
Allelopathic Influence of Eucalyptus on Common Kenyan Agricultural Crops
*Corresponding Author(s):
Brandy Garrett KlutheDepartment Of Biology, Saint Peter's University, Jersey City, NJ 07306, United States
Tel:+1 2017616428,
Email:brandygarrettkluthe@gmail.com
Abstract
Keywords
Africa; Allelopathy; Eucalyptus; Farming; Introduced species
INTRODUCTION
Several species of Eucalyptus have been introduced throughout the world. The fast growing tree has become an important source of wood for many different industries globally [6]. Farmers in Mexico have objected to the large scale Eucalyptus pulp wood plantations that have arisen since the 1990’s due to possible effects on their crops [7]. In China, Eucalyptus has become one of the most widely propagated introduced trees. It is also suspected of inhibiting crops near plantations [8]. This is a concern that is shared in many more countries where the Eucalyptus tree has been introduced; including Kenya. Several studies have used samples of collected soils from various Eucalyptus species wood lots for greenhouse experiments. These studies assessed the possible effect of the tree on the germination and growth in other plants [7]. Other studies have used leaf litter applied to the soil as a way to test the allelopathic effects of Eucalyptus on the growth and development of other plants. Predominately, the results have shown a negative effect on germination and growth, although the influence varied by tested species as well as by species of Eucalyptus used [9-13]. Variation in allelopathic influence is highlighted in the study by which examined the effect of leaf litter on three common Chinese crops - the cabbage, radish, and cucumber [13]. They found that at lower leaf litter concentrations, the cucumber actually experienced an increase in germination rates with two of the three Eucalyptus species. Conversely, the cabbage and radish were negatively affected by the leaf litter, and the impact was more pronounced with an increase in concentration. These studies were the basis for developing the concentrations and protocols for the greenhouse experiment carried out in this study. The selections of seeds used were based on commonly grown crops in Kenya, where the Eucalyptus leaves were collected. They included corn, tomato and amaranth. The latter is an indigenous plant widely consumed in Kenya while corn and tomato are introduced crop species [14].
One way for Eucalyptus to influence nearby crops is from the leaching of allelochemicals from leaves; these allelochemicals are then transported with runoff water to nearby farms. The practice of “trenching” was observed in Eucalyputs woodlots on tree plantionation in Kenya (Figure 1A). This was presumably done to prevent the allelochemicals from influencing nearby crops. This study examined a potential mode for allelopathic influence on crops-leaf litter. This was done by adding different concentrations of leaf litter to soil and by examining germination percentages of various seeds placed in aqueous solutions. It is suspected that the higher the concentration of leaf litter the greater effect on the growth and development of the plants, with shorter and less dense plants measured at higher concentrations. It is also suspected that the indigenous plants would exhibit a greater impact from the leaf litter in both the growth and development experiment and the germination experiment.

MATERIALS AND METHODS
Leaf litter greenhouse experiments
The greenhouse component of the study examined the influence of chemicals in Eucalyptus leaves on the growth and development of the seeds of three types of plants-tomato (Solanum lycopersicum L.), corn (Zea mays L.) and amaranth (Amaranthus L.). The leaves, shipped from Kenya, were ground up through a series of grinders to reach a consistency able to pass through a 1 mm filter. Commercially available top soil was purchased from a local seed coop and air dried in the greenhouse. Once the soil was completely dry, it was sifted through a filter to remove large particles in order to create an even consistency.
Planting pots and trays were obtained from a local greenhouse. The planting pots were black, plastic, six pack pots with a growing space of approximately five square centimeters. The three groups were (1) a control, (2) 1% Eucalyptus to soil mixture and (3) a 10% Eucalyptus to soil mixture. A total of one hundred and twenty seeds were used for each type of plant. Each pot contained four seeds. The soil was mixed in one hundred gram batches for each of the experimental groups. For the 1% group, one gram of ground Eucalyptus was added to 99 grams of soil. This was used to fill the pots and when the pots were half full then they were watered thoroughly. More mixed soil was added to fill each pot and then it was watered again. The seeds were then placed, four per pot, an equal distance apart. A small amount of mixed soil was then added to the top. The pots were placed in the tray and watered thoroughly. This same procedure was repeated for the 10% Eucalyptus to soil mixture. This resulted in three trays, consisting of one control, one 1% mixture and one 10% mixture. The trays consisted of ten filled pots for each seed type, with four seeds in each pot. The pots were maintained in a greenhouse located on the campus of the University of Arkansas. The greenhouse was set at 89 degrees for a daytime high temperature and 52 degrees for the night time low temperature. The greenhouse was maintained at 38% relative humidity. The pots were watered every Monday, Wednesday and Friday, with seedling counts taken every Monday and recorded.
