
Anti-Inflammatory Effect of Nandina domestica Thunb Extracts Possessing Anti-Oxidant Property by Regulating MAPKs Signaling in LPS-Induced Inflammatory Cells
*Corresponding Author(s):
Hwa-Jung ChoiDepartment Of Beauty Art, 142 Bansong Beltway (Bansong-dong), Busan 48015, Youngsan University, Republic Of Korea
Tel:+82 515407354,
Fax:+82 515407332
Email:rerived@naver.com
Abstract
Background: The regulatory mechanism of Nandina domestica leaves on various inflammatory diseases is still not clear.
Methods: In this study, the anti-inflammatory activitives and the potential mechanisms of N. Domestica Leaves extracts (NDL) were investigated in Lipopolysaccharide (LPS)-induced RAW264.7 macrophage cells. Total Flavonoid Content (TFC) and Total Phenolic Content (TPC) was determined using the sodium borohyride/chloranil-based assay and Folin−Ciocalteu reagent. Anti-oxidant activity was evaluated by DPPH radical scavenging assay. The Nitric Oxide (NO) production and inflammatory cytokines were measured by Griess reagent and RT-PCR. The expression of MAPKs was investigated by western blotting.
Results: NDL showed DPPH radical scavenging activity with dose-dependent manner at concentration of 10 μg/mL, 30 μg/mL and 100 μg/mL, respectively, with TFC of 13.70 mg/g and TPC of 89.37 mg/g. Treatment with the NDL in appropriate concentrations could reduce the production of Nitric Oxide (NO) and suppressed the mRNA expressions of interleukin (IL)-6 and IL-1β. Furthermore, NDL inhibited the phosphorylated activation of Mitogen Activated Protein Kinase (MAPK) signaling pathways, including Extracellular signal-Regulated Kinase (ERK), p38 and c-Jun N-terminal Kinase (JNK).
Conclusion: These results suggest that NDL possessing anti-oxidant activity exerted anti-inflammatory effects by down-regulating the level of inflammatory mediators via mediation of MAPK phosphorylation in LPS-induced RAW264.7 macrophages.
Keywords
Anti-inflammatory; Inflammatory cytokines; Nandina domestica; Nitric Oxide (NO)
INTRODUCTION
Oxidative stress related to the unbalance of oxidation and antioxidation in the body of human under the harmful stimulating factors [1]. Various Reactive Oxygen Species (ROS) induce oxidative damage to cells, leading to an inflammation where the tissue is mostly resistant to invasion by many factors [2,3]. Finally, oxidative stress produced by environmental imbalance generates various damage of tissue and diseases, such as tumor and inflammation [4].
Inflammation is a host response to foreign stimulus or tissue damage, which leads to the recovery of structure and function in tissue [5]. During the response, the activation of immune cells conducted by pro-inflammatory cytokines up-regulates inflammation [6]. In the process, macrophages show an important role in the immune response [7]. The important inflammatory mediators such as Nitric Oxide (NO), interleukin (IL)-6 and IL-1β can be induced by activated macrophages [8]. LPS continually triggers signal transduction events which lead to the Mitogen-Activated Protein Kinase (MAPK) signaling pathway [9].
Prevention of the various inflammatory diseases could be inhibited via repression of the over-expressed mediators [10]. Common treatment used to cure inflammatory diseases is via Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), which has reported to show various adverse effects such as pain, injuries and complication [11-13]. Hence, search of novel anti-inflammatory candidate without side effects from natural sources is of interest.
Nandina domestica Thunb is a plant species that affilated with the family Berberidaceae [14]. N. domestica has been showed efficacy in the cure of dermatophytic infections [15]. In addition, N. domestica fruits are known to possess antioxidant and anti-inflammatory efficacy [16]. However, the regulatory mechanism of N. domestica leaves on various inflammatory diseases is still not clear. In the current study, extracts of of N. Domestica Leaves (NDL) were used to investigate whether they had anti-oxidant and anti-inflammatory effects. Besides, the mechanism of the anti-inflammatory effects of these extracts was explored through analyzing three major Mitogen-Activated Protein Kinases (MAPKs) signaling including Extracellular signal-Regulated Kinase (ERK), p38 and c-Jun N-terminal Kinase (JNK) in LPS-induced RAW 264.7 macrophages.
