
An Integrated Analysis of Network Pharmacology and Molecular Docking to Explore the Active Component and the Underlying Mechanism of Cuscutae Semen in Membranous Nephropathy
*Corresponding Author(s):
Zhenghai LiGuangdong Nephrotic Drug Engineering Technology Research Center, Institute Of Consun Co. For Chinese Medicine In Kidney Diseases, Guangdong Consun Pharmaceutical Group, Guangzhou, China
Email:lizh@chinaconsun.com
Junzheng Yang
Guangdong Nephrotic Drug Engineering Technology Research Center, Institute Of Consun Co. For Chinese Medicine In Kidney Diseases, Guangdong Consun Pharmaceutical Group, Guangzhou, China
Email:yangjunzheng606403@163.com
Abstract
Aims/purpose: To analyze the bioactive components of Cuscutae Semen, the targets and the underlying mechanism in Cuscutae Semen against Membranous Nephropathy (MN), to provide the data support for Cuscutae Semen applications in MN.
Methods and results: An aggregate of 443 drug targets and 460 MN targets were obtained from the databases, there were 24 overlapped targets being determined as potential therapeutic targets of Cuscutae Semen against MN. Through centrality analysis, PTGS2 and ALOX5 is screened as core target and its bindings with kaempferol are further validated by molecular docking. By conducting Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses for drug targets, disease targets and the shared targets, arachidonic acid metabolism signaling pathway is found, indicating the potential mechanism of Cuscutae Semen in the treatment of MN.
Conclusion: Cuscutae Semen could ameliorate the renal injury in MN by targeting multi-targets and multi-pathways including arachidonic acid metabolism, those data may provide the novel insights into further study of the drug discovery in MN.
Keywords
ALOX5; Cuscutae Semen; Membranous nephropathy; Network Pharmacology; PTGS2
Introduction
Cuscutae Semen, which is called Tu-si-zi in Chinese, is a kind of dried mature seed in the Convolvulaceae family. It mainly distributes in China, Korea, Pakistan, Vietnam, India and Thailand. Cuscutae Semen has been widely prescribed by Chinese medicinal practitioners to treat oligoasthenozoospermia or dyszoospermia including Five Seeds Combo (wu zi yan zong wan, a well-known traditional Chinese herbal formula) [1,2]. It could also play the important role in a classic Chinese herbal prescription for nourishing the kidney and activating blood circulation named Bushen Huoxue recipe (BSHXR), which is effectively used to treat failed pregnancy and its complications [3]. What is more, Cuscutae Semen is also used to tonify deficiency of liver and kidney, spleen and kidney deficiency and diarrhea [4]. Specifically, it is reported that Cuscutae Semen could maintain reproduction of human beings through promoting the migration and invasion of EVT cells by increasing MMP9 expression and prevent miscarriage by activating Notch, AKT, and MAPK signaling pathways [5]; the evidences of animal studies in both sex demonstrated that Cuscutae Semen could play significant role in maintaining reproductive system functions [2,6-10]; Cuscutae Semen also has been proven that it could contribute to regulate several genes related to hearing loss [11], depression [12], liver fibrogenesis [13], leukopenia [14]. Specially, traditional clinical experiences and modern studies demonstrated that Cuscutae Semen also could help to alleviate the symptoms of kidney diseases. For example, Hui-Ting Liang et al. isolated and verified b-sitosterol, stigmasterol, astragalin, quercetin, kaempferol, apigenin, isorhamnetin, isoquercitrin, hyperoside and rutin in Cuscutae Semen by electrospray ionization ion trap mass spectrometry (ESI-MS), 1H and 13C NMR technique, and found matrine, sesamin, isorhamnetin, quercetin and beta-sitosterol had the better effect on actue and chronic kidney disease [15].
Membranous Nephropathy (MN) is a kind of autoimmune diseases and one of the leading causes of nephrotic syndrome. MN exhibits heterogenous outcomes with approximately 30% of cases progressing to end-stage renal disease and ranks second popular in primary glomerulonephritis in renal biopsy cases in China [16]. the patient often faces potential complications of nephrotic syndrome including edema and hypo-albuminemia. For its characteristic and mechanism, nowadays corticosteroids and the alkylating agents chlorambucil or cyclophosphamide are widely used, but comes with other adverse effects. New strategy still remains to investigated. traditional Chinese medicines such as Tripterygium wilfordii and Astragalus membranaceus shed light on MN treatment [17]. In this article, we explored whether Cuscutae Semen would be the new strategy treatment to MN and analyze its possible targets and obtaining potential indigents for further study through network pharmacology methods and molecular docking.
Methods
- Active ingredients and target prediction of cuscutae semen
The active ingredients of Cuscutae Semen were retrieved in TCMSP database (https://old.tcmsp-e.com/tcmsp.php). The ingredients which passed the ADME screening criteria (oral bioavailability OB≥30% and drug-likeness DL≥0.18) were imported into the TCMSP platform to analyze the targets in the Swiss Target prediction database, and the validated targets with the species “Homo sapiens” were screened and imported into the Genecard database (https://www.genecards.org/) and standardized to the officially recognized gene names.