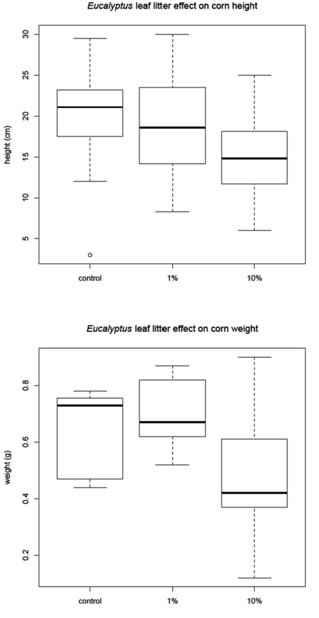
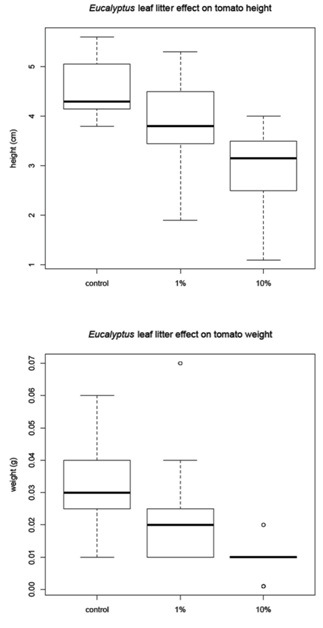
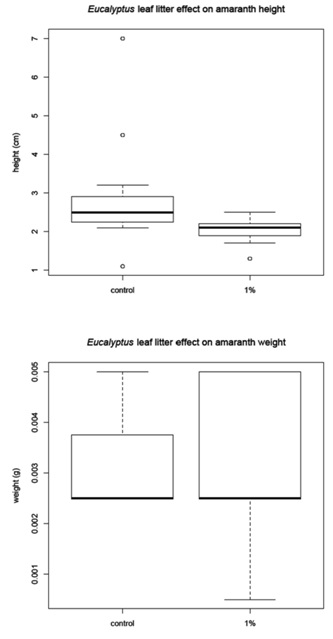
The corn was harvested 48 days after planting and the amaranth and tomato were harvested 68 days after planting. Before the plants were removed from the pots, a measurement of height was recorded for each plant (Figure 1B). The plants were then harvested, with the excess soil removed from the roots. The plants were allowed to dry for 24 hours in the greenhouse then they were placed in paper bags and moved to a drying facility for 48 hours. The dry weight was recorded for (1) the whole plant and (2) just the above ground portion of the plant (Figure 1C). The corn and tomato plants were measured individually, but the amaranth plants were measured in groups due to the small amount of plant material available.
Petri seed germination experiment
The solutions were prepared for a 10g/l solution and a 20g/l solution. The solutions were placed on a shaker table for 24 hours at 250 rev/min. The soaked leaf litter was strained through a filter and the remaining solutions were then placed in spray bottles to be used for application.
