to bradycardia. For this and other reasons, all PI ≤ 34 weeks gestational age, should be continuously monitored with chest impedance, electrocardiographic waveforms and oxygen saturation, in order to opportunely document the initiation of AOP and introduce without delays the therapeutic approach [49]. Some randomized clinical trials have shown that early caffeine initiated within the first 72 h of postnatal age is associated with a significant reduction of BPD in very and extremely PI; [50-52], however a recent randomized clinical trial, in which they tried to probe that the early onset of caffeine facilitates extubation, reduce durations of ventilation and rates of BPD; had to be interrupted at 75% enrollment, because trend towards higher mortality rates in the early caffeine group, the authors argue that a unblinded analysis revealed mortality did not differ significantly (p=.22.) between the early caffeine (9 [22%]) and control groups (5 [12%]); however they suggested caution and the need to carry out larger randomized clinical trials, before routine use is recommended [53]. This find did not occur whatsoever in the larger multi-center CAP trial with large numbers of infants studied and long term follow up [42]. Likewise in a systematic overview of meta-analysis related to caffeine, report that while the use of caffeine for the treatment of AOP is advisable, but there is currently no-good evidence to support early or higher dose administration of caffeine [54]. Therefore currently we recommend early caffeine in the at risk population.
On the other hand, the usefulness of caffeine in decreasing the failure of non-invasive ventilation has been evidenced [55]. In PI 30 -34 WGA as soon as AOP is detected, even if it is mild and in PI on mechanical or high frequency ventilation; 12-24 h before planned extubation. It has long been recommended to start caffeine to prevent apnea after surgical events with general anesthesia and before elective extubation [56].
Selection of treatment involves careful assessment of etiology, as chronic or acute pathologic processes may also impact the occurrence, severity and duration of AOP [49]. Sepsis, patent ductus arteriosus, severe intraventricular hemorrhage, necrotizing enterocolitis, anemia, electrolyte and glucose disturbances or exposure to respiratory depressant medications are associated with apnea [57] and should be differentiated form AOP. If apnea causing-pathologies have been ruled out, and apneic episodes persist, the pertinence of administering a second impregnation dose of caffeine could be evaluated, as well as increasing the maintenance dose to 8-10mg/kg [58]. Recently and based on a pharmacokinetic model, it has been recommended to increase the maintenance caffeine dose by 1 mg per kg every 1-2 weeks to a goal dose 8 mg per Kg [59].
At this point If the apnea episodes persist, CPAP or NIPPV (Nasal Intermittent Positive Pressure Ventilation) are necessary [55]. According to the clinical course, it may be necessary to initiate invasive or conventional ventilation, and do not delay it (Figure 2).
When to Discontinue Caffeine Treatment?
This is not simple. No trials have carefully addressed when to discontinue treatment in PI. However, timely discontinuation is advised to avoid unnecessary delays in discharge. The use of any specific gestational age may result in unnecessarily continuing therapy due to the large variability in resolution of apnea. Therefore, the process needs to be individualized based on the indications for starting caffeine and the clinical course of the PI.
Caffeine-treated PI frequently maintains serum levels of caffeine 5-10 days after discontinuation of the medication. Thus, monitoring for apnea recurrence before discharge for not less than7 days after stopping caffeine is recommended (Figure 2).
1. PI < 34WGA at birth with a history of severe AOP:
- • Discontinue caffeine 7 days before planned discharge.
- • Observe for a free apnea and bradycardia or a “countdown” period.
- • The event-free period need not be uniform for all infants; shorter durations may be considered for older gestational ages.
- • Consider a post menstrual age of 35-37 weeks.
2. If caffeine was used exclusively before extubation and there was no AOP subsequently:
- • Discontinue 5-7 days after extubation and observe with adequate monitoring the remainder of the hospital stay.
Monitoring Caffeine Levels and Adjustment of Caffeine Doses
The routine measurement of blood levels in the clinical management of AOP is not widely recommended. It may be helpful when dose individualization is required in order to minimize the incidence of toxic adverse effects and optimize efficacy, especially for PI who are unresponsive to therapy (breakthrough apnea, bradycardia or desaturations without other obvious disease-related etiologies). Despite different studies exploring the best minimally invasive and cost-effective methods to monitoring therapeutic ranges of caffeine in clinical practice, few have tried to develop a pharmacokinetic model to adjust caffeine dosage and none has investigated the relationship between caffeine biofluid levels in the first weeks of life and clinical outcomes, such as apnea frequency [60].