- Construction of the active ingredient-target network of cuscutae semen
Cytoscape 3.8.0 software was used to build and to analyze “active ingredient-target” network diagrams of “Cuscutae Semen.” The “node” and “edge” represented the ingredients or targets and the relationship between them. The network parameters including degree, betweenness, and closeness were analyzed using the Network Analyzer plug-in tool of Cytoscape 3.8.0. By analyzing these network parameters, the key active ingredients, targets, and their relationships in “Cuscutae Semen” were also analyzed.
- MN-related target analysis
We retrieved the GeneCards (https://www.genecards.org/) for potential targets of MN treatment using the keyword “Membrannous Nephropathy”, and then the retrieval results from the three databases were exported (the duplicates were removed to obtain the final disease targets for MN).
- PPI network construction and key target screening
Protein-protein interaction (PPI) networks were performed using Cytoscape 3.8.0. The active ingredient targets of Cuscutae Semen, and MN targets, were successively entered into Cytoscape 3.8.0 to generate the PPI network. The intersection of the two PPI networks was extracted using the Merge plugin tool of Cytoscape 3.8.0, and analyzed the properties of each node in the intersection network using CytoNCA.
- Pass-through enrichment analysis
For pass-through enrichment analysis, KEGG pathway enrichment analysis was performed; bioinformatic analysis was performed using the OECloud tools (https://cloud.oebiotech.com) for KEGG analysis.
- Molecular docking
To verify the reliability of the key targets of Cuscutae Semen in the treatment of MN, we performed molecular docking between the potential active ingredients and key targets. The 3D structures of the key target proteins (resolution< 2A) were obtained from the PDB database (https://www.rcsb.org). The 3D structures of the active ingredients were obtained through the PubChem platform. PyMOL was applied to remove ligands and water molecules. AutoDock Tools 1.5.6 was used to hydrogenate the target proteins, calculate the charge number, and determine the AD4 type of the atoms. And then, the built-in plug-ins software, autogrid 4 software and autodock 4 software were used to determine the binding energy of the best docking site between the active ingredients and target proteins. Finally, PyMOL was used to draw a molecular docking map and derive the docking hydrogen bond distance and docking target name for optimization and output.
Results
- Active ingredients and target acquisition of cuscutae semen
Retrieving the active ingredients of Cuscutae Semen in the TCSMP database according to the admission criteria (the oral bioavailability greater than 30% and higher than 0.18), 11 active ingredients of Cuscutae Semen were obtained, and sophranol was skipped because of the unknown structures. The basic information including Molecule number, Molecule name, Molecule weight, Oral Bioavailability (OB) and Drug Likeness (DL) of main active ingredients in Cuscutae Semen were shown in the table 1.
|
Mol number |
Mol name |
Mol weight |
OB(%) |
DL |
|
MOL005944 |
248.41 |
63.77 |
0.25 |
|
|
MOL001558 |
354.38 |
56.55 |
0.83 |
|
|
MOL006649 |
264.41 |
55.42 |
0.28 |
|
|
MOL000354 |
316.28 |
49.60 |
0.31 |
|
|
MOL000098 |
302.25 |
46.43 |
0.28 |
|
|
MOL005440 |
412.77 |
43.78 |
0.76 |
|
|
MOL000422 |
286.25 |
41.88 |
0.24 |
|
|
MOL000184 |
412.77 |
39.25 |
0.76 |
|
|
MOL000953 |
386.73 |
37.87 |
0.68 |
|
|
MOL005043 |
400.76 |
37.58 |
0.71 |
|
|
MOL000358 |
414.79 |
36.91 |
0.75 |
Table 1: The potential active ingredients in Cuscutae Semen.
Abbreviations: OB, oral bioavailability; DL, drug likeness.
- Potential targets analysis
The above 10 active ingredients of Cuscutae Semen in Swiss Targetprediction database (http://www.swisstargetprediction.ch/) were retrieved to predict the related biological targets. There were 460 MN-related targets and 243 drug targets were obtained; after 243 drug targets and 460 disease targets were imported into Venny 2.1, there were a total of 24 overlapped targets (Figure 1); and then we analyzed the PPI network of potential therapeutic MN targets and screened the more important targets, 24 overlapped targets were exported to construct a PPI (Protein-Protein Interaction) network. These targets are directly or indirectly related to MN. PTGS2 and MMP9 are proteins that makes most interactions with other target proteins (Table 2 & Figure 2).
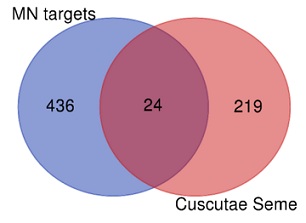 Figure 1: Venn diagrams of drug targets and disease targets.