Disposable Petri dishes were used for the germination chambers. The bottom of each Petri dish contained one 90 mm piece of filter paper. This paper was sprayed with the particular solution concentration. Ten seeds were placed on top of the paper then another piece of 90 mm filter paper was placed over the seeds. The top filter paper was sprayed again with the solution concentration to get an even moist environment. The lid of the Petri dish was placed on it, and the dish was placed in a dark cabinet. Each seed/concentration combination had 10 Petri dishes containing 10 seeds each. The Petri dishes were sprayed three times a week to maintain an even moisture environment. On the fourteenth day, the seeds were examined for germination and recorded. This was done with the use of a stereomicroscope. A seed was considered to have germinated if there was a noticeable interruption in the seed coat. The results were recorded and reported in table 1.
|
Eucalyptus paniculata |
Eucalyptus grandis |
||||||
|
Seed Type |
Control |
10g/l |
20g/l |
Seed Type |
Control |
10g/l |
20g/l |
|
Corn |
100 |
97 |
95 |
Corn |
100 |
96 |
95 |
|
Amaranth |
90 |
36 |
28 |
Amaranth |
90 |
5 |
0 |
|
Tomato |
100 |
92 |
89 |
Tomato |
100 |
86 |
86 |
RESULTS
Leaf litter greenhouse experiments
Petri seed germination experiment
The results of seed germination under the E. grandis solution are given in table 1. Ninety-six corn seeds germinated in the 10 g/l solution and 95 seeds germinate in the 20 g/l solution. The tomato seeds had 86 germinate in the 10 g/l E. grandis solution and the same number, 86, germinated in the 20 g/l solution. The amaranth seeds had 5 germinate in the 10 g/l E. grandis solution. In the 20 g/l E. grandis solution, none of the amaranth seeds germinated.
DISCUSSION
|
|
Control |
1% |
10% |
||||||||||
|
|
Height (cm) |
Weight (g) |
Height (cm) |
Weight (g) |
Height (cm) |
Weight (g) |
|||||||
|
Corn mean |
20.18 |
0.64 |
23.5 |
0.7 |
14.94 |
0.46 |
|||||||
|
Tomato mean |
4.56 |
0.03 |
3.86 |
0.02 |
2.96 |
0.01 |
|||||||
|
Amaranth mean |
2.84 |
0.003 |
2.03 |
0.003 |
0 |
0 |
|||||||
|
ANOVA |
DF |
Sum Sq |
Mean Sq |
F value |
Pf(>F) |
||||||||
|
Corn height |
2 |
405.3 |
202.65 |
7.78 |
0.000774 |
||||||||
|
Corn weight |
2 |
0.8512 |
0.4256 |
18.61 |
1.82E-07 |
||||||||
|
Tomato height |
2 |
19.75 |
9.876 |
18.92 |
1.28E-06 |
||||||||
|
Tomato weight |
2 |
0.00352 |
0.0017601 |
11.14 |
0.000126 |
||||||||
|
Amaranth height |
1 |
4.741 |
4.741 |
4.789 |
0.0375 |
||||||||
|
Amaranth weight |
1 |
2.00E-08 |
1.66E-08 |
0.01 |
0.92 |
||||||||
|
Tukey |
T1-ControlPval. |
T2-ControlPval. |
T2-T1Pval. |
||||||||||
|
Corn height |
0.34 |
0.0005 |
0.29 |
||||||||||
|
Tomato height |
0.03 |
7 |
0.004 |
||||||||||
|
Amaranth height |
0.04 |
NA |
NA |
||||||||||
|
Corn weight |
0.28 |
0.00009 |
0.0000002 |
||||||||||
|
Tomato weight |
0.15 |
0.00008 |
0.02 |
||||||||||
|
Amaranth weight |
0.92 |
NA |
NA |
||||||||||
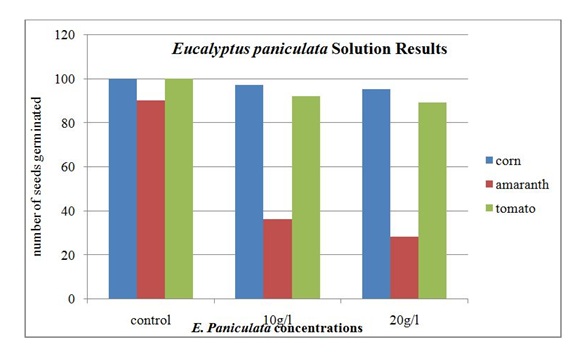
Figure 5: Seed germination results for the Eucalyptus paniculata solution on corn, amaranth and tomato seeds.