METHODS
Materials
Dulbecco’s Modified Eagle's Medium (DMEM), Fetal Bovine Serum (FBS), Penicillin-Streptomycin (PS), Phosphate Buffered Saline (PBS) and trypsin-EDTA were purchased from Gibco (BRL., Rockville, MD). Six-well plate, 96-well plate, thiazolyl blue Tetrazolium bromide (MTT), Dimethyl Sulfoxide (DMSO), Lipopolysaccharide (LPS), Nordihydroguaiaretic Acid (NDGA), ethanol, adenosine, monoclonal anti-β-actin antibody produced in mouse, agarose, ascorbic acid, 1, 1-Diphenyl-2-Picryl-Hydrazyl (DPPH) and Folin-Ciocaleu reagent were purchased from Sigma-Aldrich (St. Louis, MO, USA). Anti-rabbit lgG HRP-lonked antibody, p44/42 MAPK (Erk1/2) antibody, SAPK/JNK antibody, p38 MAPK antibody, Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) antibody, Phospho-SAPK/JNK (Thr183/Tyr185) antibody, and Phospho-p38 MAP Kinase (Thr180/Tyr182) antibody, Radio Immunoprecipitation Assay (RIPA) were purchased from Cell Signaling Technology, Inc. (Danvers, USA). Goat anti-Mouse lgG (H+L) secondary antibody were purchased from ThermoFisher SCIENTIFIC (Waltham, Co., USA). Bicinchoninic acid assay kit and SDS-PAGE gel were purchased from BioRad of USA. Enhanced chemiluminescence (Amersham Pharmacia Biotech, USA) and Griess (Promega, Madison, USA) were used in this study. RT PreMix kit and PCR PreMix kit were purchased from Bioneer of South Korea.
Extraction and preparation of Nandina domestica
Nandina domestica Thunb leaves was collected from garden at Kwangju Women’s University (Gwangju, South Korea) and dried and homogenized to obtain power. The powder (~300 g) was mixed with 3.0 L of 70% ethanol and standed at room temperature for 3 days. After filtration with filter paper (400 mesh), they were centrifuged at 1,000 rpm for 15 min and the supernatant was filtered with a whatman paper (No. 2).
Total Flavonoid Content (TFC) and Total Phenolic Content (TPC)
The TFC of each sample was determined using the sodium borohyride/chloranil-based assay [17]. TFC was expressed as milligrams of Catechin Hydrate Equivalents (CHE) per g of sample on a Dry Weight basis (mg CHE/g DW).
TPC was determined with Folin−Ciocalteu reagent according to the method of Singleton [18]. Gallic acid was used for calibration of a standard curve. TPC was determined as Gallic Acid Equivalents (GAE) and values were expressed as mg of acid/g of plant material (in GAE).
Anti-oxidant activity by DPPH radical scavenging assay
DPPH radical scavenging activity was determined using a previously reported method [19]. The concentration dependent scavenged DPPH radical was calculated from absorptions at steady state by the following equation: scavenged DPPH (%) = (1-At/Ac) ×100, where At is the absorbance of a sample at a given concentration after 30 min reaction time and Ac is the absorbance recorded 10 μL of DMSO. Ascorbic acid (AA) was used as a positive control because NDL is a natural extracts.
Cytotoxity
RAW 264.7 macrophages was obtained from the American Type Culture Collection (ATCC, Manassas, VA). RAW 264.7 macrophages were maintained in DMEM supplemented with 10% fetal bovine serum (FBS) and 0.01% antibiotic-antimycotic solution (Invitrogen, Grand Island, NY). The two cells were incubated at 37°C in a 5% CO2 atmosphere.
The cytotoxic effect of NDL were measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) tetrazolium reduction assay on RAW 264.7 macrophages [20]. Cells were plated in a 96 well plate at the density of 4×105 per well. After over night incubation, the cyctotoxic effects of NDL were evaluated with various concentrations (10 μg/mL, 30 μg/mL and 100 μg/mL) of NDL in absence or presence of LPS. After reaction and removing of MTT reagent, absorption values were read at 560 nm using a tecan microplate reader (Mannedorf, Switzerland). The measured absorbance was normalized to the absorbance of non-treated control cells.
Nitric Oxide (NO) production
Nitrite determination by Griess reaction has been widely utilized in indirect evaluation of NO production. Nitrite, a stable degradation product of NO, present in the conditioned media was determined by spectrophotometry as described previously method [21]. Raw 264.7 cells (4×105 cells/well) were cultured in a 96-well plate overnight. Cells were exposed with NDL of 10 μg/mL, 30 μg/mL and 100 μg/mL for 12 hr, and then treated with LPS of 1 μg/mL for 24 h. One hundred μL of the conditioned media was incubated with the same volume of Griess reagent [0.1% (w/v) N-(1-naphthyl)-ethylenediamine and 1% (w/v) sulfanilamide in 5% (v/v) phosphoric acid] for 10 min at room temperature. Absorbance at 546 nm was measured using a microplate reader and the nitrite concentration determined by using a standard curve of sodium nitrite made up in DMEM free of phenol red. NDGA is a natural lignan with recognized antioxidant and estimated that NDGA comprises approximately 5% to 10% of the dry weight of the leaves, and this corresponds to 80% of all the phenolic compounds in the resin [22,23]. Cell culture and animal model studies have demonstrated that NDGA has biological properties, including anticarcinogenic, antidiabetic, antiviral, antioxidant, and anti-inflammatory activities [24]. Therefore, NDGA is a positive control in this study.