Figure 1: Venn diagrams of drug targets and disease targets.
|
name |
NCC |
Degree |
Cloneness |
Betweenness |
Clustrering Coefficient |
|
MMP9 |
12 |
12 |
15.66667 |
83.56905 |
0.36364 |
|
PTGS2 |
11 |
11 |
15.33333 |
94.70952 |
0.4 |
|
MPO |
9 |
9 |
14.16667 |
31.21429 |
0.5 |
|
HIF1A |
9 |
9 |
14 |
32.26667 |
0.52778 |
|
XDH |
8 |
8 |
13.83333 |
62.77619 |
0.46429 |
|
NOS2 |
6 |
6 |
12.5 |
1.4 |
0.86667 |
|
MMP2 |
6 |
6 |
12.33333 |
5.43333 |
0.73333 |
|
MME |
5 |
6 |
12.25 |
37.85952 |
0.26667 |
|
ALOX5 |
5 |
5 |
11.66667 |
7.01667 |
0.7 |
|
AKR1B1 |
2 |
5 |
12 |
51.9 |
0.2 |
|
NR3C1 |
4 |
4 |
11.33333 |
0 |
1 |
|
FABP1 |
3 |
4 |
10.75 |
41.3 |
0.33333 |
|
PTK2 |
4 |
4 |
10.66667 |
2.56667 |
0.66667 |
|
DPP4 |
4 |
4 |
11.25 |
14.65476 |
0.5 |
|
ALK |
2 |
3 |
10.25 |
3.33333 |
0.33333 |
|
TTR |
2 |
2 |
9.08333 |
0 |
1 |
|
SLC5A2 |
2 |
2 |
8.28333 |
0 |
1 |
|
CXCR1 |
2 |
2 |
9.5 |
0 |
1 |
|
PLA2G1B |
2 |
2 |
9.41667 |
0 |
1 |
|
FABP3 |
1 |
1 |
7.2 |
0 |
0 |
|
PRKCD |
1 |
1 |
7.75 |
0 |
0 |
Table 2: Common genes of drug targets and disease targets in figure 1.
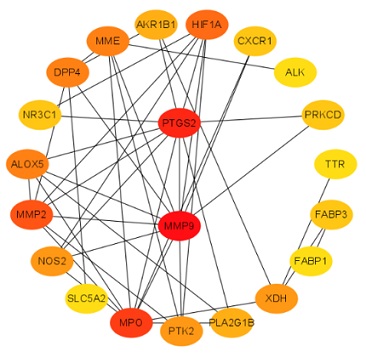 Figure 2: The active ingredient-target network map in Cuscutae Semen. The hexagon represents the target of the action of the active ingredient; the quadrilateral represents the 21 active ingredients (3 genes were skipped for they are could not be translated into proteins).
Figure 2: The active ingredient-target network map in Cuscutae Semen. The hexagon represents the target of the action of the active ingredient; the quadrilateral represents the 21 active ingredients (3 genes were skipped for they are could not be translated into proteins).
- Visualization of MN pathway enrichment analysis for cuscutae semen treatment on MN
24 targets were imported into OECloud tools for KEGG pathway enrichment analysis. The results showed that KEGG enrichment, involved 15 pathways. The top 14 entries of KEGG significance, according to the Log10 (P) values, were plotted in bubble diagrams, as figure 3 shows. The KEGG analysis mainly contained pathways in cancer, arachidonic acid metabolism, drug metabolism,endocrine resistance and so on in Membranous Nephropathy.
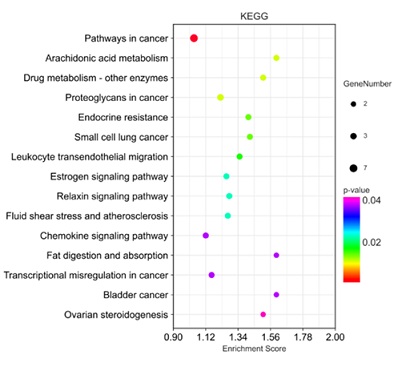 Figure 3: Bubble chart of enrichment bubbles of KEGG analysis targets for the treatment of MN by Cuscutae Semen.
Figure 3: Bubble chart of enrichment bubbles of KEGG analysis targets for the treatment of MN by Cuscutae Semen.
- Active compounds analysis
In order to predict the effective monomer components for further research, we firstly set specific one extract and the subset of figure 2 to explore which extract would be effective to membrane nephropathy, the results showed Venn figures of top three extracts, as shown in figure 4. According to table 3, kaempferol, isorhamnetin and quercetin may be important components of Cuscutae Semen in regulating the development of membranous nephropathy because these extracts share most common targets.
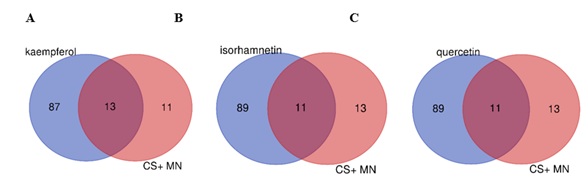 Figure 4: A subset of predicted components of (A) kaempferol, (B) isorhamnetin, and (C) quercetin and components of Cuscutae Semen that intersect with targets of MN respectively.
Figure 4: A subset of predicted components of (A) kaempferol, (B) isorhamnetin, and (C) quercetin and components of Cuscutae Semen that intersect with targets of MN respectively.
|
Component |
Kaempferol |
Isorhamnetin |
Quercetin |
|||||
|
Common Targets |
|
|
|
Table 3. Common targets of each component in figure 4.