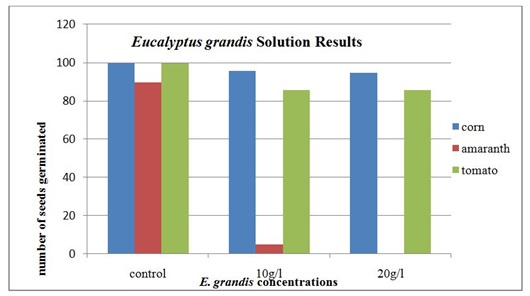
The amaranth was the only seed that displayed a statistically significant difference in the germination percentages from the control group. This was verified using a chi-square analysis. The results were significant for both Eucalyptus species as well as in both concentrations. The corn, tomato and Eucalyptusare all introduced species to Kenya, while the amaranth is a native species. This may provide some insight into the why the amaranth is more susceptible to the secondary metabolites that are found in the Eucalyptus leaves. The recent interaction between the two species has not been a long enough time for defense mechanisms to evolve to combat the allelopathic effects of the Eucalyptus tree. The evidence from this study also indicated that allelochemicals found in Eucalyptus could contribute to lower crop yields in rural farming areas and have a significant impact on food security for subsistence farmers.
ACKNOWLEDGMENT
REFERENCES
- Whittaker RH, Feeny PP (1971) Allelochemics: chemical interactions between species. Science 171: 757-70.
- Willis RJ (2010) History of Allelopathy. Springer, Dordrecht, The Netherlands.
- Hejl AA, Einhellig FA, Rasmussen JA (1993) Effects of juglone on growth, photosynthesis, and respiration. J Chem Ecol 19: 559-68.
- Jose S (2002) Black walnut allelopathy: current state of the science. Chemical Ecology of Plants: Allelopathy in Aquatic and Terrestrial Ecosystems 149-172.
- Del Moral R, Muller CH (1970) The Allelopathic Effects of Eucalyptus camaldulensis. The American Midland Naturalist 83: 254-282.
- Bennett BM (2010) The El Dorado of Forestry: The Eucalyptus in India, South Africa, and Thailand, 1850-2000*. International Review of Social History 55: 27-50.
- Espinosa-Garcia FJ, Martinez-Hernandez E, Quiroz-Flores A (2008) Allelopathic potential of Eucalyptus spp plantations on germination and early growth of annual crops. Allelopathy Journal 21: 25-38.
- Zhang D-J, Zhang J, Yang W-Q, Wu F-Z (2010) Potential allelopathic effect of Eucalyptus grandis across a range of plantation ages. Ecological Research 25: 13-23.
- Li Y, Hu T, Duan X, Zeng F, Chen H, et al., (2013) Effects of Decomposing Leaf Litter of Eucalyptusgrandis on the Growth and Photosynthetic Characteristics of Lolium perenne. J AGR SCI 5.
- Bughio FA, Mangrio SM, Abro SA, Jahangir TM, Bux H (2013) Physio-morphological responses of native Acaia Nilotica to Eucalyptus allelopathy. Pak J Bot 45: 97-105.
- Dadkhah A (2013) Allelopathic effect of sugar beet (Beta vulgaris) and Eucalyptus (Eucalyptuscamaldulensis) on seed germination and growth of Portulaca oleracea. Russian Agricultural Sciences 39: 117-123.
- Niakan M, Saberi K (2009) Effects of Eucalyptus Allelopathy on Growth Characters and Antioxidant Enzymes Activity in Phalaris Weed. Asian Journal of Plant Sciences 8: 440-446.
- Zhang C, Fu S (2010) Allelopathic effects of leaf litter and live roots exudates of Eucalyptus species on crops. Allelopathy Journal 26: 91-99.
- Ndenga EA, Achigan-Dako EG, Mbugua G, Maye D, Ojanji W (2013) Agricultural Diversification with Indigenous Vegetables for Cash Cropping and Nutrition: Examples from Rift Valley and Central Provinces in Kenya. International Society for Horticultural Science, Belgium.
Citation: Kluthe BG, Ali MBH, Stephenson SL (2018) Allelopathic Influence of Eucalyptus on Common Kenyan Agricultural Crops. J Agron Agri Sci 1: 002.
Copyright: © 2018 Mourad Ben Hassine Ben Ali, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