RT-PCR analysis
Levels of cytokines (IL-6 and IL-1β) in intercellular were analyzed by RT-PCR. The RAW 264.7 cells were seeded in 6-well plates (4×105 cells/well), treated for 2 h in media containing NDL of 10 μg/mL, 30 μg/mL and 100 μg/mL, and were then incubated for 4 h with Lipopolysaccharide (LPS) of 1 μg/mL. After removing supernatant, the cells washed with 1 × PBS, and then harvested.
Total RNA was isolated using the TRIzol reagent kit (Invitrogen, Carlsbad, CA, USA). A total of 1 mg of RNA was used for the Reverse Transcription (RT) reaction, which also contained the AccuPower RT premix and random hexamers. Total cDNA corresponding to 1 mg of RNA was used in PCR reactions. The following PCR primers were showed in table 1. Amplification products were resolved by 1.5% agarose gel electrophoresis. Gels were stained with ethidium bromide and photographed under ultraviolet light. All the primers were purchased from Bioneer (Daejeon, South Korea).
|
Gene |
Sequence |
|
IL-6 (230 bp) |
Sense: 5’- AGC CAG AGT CCT TCA GAG AGA-3’ Anti-sense: 5’- TAA CGC ACT AGG TTT GCC GAG-3’ |
|
IL-1β (563 bp) |
Sense: 5’- ATG GCA ATG TTC CTG AAC TCA ACT-3’ Anti-sense: 5’- CAG GAC AGG TAT AGA TTC TTT CCT TT-3’ |
|
β-actin (349 bp) |
Sense: 5’- TGG AAT CCT GTG GCA TCC ATG AAA C-3’ Anti-sense: 5’- TAA AAC GCA GCT CAG TAA CAG TCC G-3’ |
Table 1: List of primer sequences used for RT-PCR.
Western blotting analysis
To observation of effect of NDL on expression of ERK, JNK and p38 MAP kinse in Raw 264.7 cells, western blot was conducted was performed as described previously [25]. Raw 264.7 cells (4×105 cells) were incubated in 6-well plates for 24 h. After washing 2 times with 1 × phosphate buffered salin (PBS, 1 mL), the cells were contained with 1 × PBS (2 mL) in serum-free media. After removing 1 × PBS, cells were incubated for 12 hr in 2% serum-media containing N. domestica extracts of 10 μg/mL, 30 μg/mL and 100 μg/mL, respectively, and LPS of 1 μg/mL for 1 h. NDGA was used as a positive control. Harvested cells were lysed by using Radio Immunoprecipitation Assay (RIPA) buffer mixed with protease and phosphatase inhibitor cocktail (Gendepot, USA). The concentrations of each protein sample were measured by Bicinchroninic Acid (BCA) protein assay kit (Thermo Fisher, USA). Proteins were separated using SDS-PAGE and transferred to Polyvinylidene Fluoride (PVDF) (Millipore, Billerica, MA) membranes. The PVDF membranes were incubated with a blocking buffer Tris-Buffered Saline (TBS)-T mixed with 5% skimmed milk for 1h at RT. The PVDF membranes were incubated with primary antibodies (1:1000) at 4°C overnight. The PVDF membranes were washed and incubated with secondary antibodies, conjugated with horseradish peroxidase (1:10000), for 2h at RT. Bands of proteins were detected using Enhanced Chemiluminescence (ECL) method and the bands of proteins were analyzed into levels of proteins by image J 1.47 software.
Statistical analysis
All measurements were made in triplicate and all values are represented as mean±SD. The results were subjected to an Analysis of Variance (ANOVA) using the Tukey test to analyze differences; p < 0.05 was considered significant.
RESULTS
TFC, TPC and DPPH radical scavenging activity of NDL
TFC and TPC of NDL are presented in table 2. The TFC of the NDL was 13.70 mg/g. The TPC of the NDL also showed 89.37 mg/g.