- Molecular docking
Molecular docking is a tool for predicting how a protein interacts with small molecules (ligands) using molecular computational methods [1]. In this study, according to the above results, we chose kaempferol as ligand and PTGS2 AKR1B1, ALOX5, PTK2 and CFTR as proteins to perform the molecular docking. The results indicated that the receptor-ligand interaction between drugs and proteins includes hydrophobic interactions and polar interactions. According to table 4 and figure 5, AKR1B1 have strong binding interactions with kaempferol. What is more, we observed that PTGS2 and ALOX5 binds to kaempferol obviously, which revealed that Cuscutae Semen is involved with arachidonic acid metabolism pathway. This is coincident with what we obtained in figure 3.
|
ligand |
Target proteins |
Affinity (kcal/mol) |
|
kaempferol |
PTGS2 |
-8.2 |
|
AKR1B1 |
-13.2 |
|
|
ALOX5 |
-11.5 |
|
|
PTK2 |
-8.2 |
|
|
CFTR |
-10.9 |
Table 4: Results of Molecular Docking Between ingredients of Cuscutae Semen and the predicted Targets.
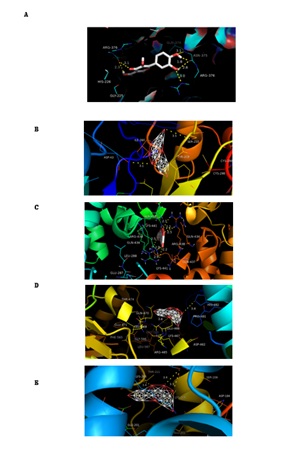 Figure 5. Molecular models of the binding of kaempferol, extracts of Cuscutae Semen, to the PTGS2 and kaempferol binding to PTGS2 (A), AKR1B1(B), ALOX5(C), PTK2(D) and CFTR(E) shown as 3D diagrams.
Figure 5. Molecular models of the binding of kaempferol, extracts of Cuscutae Semen, to the PTGS2 and kaempferol binding to PTGS2 (A), AKR1B1(B), ALOX5(C), PTK2(D) and CFTR(E) shown as 3D diagrams.
(Abbreviation: PTGS2: Cyclooxygenase-2; AKR1B1: Aldose reductase; ALOX5: Arachidonate 5-lipoxygenase; PTK2: Focal adhesion kinase 1; CFTR: Cystic fibrosis transmembrane conductance regulator)
Discussion
Based on the above speculation, we browse the literature and found that components in table 1 and figure 4 (but not limited to) have certain reports in the treatment of different kinds of kidney diseases. It is reported that kaempferol is the precursor of coenzyme Q (CoQ) cycle in cells in kidney, and protects renal tissue from oxidative stress by increasing the content of coenzyme, which clarifies the regulated role of kaempferol in CoQ cycle, providing a molecular basis for recognizing kaempferol's participation in the regulation of intracellular oxidative stress signaling. Kaempferol also shows protective effect in a unilateral ureteral obstruction model induced fibrosis via activating BMP 7-Smad 1/5 pathway in renal fibroblasts, thus reduces interstization of renal fibroblasts, and inhibits interstitium of renal tubular epithelial cells through hedgehog pathway [18-20]. Moreover, kaempferol suppresses tubular epithelial cell apoptosis, autophagy response [21], inflammatory response and decreased oxidative stress injury caused by cisplatin, cadmium chloride, calcium chloride [22], and carbon tetrachloride [23], which are negatively regulated by NF-κB pathway [24,25] to alleviate acute and chronic renal injury. In addition, kaempferol effectively inhibits podocyte apoptosis and promotes the transformation of M1-macrophages to M2-macrophages in diabetic nephropathy animals [26], thus reducing cytokines production [27,28] such as TGF- β and IL-6; on the other hand, kaempferol also increase the activity of nitric oxide by increasing the production of GSH and SOD [29]. A large number of literatures reported the significant effect of kaempferol on inhibiting ROS generation and inhibiting apoptosis in injured kidney. Cechinel-Zanchett et al. reported that kaempferol promotes sodium and chloride excretion and diuresis in a hypertensive rat model [30]. Overall, a large body of reports suggests that kaempferol is involved in the regulation of [31] of inflammatory response in renal disease, thereby exerting a renal protective effect. The current report of isorhamnetin mainly focus on its treatment of osteoporosis [32], alleviating obesity and diabetes [33,34], breast tumors and other gynecological tumors [35] It is said that it plays a protective role in cardiovascular disease and neurological diseases, especially the effects of inhibiting proliferation and differentiation and antioxidant, anti-inflammation and so on [36]. isorhamnetin also involved in regulating the production advanced glycation products (AGEs, advanced glycoxal end-products) in renal and hepatic cells [36]; besides, isorhamnetin is involved with AChE/BChE/COX2/NOX [37]. Diabetic rats treated with isorhamnetin lower fasting blood glucose and increase the content of renal autophagosomes [38], suggesting its important effect in treating diabetic nephropathy. A large number of reports have clarified that quercetin alleviates the development of various types of kidney diseases. It stops nephrotoxic damage in rats caused by 5-fluorodixine, cisplatin [39] and novel coronavirus N protein [40], down-regulates the level of KIM-1 and NGAL in the kidney and serum and deactivates RAS system [41,42] in kidney cortical renal tubular epithelial cells [43]; Treating STZ-induced diabetic rats with quercetin approves endothelial dysfunction [44] by deactivating NRF2/HO-1 pathway, thus reducing ferroptosis [45], inflammatory response and tissue oxidative stress [46,47] in renal tubular epithelial cells and glomerular mesangial cells [48-50]. Besides, quercetin has been reported that it participates in regulating the metabolism of fructose and purine, so as to be a potential to alleviating hyperuricemia and gout [51]. Quercetin is a new strategy treatment of acute and chronic renal diseases nowadays; further study remains to be elucidated.