Based on pharmacokinetic information, and on the postnatal improvement of metabolic pathways associated with a progressive shortening of caffeine half-life in PI, it is suggested to adjust the maintenance dose through time. Koch et al [61], developed simulation models of caffeine concentrations, proposing the need of adjusting the maintenance doses through time, increasing the dose of caffeine citrate by 1 mg/kg/day every 1-2 weeks. This is not associated with supra-therapeutic levels; it decreases the risk of sub-therapeutic levels and may increase the short and long-term beneficial effects of caffeine treatment. They recommended the administration of 6 mg/kg/day in the second week of life, 7 mg/kg/day in weeks 3-4 and 8 mg/kg/day in weeks 5-8.
Potential Side Effects of Caffeine
No deaths have been reported in relation to overdose of caffeine in neonates. However, caffeine has a diuretic effect and a potential effect on heart rate, left ventricular output, stroke volume and irritability. Likewise, caffeine has calciuric and osteoclastogenic effects and the cumulative dose and duration of therapy has been associated with osteopenia of prematurity [62].
The effects of neonatal caffeine therapy in adults born preterm are uncertain. A recent study on mice pups randomized to caffeine (20 mg/kg/d) or saline by intraperitoneal injection for 10 days after birth, evaluated the impact of neonatal caffeine on systemic blood pressure, vessel reactivity, and response to stress in adult mice. No differences were noted in systolic, diastolic, and mean blood pressures between the two groups at 8 and 12 weeks of age. However, norepinephrine-induced vasoconstriction was substantially higher in aortic rings in caffeine treated male mice, that also had substantially higher fecal corticosterone and urinary 8-hydroxy-deoxyguanosine at 14 weeks, suggestive of chronic stress. On the other hand, female mice exposed to caffeine had a more significant vasodilator responses to nitric oxide donors in aortic rings and had significantly lower body weight over-time, which suggests gender-specific effects of caffeine. Altered vessel reactivity and chronic stress in the presence of other risk factors may predispose to the development of systemic hypertension with sex-specific vulnerability in adults born preterm [63].
Summary
Based on high quality of evidence, caffeine has been demonstrated to be efficacious and safe to reduce AOP and to stimulate breathing in PI. It also reduces the incidence of BPD, increases success of non-invasive ventilation, and decreases extubation failure. The early initiation of caffeine and the administration of high doses are currently proposed, but there is limited date to support these practices. Apparently, the PI with AOP and those who needs ventilatory support and are in the weaning process; are the best candidates for caffeine. Optimizing therapeutic approaches for apnea of prematurity within a wide margin of safety will favorably impact outcomes of preterm infants.
Compliance with Ethical Standards
Conflict of Interest
The authors declare no conflict of interest in this work.
Funding
There is no funding source from agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Acknowledgement
The authors are thankful to SIBEN for the support and sponsorship.
Informed consent
Not necessary as it is a review article.
References
- Dobson NR, Hunt CE (2018) Caffeine: An evidence-based success story based in VLBW pharmacotherapy. Pediatr Res 85: 333-340.
- Eichenwald EC (2016) Committee on fetus and newborn, American academy of pediatrics, apnea of prematurity. Pediatrics 137: 20153757.
- Kuzemko JA, Paala J (1973) Apnoeic attacks in the newborn treated with aminophylline. Arch Dis Child 48: 404-406.
- Shannon DC, Gotay F, Stein IM, Rogers MC, Todres D, et al. (1976) Prevention of apnoea and bradycardia in low-birth-weight infants. Pediatrics 55: 589-594.
- Bednarek FJ, Roloff DW (1976) Treatment of apnea of prematurity with aminophylline. Pediatrics 58: 335-339.
- Lyon AJ, McIntoch N (1985) Rectal aminophylline in the management of apnoea of prematurity. Arch Dis Child 60: 38-41.
- Aranda JV, Gorman W, Bergsteinsson H, Gunn T (1977) Efficacy of caffeine in treatment of apnea in the low-birth-weight infant. J Pediatr 90: 467-472.
- Zulgarnain A, Hussain M, Suleri KM, Ali Ch Z (2019) Comparison of caffeine versus theophylline for apnea of prematurity. Pak J Med Sci 35:113-116.
- Henderson-Smart DJ, Steer PA (2010) Caffeine versus theophylline for apnea in preterm infants. Cochrane Database Syst Rev 1: 000273.
- Joshi S, Kotecha S (2007) Lung growth and development. Early Hum Dev 83: 789-794.
- Cloutier M, Tremblay M, Piedbouef B (2010) ROCK2 is involved in accelerated fetal lung development induced by in vivo lung distension. Pediatr Pulmonol 45: 966-976.
- Di Fiore JM, Martin RJ, Gauda EB (2013) Apnea of prematurity perfect storm. Respir Physiol Neurobiol 189: 213-222.
- Feldman JL, Del Negro CA, Gray PA (2013) Understanding the rhythm of breathing: So near yet so far. Annu Rev Physiol 75: 423-452.
- Poets CF (2010) Apnea of prematurity: What can observational studies tell us about pathophysiology? Sleep Med 11: 701-707.