It was demonstrated that NDL has excellent DPPH radical scavenging activity with dose-dependant manner at concentration of 10 μg/mL, 30 μg/mL and 100 μg/mL, respectively. Also, Ascorbic Acid (AA) showed significantly DPPH radical scavenging activity with 86.8 % at concentration of 500 μM (Figure 1). The DPPH radical scavenging activity in NDL was lower when compared to the levels in AA (Figure 1).
|
Material |
TFC |
TPC |
|
(mg/g) |
(mg/g) |
|
|
Nandina domestica extracts |
13.70 ± 0.96 |
89.37 ± 1.24 |
Table 2: Total flavonoid content (TFC) and total phenolic content (TPC) of Nandina domestica extracts.
Results are presented as the mean IC50 values obtained from three independent experiments carried out in triplicate ± S.D.
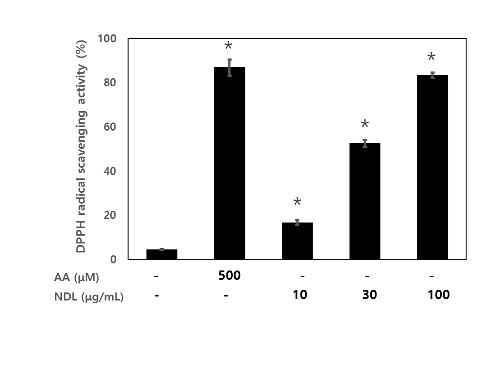 Figure 1: DPPH radical scavenging activity of NDL. NDL were treated with concentration of 10 μg/mL, 30 μg/mL and 100 μg/mL and AA treated with concentration of 500 μM. * p<0.05 as compared with the LPS group from three separate experiments. AA, ascorbic acid; NDL, extracts of of N. domestica leaves.
Figure 1: DPPH radical scavenging activity of NDL. NDL were treated with concentration of 10 μg/mL, 30 μg/mL and 100 μg/mL and AA treated with concentration of 500 μM. * p<0.05 as compared with the LPS group from three separate experiments. AA, ascorbic acid; NDL, extracts of of N. domestica leaves.
Effects of NDL on cell viability
The cytotoxicity of NDL in RAW 264.7 cells was measured by MTT assay (Figure 2). The results showed that NDL did not affect cell viability at a concentration of 100 μg/mL to 100 μg/mL regardless of the presence of LPS for 24 h. Therefore, a concentration of 10 to 100 μg/mL of NDL was used in all experiments.
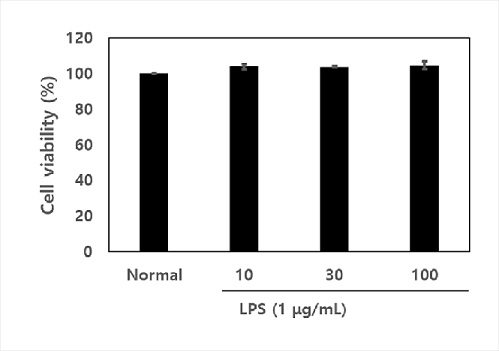 Figure 2: Effects of NDL on cell viability. The cytotoxic effect of NDL were measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) tetrazolium reduction assay on RAW 264.7 macrophages. NDL were treated with concentration of 10 μg/mL, 30 μg/mL and 100 μg/mL. The data are displayed with mean ± standard deviation (n=3). Normal is cell vaibility of RAW 264.7 macrophages without treatment of NDL in absence of LPS.
Figure 2: Effects of NDL on cell viability. The cytotoxic effect of NDL were measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) tetrazolium reduction assay on RAW 264.7 macrophages. NDL were treated with concentration of 10 μg/mL, 30 μg/mL and 100 μg/mL. The data are displayed with mean ± standard deviation (n=3). Normal is cell vaibility of RAW 264.7 macrophages without treatment of NDL in absence of LPS.
Effects of NDL on NO production in LPS-stimulated cells
The potential anti-inflammatory effects of NDL on LPS-stimulated NO production wasexamined in RAW 264.7 macrophages by pre-treating cells with various concentrations of NDL for 12 h before stimulation with 1 μg/mL LPS for 24 h. NO concentrations in the culture medium were measured by Griess reagent. As shown in figure 3, NO production was remarkably induced in LPS-stimulated RAW 264.7 macrophages, while pretreatment with NDL prevented this increase in a dose-dependent manner and pretreatment of NDL (100 μg/mL) significantly decreased LPS-induced NO proudction. This inhibitory effect was achieved with non-cytotoxic concentrations of NDL.