Matrine has been reported to significantly reduce blood glucose, urinary albumin content and improve renal function in KKAY mice [52]. In doxorubicin-treated rats, scientists observed that matrine inhibits the activation of Treg/Th 17 cells by down-regulating Foxp3 and Rorγt, so as to decrease levels of IL-6, IL-10, TGF- β, and IL-10 [53]. Meanwhile, for acute kidney injury caused by cisplatin, the treatment of matrine inhibits NF-kB signaling pathway, reducing oxidative stress production, improving mitochondrial function by inhibiting SIRT 3-OPA 1 pathway, thus alleviating symptoms of acute kidney injury [54]. Matrine also reveals antifibrotic effect in glomerulonephritis rats [55]. The role of sesamin in treating kidney disease is relatively rarely reported. As we can see, sesamin alleviates kidney injury induced by immunosuppressants cyclophosphamide and fluoride [56], its intervention improves kidney function, increases the amount of antioxidants and reduce oxidants, meanwhile inhibiting apoptotic [57]. Sesamin also approves hyperlipidemia rats by downregulating α-SMA and Col-IV, thus inhibiting renal fibrosis [58]. In the 2-kidney 1 clip-induced hypertensive rat model and high fructose-drink rat model [59], sesamin treatment was found to improve endothelial cell dysfunction and decrease blood pressure [60] Meanwhile, sesamin was reported to be associated with regulating macrophages. Mouse macrophages stimulated by LPS shows lowering ubiquitination of HO-1 and less M1-macrophages after semamin treatment [61], which indicates its anti-inflamation effect.
Network pharmacology analysis in this article confirmed that kaempferol, quercetin, isorhamnetin, matrine and sesamin in Cuscutae Semen may be devoted to new treatment strategy to MN. Moreover, we found that quercetin and isorhamnetin is widely studied and has been clarified effective in the inflammatory response of kidney and immune system, and sesamin and matrine are rarely reported to treat kidney disease. They are potential therapeutic agents for nephropathy due to their anti-fibrosis, anti-inflammatory and anti-oxidative stress injury effects.
Acknowledgement
None.
Conflict of Interests
There is no conflict of interest in this article.
Funding
None.
References
- Bai X, Tang Y, Li Q, Liu D, Liu G, et al. (2021) An Integrated Analysis of Network Pharmacology, Molecular Docking, and Experiment Validation to Explore the New Candidate Active Component and Mechanism of Cuscutae Semen-Mori Fructus Coupled-Herbs in Treating Oligoasthenozoospermia. Drug Des Devel Ther 15: 2059-2089.
- Guan S, Zhu Y, Wang J, Dong L, Zhao Q, et al. (2020) A combination of Semen Cuscutae and Fructus Lycii improves testicular cell proliferation and inhibits their apoptosis in rats with spermatogenic dysfunction by regulating the SCF/c-kit--PI3K--Bcl-2 pathway. J Ethnopharmacol 251: 112525.
- Ding J, Tan X, Song K, Ma W, Xiao J, et al. (2018) Bushen Huoxue Recipe Alleviates Implantation Loss in Mice by Enhancing Estrogen-Progesterone Signals and Promoting Decidual Angiogenesis Through FGF2 During Early Pregnancy. Front Pharmacol 9: 437.
- Fan RH, Liu CG, Zhang Z, Xing MQ, Han YM, et al. (2022) Metabolomics analysis of Semen Cuscutae protection of kidney deficient model rats using ultra high-performance liquid chromatography-quadrupole time-of-flight Mass Spectrometry. J Pharm Biomed Anal 207: 114432.
- Gao F, Zhou C, Qiu W, Wu H, Li J, et al. (2018) Total flavonoids from Semen Cuscutae target MMP9 and promote invasion of EVT cells via Notch/AKT/MAPK signaling pathways. Sci Rep 8: 17342.
- Han S, Dai Y, Sun L, Xing Y, Ding Y, et al. (2023) Molecular mechanism of Cuscutae semen-radix rehmanniae praeparata in relieving reproductive injury of male rats induced with tripterygium wilfordii multiglycosides: A tandem mass tag-based proteomics analysis. Front Pharmacol 14: 1050907.