- Mathew OP (2011) Apnea of prematurity: Pathogenesis and management strategies. J Perinatol 31: 302-310.
- Arora P (2012) Pathogenesis and management of apnea of prematurity: A brief overview. J Neonatal Biol 1: 2
- Alvaro RE (2018) Control of breathing and apnea of prematurity. Neo Reviews 19: 224-234.
- Cardot V, Chardon K, Tourneux P, Micallef S, Stéphan E, et al. (2007) Ventilatory response to a hyperoxic test is related to the frequency of short apneic episodes in late preterm neonates. Pediatr Res 62: 591-596.
- Becker HF, Polo O, McNamara SG, Berthon-Jones M, Sulliban CE (1996) Effect of Different Levels of Hyperoxia on Breathing in Healthy Subjects. J Appl Physiol 81: 1683-1690.
- Lofaso F, Dauger S, Matrot B, Vardon G, Gaultier C, et al. (2007) Inhibitory effects of repeated hyperoxia on breathing in newborn mice. Eur Respir J 29: 18-24.
- Sola A (2015) Oxygen saturation in the newborn and the importance of avoiding hyperoxia-induced damage. Neo Reviews 16: 393-405.
- Zhao J, Gonzalez F, Mu D (2011) Apnea of prematurity: From cause to treatment. Eur J Pediatr 170: 1097-1105.
- Ballout RA, Foster JP, Kahale LA, Badr L (2017) Body positioning for spontaneously breathing preterm infants with apnoea. Cochrane Database Syst Rev 1: 00495.
- Eichenwald EC (2016) Commitee on fetus and newborn. Apnea of prematurity. Pediatrics 137: 20153757.
- Reher C, Kuny KD, Pantalitschka T, Urschitz MS, Poets CF (2008) Randomised crossover trial of different postural interventions on bradycardia and intermittent hypoxia in preterm infants. Arch Dis Child Fetal Neonatal Ed 93: 289-291.
- Poets CF (2010) Interventions for apnoea of prematurity: A personal view. Acta Paediatrica 99: 172-177.
- Rostas SE, McPherson Ch (2019) Caffeine therapy in preterm infants: The dose (and timing) make the medicine. Neonatal Netw 38: 365-374.
- Natarajan G, Lulic-Botica M, Aranda JV (2007) Pharmacology review: Clinical pharmacology of caffeine in the newborn, NeoReview 8: e214-e221.
- Lista G, Fabbri L, Polackova R, Kiechl-Kohlendorfer U, Papagaroufalis K, et al. (2016) The real-world routine use of caffeine citrate in preterm infants: A europeian post-authorization safety study. Neonatology 109: 221-227.
- Du L, Tonf X, Chen Ch, Gao X, Gagnatelli A, et al. (2020) Caffeine citrate for apnea of prematurity: A prospective, open-label, single-arm study in chinese neonates. Front Pediatr 8: 76.
- Henderson-Smart DJ, Davis PG (2003) Prophylactic methilxanthines for extubación in preterm infants. Cochrane Database Syst Rev 1: 000139.
- Poets CF, Roberts RS, Schmidt B, Whyte RK, Asztalos EV, et al. (2015) Nelson H. Association between intermittent hypoxemia or bradycardia and late death or disability in extremely preterm infants. JAMA 314: 595-603.
- Dobson NR, Hunt CE (2013) Pharmacology review: Caffeine use in neonates: Indications, pharmacokinetics, clinical effects, outcomes. Neo Reviews 14: 540.
- Wilson C (2018) The clinical toxicology of caffeine: A review and case study. Toxicol Report 5: 1140-1152.
- Rossor T, Bhat R, Ali K, Peacock J, Rafferty GF, et al. (2018) The effect of caffeine on the ventilatory response to hypercarbia in preterm infants. Pediatr Res 83: 1152-1157.
- Shrestha B, Jawa G (2017) Caffeine citrate-is it a silver bullet in neonatology? Pediatr Neonatol 58: 291-397.
- Pacifici GM (2104) Clinical pharmacology of caffeine citrate in preterm infants. Medical Express 1: 243-250.
- Kreutzer K, Bassler D (2014) Caffeine for apnea of prematurity: A neonatal success story. Neonatology 105: 332-336.
- Jandova Z, Gill ZC, Lim NM, Mobley DL, Oostenbrink Ch (2019) Binding modes and metabolism of caffeine. Chem Res Toxicol 32: 1374-1383.
- Yu T, Balch AH, Ward RM, Korgenski EK, Sherwin CMT (2016) Incorporating pharmacodynamic consideration into caffeine Therapeutic drurg monitoring in preterm neonates. BMC PharmacolToxicol 17: 22-23.
- Moschino L, Zivanovic S, Hartley C, Trevisanuto D, Baraldi E, et al. (2020) Caffeine in preterm infants: Where are we in 2020? ERJ Open Res 6: 00330-2019.
- Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, et al. (2006) Caffeine therapy for apnea of prematurity. N Engl J Med 354: 2112-2121.
- Davis PG, Schmidt B, Roberts RS, Doyle LW, Asztalos E, et al. (2010) Caffeine for apnea of prematurity trial: Benefits may vary in subgroups.J Pediatr 156: 382-387.
- Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, et al. (2007) Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med 357: 1893-1902.
- Schmidt B, Anderson PJ, Doyle LW, Dewey D, Grunau RE, et al. (2012) Survival without disability to age 5 years after neonatal caffeine for Apnea of Prematurity. JAMA 307: 275-282.
- Mürner-Lavanchy IM, Doyle LW, Schmidt B, Roberts RS, Asztalos EV, et al. (2018) Anderson PJ. Neurobehavioral outcomes 11 years after neonatal caffeine therapy for apnea of prematurity. Pediatrics 2018; 141(5): e20174047.
- Kumar VHS, Lipshultz SE (2019) Caffeine and clinical outcomes in premature neonates. Children 6: 118.
- Endesfelder S, Strau BE, Scheuer T, Schmitz T, Buhrer C (2019) Antioxidative effects of caffeine in a hyperoxia-based rat model of bronchopulmonary dysplasia. Resp Res 20: 88.
- Fairchild K, Mohr M, Paget-Brown A, Tabacaru C, Lake D, et al. (2016) Clinical associations of immature breathing in preterm infants: Part 1-central apnea. Pediatr Res 80: 21-27.
- Schoen K, Yu T, Stockmann C, Spigarelli MG, Sherwin CMT (2014) Use of methylxanthine therapies for the treatment and prevention of apnea of prematurity. Paediatr Drugs 16: 169-177.
- Kua KP, Lee SWH (2017) Systematic review and meta-analysis of clinical outcomes of early caffeine therapy in preterm neonates. Br J ClinPharmacol 83: 180-191.
- Fakoor Z, Makooie AA, Joudi Z, Asl RG (2019) The effect of venous caffeine on the prevention of apnea of prematurity in the very preterm infants in the neonatal intensive care unit of ShahidMotahhari Hospital, Urmia, during a year. J Ady Pharm Technol Res 10: 16-19.
- Amaro CM, Bello JA, Jain D, Ramnath A, D'Ugard C, et al. (2018) Early caffeine and weaning from mechanical ventilation in preterm infants: A randomized, placebo-controlled trial. J Pediatr 196: 52-57.
- Alhersh E, Abushanab D, Al-Shaibi S, Al-Bradiyeh D (2020) Caffeine for the treatment of apnea in the neonatal intensive unit: A systematic overview of meta-analysis. Pediatr Drugs 22: 399-408.
- Dobson NR, Patel RM (2016) The role of caffeine in non-invasive respiratory support. Clin Perinatol 43: 773-782.
- Henderson-Smart DJ, Steer PA (2001) Prophylactic caffeine to prevent postoperative apnoea following general anaesthesia in preterm infants. Cochrane Database Syst Rev 4: 00048.
- Zagol K, Lake DE, Vergales B, Moorman ME, Paget-Brown A, et al. (2012) Anemia, apnea of prematurity and blood transfusion. J Pediatr 161: 417-421.
- Vliegenthart R, Miedema M, Hutten GJ, van Kaam AH, Onland W (2018) High versus standard dose caffeine for apnoea: A systematic review. Arch Dis Child Fetal Neonatal Ed 103: 523-529.
- Saroha V, Patel RM (2020) Caffeine for preterm infants: Fixed standard dose, adjustments for age or high dose? Sem Fetal Neo Medicine 25: 101178.
- Wilhelm AJ, den Burger JC, Swart EL (2014) Therapeutic drug monitoring by dried blood spot: Progress to date and future directions. Clin Pharmacokinet 53: 961-973.
- Koch G, Datta AN, Jost K, Schulzke SM, van den Anker J, et al. (2017) Caffeine citrate dosing adjustments to assure stable caffeine concentrations in preterm neonates. J Pediatr 191: 50-56.
- Ali E, Rockman-Greenberg Ch, Moffatt M, Narvey M, Reed M, et al. (2018) Caffeine is a risk factor for osteopenia of prematurity in preterm infants: A cohort study. BMC Pediatrics 18:
- Singh AP, Chandrasekharan P, Gugino S, BerkelhamerS, Wang H, et al. (2020) Effects of neonatal caffeine administration on vessel reactivity in adult mice. Am J Perinatol 2.
Citation: Lemus-Varela L, Sola A (2021) Algorithm for the Therapeutic Approach to Apnea of Prematurity. J Neonatol Clin Pediatr 8: 068.
Copyright: © 2021 Lourdes Lemus-Varela PhD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