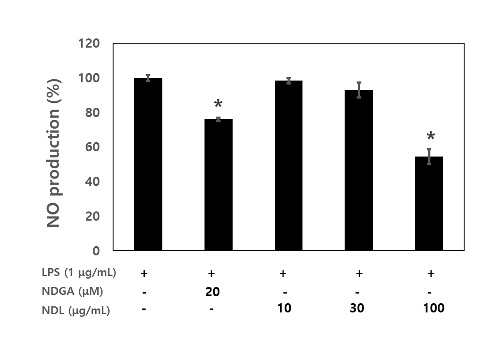 Figure 3: Effects of NDL on NO production in LPS-stimulated RAW 264.7 cells. Cells were co-incubated with the indicated concentrations of NDL (10 μg/mL, 30 μg/mL and 100 μg/mL) and LPS (1 μg/mL) for 24 h. The culture supernatants were subsequently isolated and analyzed for nitrite production. NDGA (20 μM) was used as positive control. * p < 0.05 as compared with the LPS group from three separate experiments.
Figure 3: Effects of NDL on NO production in LPS-stimulated RAW 264.7 cells. Cells were co-incubated with the indicated concentrations of NDL (10 μg/mL, 30 μg/mL and 100 μg/mL) and LPS (1 μg/mL) for 24 h. The culture supernatants were subsequently isolated and analyzed for nitrite production. NDGA (20 μM) was used as positive control. * p < 0.05 as compared with the LPS group from three separate experiments.
Effects of NDL on inflammatory cytokine (IL-6 and IL-1β) mRNA in LPS-stimulated cells
To study the effects of NDL on LPS-induced inflammatory cytokines production, such as IL-6 and IL-1β production in RAW 264.7 cells, cells were pretreated for 2 h with DNL (10 μg/mL, 30 μg/mL and 100 μg/mL), followed by treatment with LPS (1 μg/mL) for 4 h. The production of IL-6 and IL-1β induced by LPS was evaluated by RT-PCR.
LPS treatment was found to significantly increase IL-6 and IL-1β mRNA levels, as evidenced by RT-PCR analysis, whereas NDL blocked the expression of IL-6 and IL-1β mRNA (Figure 4A and 4B). Pretreatment NDL (30 μg/mL and 100 μg/mL) significantly decreased LPS-induced IL-6 and IL-1β mRNA showing a dose-dependent manner. Our result showed that DNL dose-dependently blocked the mRNA of the inflammatory cytokine IL-6 and IL-1β (Figure 4).
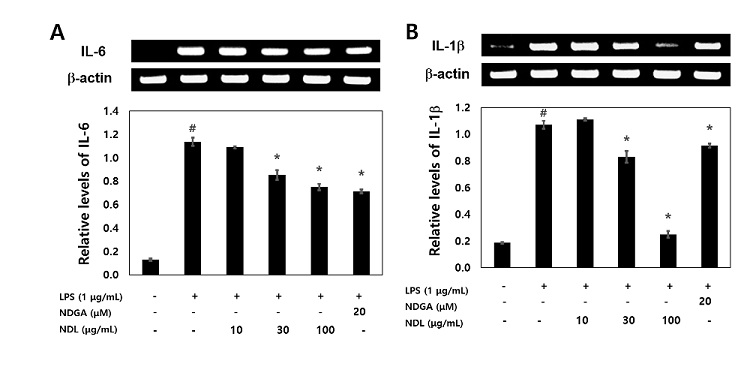 Figure 4: Effects of NDL on inflammatory cytokine (IL-6 and IL-1β) mRNA in LPS-stimulated cells. The RAW 264.7 cells treated for 2 h in media containing NDL of 10 μg/mL, 30 μg/mL and 100 μg/mL, and were then incubated for 4 h with LPS of 1 μg/ mL. Total RNA was isolated using the TRIzol reagent kit. Levels of cytokines (IL-6 and IL-1β) in intercellular were analyzed by RT-PCR. #Significantly different from normal (p<0.05). NDGA (20 μM) was used as positive control. *Significantly different from LPS (p<0.05). The data are displayed with mean ± standard deviation (n=3).
Figure 4: Effects of NDL on inflammatory cytokine (IL-6 and IL-1β) mRNA in LPS-stimulated cells. The RAW 264.7 cells treated for 2 h in media containing NDL of 10 μg/mL, 30 μg/mL and 100 μg/mL, and were then incubated for 4 h with LPS of 1 μg/ mL. Total RNA was isolated using the TRIzol reagent kit. Levels of cytokines (IL-6 and IL-1β) in intercellular were analyzed by RT-PCR. #Significantly different from normal (p<0.05). NDGA (20 μM) was used as positive control. *Significantly different from LPS (p<0.05). The data are displayed with mean ± standard deviation (n=3).