- Karna KK, Choi BR, You JH, Shin YS, Soni KK, et al. (2019) Cross-talk between ER stress and mitochondrial pathway mediated adriamycin-induced testicular toxicity and DA-9401 modulate adriamycin-induced apoptosis in Sprague-Dawley rats. Cancer Cell Int 19: 85.
- Ke J, Duan R (2013) Effects of flavonoids from semen cuscutae on the hippocampal-hypothalamic-pituitary-ovarian sex hormone receptors in female rats exposed to psychological stress. Clin Exp Obstet Gynecol 40: 271-274.
- Li L, Chen B, An T, Zhang H, Xia B, et al. (2021) BaZiBuShen alleviates altered testicular morphology and spermatogenesis and modulates Sirt6/P53 and Sirt6/NF-κB pathways in aging mice induced by D-galactose and NaNO(2). J Ethnopharmacol 271: 113810.
- Liu CX, Hu SQ, Liu DL, Xu YH, Hu K, et al. (2023) The effect of semen cuscutae flavonoid on Sertoli cells and blood-testis barrier in male infertility: integrating network pharmacology and experimental verification. Pharm Biol 61: 986-999.
- Hong BN, Shin SW, Nam YH, Shim JH, Kim NW, et al. (2023) Amelioration of Sensorineural Hearing Loss through Regulation of Trpv1, Cacna1h, and Ngf Gene Expression by a Combination of Cuscutae Semen and Rehmanniae Radix Preparata. Nutrients 15: 1773.
- Hou L, Yang L, Zhu C, Miao J, Zhou W, et al. (2023) Cuscutae semen alleviates CUS-induced depression-like behaviors in mice via the gut microbiota-neuroinflammation axis. Front Pharmacol 14: 1107781.
- Kim EY, Kim EK, Lee HS, Sohn Y, Soh Y, et al. (2007) Protective effects of Cuscutae semen against dimethylnitrosamine-induced acute liver injury in Sprague-Dawley rats. Biol Pharm Bull 30: 1427-1431.
- Li J, Niu J, Yang M, Ye P, Zhai J, et al. (2019) Using single-patient (n-of-1) trials to determine effectiveness of traditional Chinese medicine on chemotherapy-induced leukopenia in gastric cancer: a feasibility study. Ann Transl Med 7: 124.
- Liang HT, Xiao PT, Jiang ZM, Wang JW, Liu EH, et al. (2022) Spectrum-Effect Relationships Between High-Performance Liquid Chromatography Fingerprints and Hepatoprotective Activities of Cuscutae Semen. J AOAC Int 105: 1447-1459.
- Moszczuk B, Kiryluk K, Paczek L, Mucha K (2021) Membranous Nephropathy: From Research Bench to Personalized Care. J Clin Med 10: 1205.
- Miao H, Zhang Y, Yu X, Zou L, Zhao Y (2022) Membranous nephropathy: Systems biology-based novel mechanism and traditional Chinese medicine therapy. Front Pharmacol 13: 969930.
- Ji X, Cao J, Zhang L, Zhang Z, Shuai W, et al. (2020) Kaempferol Protects Renal Fibrosis through Activating the BMP-7-Smad1/5 Signaling Pathway. Biol Pharm Bull 43: 533-539.
- Guan Y, Quan D, Chen K, Kang L, Yang D, et al. (2023) Kaempferol inhibits renal fibrosis by suppression of the sonic hedgehog signaling pathway. Phytomedicine 108: 154246.
- Zhang Y, Qin X, Yang Y, Li J, Li X, et al. (2023) Ginkgo biloba extract attenuates cisplatin-induced renal interstitial fibrosis by inhibiting the activation of renal fibroblasts through down-regulating the HIF-1α/STAT3/IL-6 pathway in renal tubular epithelial cells. Phytomedicine 115: 154809.
- Shao YF, Tang BB, Ding YH, Fang CY, Hong L, et al. (2023) Kaempferide ameliorates cisplatin-induced nephrotoxicity via inhibiting oxidative stress and inducing autophagy. Acta Pharmacol Sin 44: 1442-1454.
- Yuan P, Sun X, Liu X, Hutterer G, Pummer K, et al. (2021) Kaempferol alleviates calcium oxalate crystal-induced renal injury and crystal deposition via regulation of the AR/NOX2 signaling pathway. Phytomedicine 86: 153555.
- Barakat H, Alkabeer IA, Althwab SA, Alfheeaid HA, Alhomaid RM, et al. (2023) Nephroprotective Effect of Fennel (Foeniculum vulgare) Seeds and Their Sprouts on CCl(4)-Induced Nephrotoxicity and Oxidative Stress in Rats. Antioxidants (Basel) 12: 325.
- Wang Z, Sun W, Sun X, Wang Y, Zhou M, et al. (2020) Kaempferol ameliorates Cisplatin induced nephrotoxicity by modulating oxidative stress, inflammation and apoptosis via ERK and NF-κB pathways. AMB Express 10: 58.
- Alshehri AS, El-Kott AF, El-Kenawy AE, Zaki MSA, Morsy K, et al. (2022) The ameliorative effect of kaempferol against CdCl(2)- mediated renal damage entails activation of Nrf2 and inhibition of NF-kB. Environ Sci Pollut Res Int 29: 57591-57602.
- Li Y, Zheng D, Shen D, Zhang X, Zhao X, et al. (2020) Protective Effects of Two Safflower Derived Compounds, Kaempferol and Hydroxysafflor Yellow A, on Hyperglycaemic Stress-Induced Podocyte Apoptosis via Modulating of Macrophage M1/M2 Polarization. J Immunol Res 2020: 2462039.
- Sharma D, Gondaliya P, Tiwari V, Kalia K (2019) Kaempferol attenuates diabetic nephropathy by inhibiting RhoA/Rho-kinase mediated inflammatory signalling. Biomed Pharmacother 109: 1610-1619.
- Sharma D, Kumar Tekade R, Kalia K (2020) Kaempferol in ameliorating diabetes-induced fibrosis and renal damage: An in vitro and in vivo study in diabetic nephropathy mice model. Phytomedicine 76: 153235.
- Shill MC, Bepari AK, Khan M, Tasneem Z, Ahmed T, et al. (2021) Therapeutic Potentials of Colocasia affinis Leaf Extract for the Alleviation of Streptozotocin-Induced Diabetes and Diabetic Complications: In vivo and in silico-Based Studies. J Inflamm Res 14: 443-459.
- Cechinel-Zanchett CC, Mariano LNB, Boeing T, Costa JC, Silva LM, et al., (2020) Diuretic and Renal Protective Effect of Kaempferol 3-O-Alpha-l-rhamnoside (Afzelin) in Normotensive and Hypertensive Rats. J Nat Prod 83: 1980-1989.
- Sheng H, Zhang D, Zhang J, Zhang Y, Lu Z, et al. (2022) Kaempferol attenuated diabetic nephropathy by reducing apoptosis and promoting autophagy through AMPK/mTOR pathways. Front Med (Lausanne) 9: 986825.
- Yang Y, Wei Q, An R, Zhang HM, Shen JY, et al. (2022) Anti-osteoporosis effect of Semen Cuscutae in ovariectomized mice through inhibition of bone resorption by osteoclasts. J Ethnopharmacol 285: 114834.
- González-Arceo M, Gomez-Lopez I, Carr-Ugarte H, Eseberri I, González M, et al. (2022) Anti-Obesity Effects of Isorhamnetin and Isorhamnetin Conjugates. Int J Mol Sci 24: 299.
- Kalai FZ, Boulaaba M, Ferdousi F, Isoda H (2022) Effects of Isorhamnetin on Diabetes and Its Associated Complications: A Review of In Vitro and In Vivo Studies and a Post Hoc Transcriptome Analysis of Involved Molecular Pathways. Int J Mol Sci 23: 704.
- Wendlocha D, Krzykawski K, Mielczarek-Palacz A, Kubina R, et al. (2023) Selected Flavonols in Breast and Gynecological Cancer: A Systematic Review. Nutrients 15: 2938.
- Gong G, Guan YY, Zhang ZL, Rahman K, Wang SJ, et al. (2020) Isorhamnetin: A review of pharmacological effects. Biomed Pharmacother 128: 110301.
- Hogan IA, Kuo YC, Abubakar AN, Lawal B, Agboola AR, et al. (2023) Attenuation of hyperglycemia-associated dyslipidemic, oxidative, cognitive, and inflammatory crises via modulation of neuronal ChEs/NF-κB/COX-2/NOx, and hepatorenal functional deficits by the Tridax procumbens extract. Biomed Pharmacother 158: 114114.
- Matboli M, Ibrahim D, Hasanin AH, Hassan MK, Habib EK, et al. (2021) Epigenetic modulation of autophagy genes linked to diabetic nephropathy by administration of isorhamnetin in Type 2 diabetes mellitus rats. Epigenomics 13: 187-202.
- Muñoz-Reyes D, Casanova AG, González-Paramás AM, Martín Á, Santos-Buelga C, et al. (2022) Protective Effect of Quercetin 3-O-Glucuronide against Cisplatin Cytotoxicity in Renal Tubular Cells. Molecules 27: 1319.
- Wu W, Wang W, Liang L, Chen J, Wei B, et al. (2023) Treatment with quercetin inhibits SARS-CoV-2 N protein-induced acute kidney injury by blocking Smad3-dependent G1 cell-cycle arrest. Mol Ther 31: 344-361.
- Ali HH, Ahmed ZA, Aziz TA (2022) Effect of Telmisartan and Quercetin in 5 Fluorouracil-Induced Renal Toxicity in Rats. J Inflamm Res 15: 6113-6124.
- Luo M, Liu Z, Hu Z, He Q (2022) Quercetin improves contrast-induced acute kidney injury through the HIF-1α/lncRNA NEAT1/HMGB1 pathway. Pharm Biol 60: 889-898.
- Muñoz-Reyes D, Morales AI, Prieto M (2021) Transit and Metabolic Pathways of Quercetin in Tubular Cells: Involvement of Its Antioxidant Properties in the Kidney. Antioxidants (Basel) 10: 909.