Effects of NDL on the phosphorylation of ERK, JNK and p38 in LPS-stimulated cells
Three MAPKs (ERK, JNK and p38) are known to be activated by LPS. Thus, we investigated the effect of DNL on the phosphorylation of ERK, JNK and p38. After cells were pretreated with the indicated concentrations of NDL for 12 h and stimulated with LPS (1 μg/mL) for 1 min, the expression of ERK, JNK and p38 was analyzed by Western blotting. As shown in figure 5, western blot analysis showed that LPS leads to phosphorylation of ERK, JNK and p38. NDL treatment obviously inhibited phosphorylation of ERK, JNK and p38.
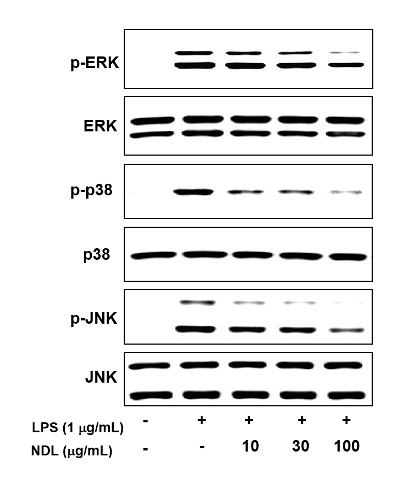 Figure 5: Effects of NDL on the phosphorylation of ERK, JNK and p38 in LPS-stimulated cells. The RAW 264.7 cells were pretreated with the indicated concentrations of NDL for 12 hr and stimulated with LPS (1 μg/mL) for 1 min, the expression of ERK, JNK and p38 was analyzed by Western blotting. NDGA (20 μM) was used as positive control.
Figure 5: Effects of NDL on the phosphorylation of ERK, JNK and p38 in LPS-stimulated cells. The RAW 264.7 cells were pretreated with the indicated concentrations of NDL for 12 hr and stimulated with LPS (1 μg/mL) for 1 min, the expression of ERK, JNK and p38 was analyzed by Western blotting. NDGA (20 μM) was used as positive control.
DISCUSSION
A large class of polyphenolic compounds is integral components abundant in our daily diets, such as: vegetables, fruits, and plant-derived beverages [26]. They are effective scavengers of ROS and their ROS scavenging activity in vitro is related to the number and position of the hydroxyl groups [27-29]. In a article previously published by Aktumsek and co-workers, the methanol extract of Centaurea pulcherrima var. pulcherrima showed the superior free radical scavenging activity, and reducing power [30]. A significant relationship between antioxidant capacity and total flavonoids and phenolic components was found. The results of the present study in part indicated the contribution of flavonoids or phenolics towards antioxidant capacity. In the our study, NDL showed DPPH radical scavenging activity with dose-dependant manner at concentration of 10 μg/mL, 30 μg/mL and 100 μg/mL, respectively, with TFC of 13.70 mg/g and TPC of 89.37 mg/g.
Macrophages are an important cells in the tissue immune system, and they are also the important cells to regulate the care of wounded tissue [31]. In wound care, macrophages secrete proinflammatory factors to operate inflammation [32]. Inflammatory cells produce ROS which take part in oxidative stress; the contrary, ROS can lead to generationg expression of inflammatory cytokines [3]. Therefore, oxidative stress and inflammation can extend each other's role and accelerate tissue damage. Thus, the function of oxidative stress in inflammatory damages has attracted increasing attention.
A large amount of NO produced by LPS is related to inflammatory reaction. Furthermore, NO can interact with other radicals to generate cytotoxic molecules. Hence, inhibition of NO production is an anti-inflammatory treatment. In this study, treatment with NDL after the LPS activation could significantly suppress the secretion of NO. Besides, ROS is an important molecular target of inflammatory disease. Excessive production of ROS in LPS-induced macrophages can cause proinflammatory response by upregulating proinflammatory cytokines and functioning as a secondary messenger in following progression [33]. In our study, LPS did increase the NO production in macrophages. Compared with only LPS treatment group, NDL markedly downregulated NO production showing DPPH radical scavenging activity.