- Chellian J, Mak KK, Chellappan DK, Krishnappa P, Pichika MR (2022) Quercetin and metformin synergistically reverse endothelial dysfunction in the isolated aorta of streptozotocin-nicotinamide- induced diabetic rats. Sci Rep 12: 21393.
- Feng Q, Yang Y, Qiao Y, Zheng Y, Yu X, et al. (2023) Quercetin Ameliorates Diabetic Kidney Injury by Inhibiting Ferroptosis via Activating Nrf2/HO-1 Signaling Pathway. Am J Chin Med 51: 997-1018.
- Abdou HM, Abd Elkader HAE (2022) The potential therapeutic effects of Trifolium alexandrinum extract, hesperetin and quercetin against diabetic nephropathy via attenuation of oxidative stress, inflammation, GSK-3β and apoptosis in male rats. Chem Biol Interact 352: 109781.
- Tang L, Li K, Zhang Y, Li H, Li A, et al. (2020) Quercetin liposomes ameliorate streptozotocin-induced diabetic nephropathy in diabetic rats. Sci Rep 10: 2440.
- Lei D, Chengcheng L, Xuan Q, Yibing C, Lei W, et al. (2019) Quercetin inhibited mesangial cell proliferation of early diabetic nephropathy through the Hippo pathway. Pharmacol Res 146: 104320.
- Liu Y, Li Y, Xu L, Shi J, Yu X, et al. (2021) Quercetin Attenuates Podocyte Apoptosis of Diabetic Nephropathy Through Targeting EGFR Signaling. Front Pharmacol 12: 792777.
- Wan H, Wang Y, Pan Q, Chen X, Chen S, et al. (2022) Quercetin attenuates the proliferation, inflammation, and oxidative stress of high glucose-induced human mesangial cells by regulating the miR-485-5p/YAP1 pathway. Int J Immunopathol Pharmacol 36: 20587384211066440.
- Nutmakul T (2022) A review on benefits of quercetin in hyperuricemia and gouty arthritis. Saudi Pharm J 30: 918-926.
- Tang S, Wang C, Li YH, Niu TY, Zhang YH, et al. (2020) Structure-activity relationship and hypoglycemic activity of tricyclic matrines with advantage of treating diabetic nephropathy. Eur J Med Chem 201: 112315.
- Xu Y, Lin H, Zheng W, Ye X, Yu L, et al. (2016) Matrine ameliorates adriamycin-induced nephropathy in rats by enhancing renal function and modulating Th17/Treg balance. Eur J Pharmacol 791: 491-501.
- Yuan L, Yang J, Li Y, Yuan L, Liu F, et al. (2022) Matrine alleviates cisplatin-induced acute kidney injury by inhibiting mitochondrial dysfunction and inflammation via SIRT3/OPA1 pathway. J Cell Mol Med 26: 3702-3715.
- Zhang HW, Jin Y (2004) Renoprotective effects of matrine on experimental glomerulosclerosis in rats]. Zhonghua Er Ke Za Zhi 42: 737-740.
- Cao J, Chen J, Xie L, Wang J, Feng C, et al. (2015) Protective properties of sesamin against fluoride-induced oxidative stress and apoptosis in kidney of carp (Cyprinus carpio) via JNK signaling pathway. Aquat Toxicol 167: 180-190.
- Alshahrani S, Thubab HMA, Zaeri AMA, Anwer T, Ahmed RA, et al. (2022) The Protective Effects of Sesamin against Cyclophosphamide-Induced Nephrotoxicity through Modulation of Oxidative Stress, Inflammatory-Cytokines and Apoptosis in Rats. Int J Mol Sci 23: 11615.
- Zhang R, Yu Y, Deng J, Zhang C, Zhang J, et al. (2016) Sesamin Ameliorates High-Fat Diet-Induced Dyslipidemia and Kidney Injury by Reducing Oxidative Stress. Nutrients 8: 276.
- Wu XQ, Kong X, Zhou Y, Huang K, Yang JR, et al. (2012) Sesamin exerts renoprotective effects by enhancing NO bioactivity in renovascular hypertensive rats fed with high-fat-sucrose diet. Eur J Pharmacol 683: 231-237.
- Kong X, Yang JR, Guo LQ, Xiong Y, Wu XQ, et al. (2009) Sesamin improves endothelial dysfunction in renovascular hypertensive rats fed with a high-fat, high-sucrose diet. Eur J Pharmacol 620: 84-89.
- Fukunaga M, Ohnishi M, Shiratsuchi A, Kawakami T, Takahashi M, et al. (2014) Sesamin increases heme oxygenase-1 protein in RAW 264.7 macrophages through inhibiting its ubiquitination process. Eur J Pharmacol 741: 214-221.
Citation: Lai Y, Liang B, Li M, Lin Z, Duan T, et al. (2023) An Integrated Analysis of Network Pharmacology and Molecular Docking to Explore the Active Component and the Underlying Mechanism of Cuscutae Semen in Membranous Nephropathy. J Altern Complement Integr Med 9: 424.
Copyright: © 2023 Ying Lai, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