Pro-inflammatory cytokines, such as TNF-α and IL-6, play crucial roles in the development of inflammatory diseases and are involved in the innate immunity and autoimmune diseases [34,35]. MAPKs, including ERK, JNK, and p38, also take part in the regulation of the expression of inflammation-related genes, leading to the overproduction of pro-inflammatory cytokines [36,37]. Western blot analysis showed that NDL inhibited LPS-induced phosphorylation of p38, ERK and JNK in RAW 264.7 cells. This data demonstrated that the anti-inflammatory mechanisms of DNL were likely via the inhibition of MAPK signaling pathways. Collectively, our results show that NDL decrease NO production in LPS-exposed RAW 264.7 cells showing anti-oxidant activity. The inhibitory activity of NDL occurred by the suppression of IL-6 and IL-1β expression via the reduction of MAPK phosphorylation. This anti-inflammatory effect was linked to MAPK signaling. NDL has an inhibitory effect on the activation of phosphate-p38, ERK and JNK. In addition, we confirmed that NDL suppresses pro-inflammatory cytokines, IL-6 and IL-1β. Furethemore, anti-inflammatory of NDL was correlated with anti-oxidant activity.
CONCLUSION
NDL showed DPPH radical scavenging activity and reduced the production of Nitric Oxide (NO) and suppressed the mRNA expressions of interleukin (IL)-6 and IL-1β. Furthermore, NDL inhibited the phosphorylated activation of Mitogen Activated Protein Kinase (MAPK) signaling pathways, including Extracellular signal-Regulated Kinase (ERK), p38 and c-Jun N-terminal Kinase (JNK). Based on the these results, the therapeutic effects of NDL against inflammatory diseases will need to be studied using animal models and isolated single molecule showing anti-inflammatory effect.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
AUTHOR CONTRIBUTIONS
Mi-Young Yun, the conception or design, or the acquisition, analysis, or interpretation of data for the work; Wang Zhe, drafting the article or revising it critically for important intellectual content; Sung-Min Park, agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; Hwa-Jung Choi, final approval of the version to be published.
REFERENCES
- Cho YH, Kim NH, Khan I, Yu JM, Jung HG, et al. (2016) Anti-inflammatory potential of quercetin-3-O-β-D-(“2”-galloyl)-glucopyranoside and quercetin isolated from Diospyros kakicalyx via suppression of MAP signaling molecules in LPS-induced RAW 264.7 macrophages. J Food Sci 81: 2447-2456.
- Lee YM, Song BC, Yeum KJ (2015) Impact of volatile anesthetics on oxidative stress and inflammation. Biomed Res Int 2015: 242709.
- Wang Y, Branicky R, Noë A, Hekimi S (2018) Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol 217: 1915-1928.
- Tang J, Diao P, Shu X, Li L, Xiong L (2019) Quercetin and quercitrin attenuates the inflammatory response and oxidative stress in LPS-induced RAW264.7 cells: In Vitro assessment and a theoretical model. Biomed Res Int 2019: 7039802.
- Lawrence T, Willoughby DA, Gilroy DW (2002) Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat Rev Immunol 2: 787-795.
- Tao JY, Zheng GH, Zhao L, Wu JG, Zhang XY, et al. (2009) Anti-inflammatory effects of ethyl acetate fraction from Melilotus suaveolens Ledeb on LPS-stimulated RAW 264.7 cells. J Ethnopharmacol 123: 97-105.
- Carralot JP, Kim TK, Lenseigne B, Boese AS, Sommer P, et al. (2009) Automated high-throughput siRNA transfection in raw 264.7 macrophages: a case study for optimization procedure. J Biomol Screen 14: 151-160.
- Nakagawa S, Arai Y, Kishida T, Hiraoka N, Tsuchida S, et al. (2012) Lansoprazole inhibits nitric oxide and prostaglandin E(2) production in murine macrophage RAW 264.7 cells. Inflammation 35: 1062-1068.
- Dong C, Davis RJ, Flavell RA (2002) MAP kinases in immune responses. Annu Rev Immunol 20: 55-72.
- Yoon SB, Lee YJ, Park SK, Kim HC, Bae H, et al. (2009) Anti-inflammatory effects of Scutellaria baicalensis water extract on LPS-activated RAW 264.7 macrophages. J Ethnopharmacol 125: 286-290.
- Bjarnason I, Hayllar J, MacPherson A, Russell AS (1993) Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans. Gastroenterology 104: 1832-1847.
- Chandra S, Chatterjee P, Dey P. Bhattacharya S (2012) Evaluation of anti-inflammatory effect of Ashwagandha: a preliminary study in vitro. Phcog J 4: 47-
- Singh G, Triadafilopoulos G (1999) Epidemiology of NSAID induced gastrointestinal complications. J Rheumatol 56: 18-24.
- Jo A, Yoo HJ, Lee M (2019) Robustaflavone isolated from Nandina domestica using bioactivity-guided fractionation downregulates inflammatory mediators. Molecules 24: 1789.
- Bajpai VK, Yoon JI, Kang SC (2009) Antifungal potential of essential oil and various organic extracts of Nandina domestica against skin infectious fungal pathogens. Appl Microbiol Biotechnol 83: 1127-1133.
- Ueki T, Akaishi T, Okumura H, Abe K (2012) Extract from Nandina domestica inhibits lipopolysaccharide-induced cyclooxygenase-2 expression in human pulmonary epithelial A549 cells. Biol Pharm Bull 35: 1041-1047.
- He XJ, Liu D, Liu RH (2008) Sodium borohydride/chloranil-based assay for quantifying total flavonoids. J Agri Food Chem 56: 9337-9344.
- Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol 299: 152-178.
- Yen GC, Chen HY (1995) Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agric Food Chem 43: 27-37.
- Mosmann T (1983) Rapid colorimetric assays for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55-63.
- Srisook K, Palachot M, Mongkol N, Srisook E, Sarapusit S (2011) Anti-inflammatory effect of ethyl acetate extract from Cissus quadrangularis Linn may be involved with induction of heme oxygenase-1 and suppression of NF-κB activation. J Ethnopharmacol 133: 1008-10
- Arteaga S, Andrade-Cetto A, Cárdenas R (2005) Larrea tridentata (Creosote bush), an abundant plant of Mexican and US-American deserts and its metabolite nordihydroguaiaretic acid. J Ethnopharmacol 98: 231-239.
- Heron S, Yarnell E (2001) The safety of low-dose Larrea tridentata (DC) coville (Creosote Bush or Chaparral): a retrospective clinical study. J Altern Complement Med 7: 175-185.
- Lü JM, Nurko J, Weakley SM, Jiang J, Kougias P, et al. (2010) Molecular mechanisms and clinical applications of nordihydroguaiaretic acid (NDGA) and its derivatives: an update. Med Sci Monit 16: 93-100.
- Baumann B, Weber CK, Troppmair J, Whiteside S, Israel A, et al. (2000) Raf induces NF-κB by membrane shuttle kinase MEKK1, a signaling pathway critical for transformation. Proc Natl Acad Sci USA 97: 4615-4620.
- Choi HJ, Song JH, Park KS, Kwon DH (2009) Inhibitory effects of quercetin 3-rhamnoside on influenza A virus replication. Eur J Pharm Sci 37: 329-3
- Terao J (2009) Dietary flavonoids as antioxidants. Forum Nutr 61: 87-
- Tournaire C, Croux S, Maurette MT, Beck I, Hocquaux M, et al. (1993) Antioxidant activity of flavonoids: efficiency of singlet oxygen quenching. J Photochem Photobiol 19: 205-2
- Wolfe KL, Liu RH (2008) Structure-activity relationships of flavonoids in the cellular antioxidant activity assay. J Agric Food Chem 56: 8404-84
- Aktumsek A, Zengin G, Guler GO, Cakmak YS, Duran A (2013) Assessment of the antioxidant potential and fatty acid composition of four Centaurea L. taxa from Turkey. Food Chem 141: 91-9
- Palmieri B, Vadalà M, Laurino C (2017) Review of the molecular mechanisms in wound healing: new therapeutic targets? J Wound Care 26: 765-775.
- Meglio PD, Perera GK, Nestle FO (2011) The multitasking organ: recent insights into skin immune function. Immunity 35: 857-869.
- Yu A, Wang J, Xue X, Wang Y (2010) Theoretical study of the peripheral disulfide bridge substituent effects on the antioxidant properties of naphthyridine diol derivatives. J Phys Chem A 114: 1008-1016.
- De Gonzalo-Calvo D, Neitzert K, Fernández M,Vega-Naredo I, Caballero B, et al. (2010) Differential inflammatory responses in aging and disease: TNF-α and IL-6 as possible biomarkers. Free Radic Biol Med 49: 733-737.
- Li A, Zhang XX, Shu M, Wu MJ, Wang J, et al. (2017) Arctigenin suppresses renal interstitial fibrosis in a rat model of obstructive nephropathy. Phytomedicine 30: 28-41.
- Arthur JSC, Ley SC (2013) Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol 13: 679-692.
- Qiu J, Chi G, Wu Q, Ren Y, Chen C, et al. (2016) Pretreatment with the compound asperuloside decreases acute lung injury via inhibiting MAPK and NF-κB signaling in a murine model. Int Immunopharmacol 31: 109-115.
Citation: Yun M-Y, Zhe W, Park S-M, Choi H-J (2020) Anti-Inflammatory Effect of Nandina domestica Thunb Extracts Possessing Anti-Oxidant Property by Regulating MAPKs Signaling in LPS-Induced Inflammatory Cells. J Altern Complement Integr Med 6: 114.
Copyright: © 2020 Mi-Young Yun, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

