
Anticarcinogenic Activity of Plant Bioactivity Compounds
*Corresponding Author(s):
Buyandelger NyamdavaaInner Mongolia University For The Nationalities, Mongolian Medicine College, Tongliao, China
Email:v_ik@yahoo.com
Abstract
Cancer is the first leading cause of mortality in the world. Patients who are diagnosed at earlier stage are good related with diagnosed at late stage, there are no choice of treatment (cancer patients who are at a late stage are unable to cope with chemical therapy) at the late stage. It has been proven plant bioactive compounds are able to prevent cancer, inhibit NF-kB, Nrf2, PI3K, STAT, Wnt, signaling pathways, angiogenesis and induce cancer cell death by several studies those are in vivo and in vitro. Therefore, we are going to study the possibilities of using plant bioactive compounds for cancer treatment.
Keywords
Angiogenesis; Cancer stem cell; Plant bioactive compounds; Signaling pathways
INTRODUCTION
In the earth, by 2018, approximately 18,1 million population are diagnosed with cancer and mortality rate is 9,6 million [1]. Successful cancer treatment has not been achieved yet although there has been progress. Furthermore, the standard treatment of cancer (chemotherapy, radiotherapy, immunotherapy) has high cost, treatment quite often causes suffering, and various side effects for patients [2]. Due to that, there is need to search a new approach which is less toxic, highly effective and has low cost. Plant bioactive compounds are able to act against bacteria and virus and inhibit cancer cell signaling pathways. Since thousands of years ago, plants have been used for prevention and treatment of cancer various diseases. The Red Emperor, or Shen Nung of the ancient Chinese emperor, compiled the first medicinal herbal literature, the Pentsao in 2,800 B.C. In United States, 50-60% of cancer patients get combination of standard cancer treatment and additional herbal medicine. These additional therapeutic products (EGCG, idroxycerol, resveratrol, sulforaphane etc) are the target therapy for colon cancer, breast cancer, lung cancer, liver cancer, NF-κB, Hedgehog, Wnt pathways and angiogenesis [3,4]. Due to anti-cancer plant bioactive compounds have higher probability to affect cancer stem cell, there might be a chance to make new cancer treatment approach to cancer stop or eliminate, which is the combination of plant bioactive compounds and chemotherapy. In the review, study on the effect of plant bio-activie compounds for anti-cancer service will be studied.
MATERIALS AND METHODS
We have performed our search by using key word “lung cancer, cancer stem cell, signaling pathway” from www.Pubmed.gov and other medical journals. Performed our study (Table 1) by translating materials that came out from key word search, and by using decomposition analysis, summary conclusion methods.
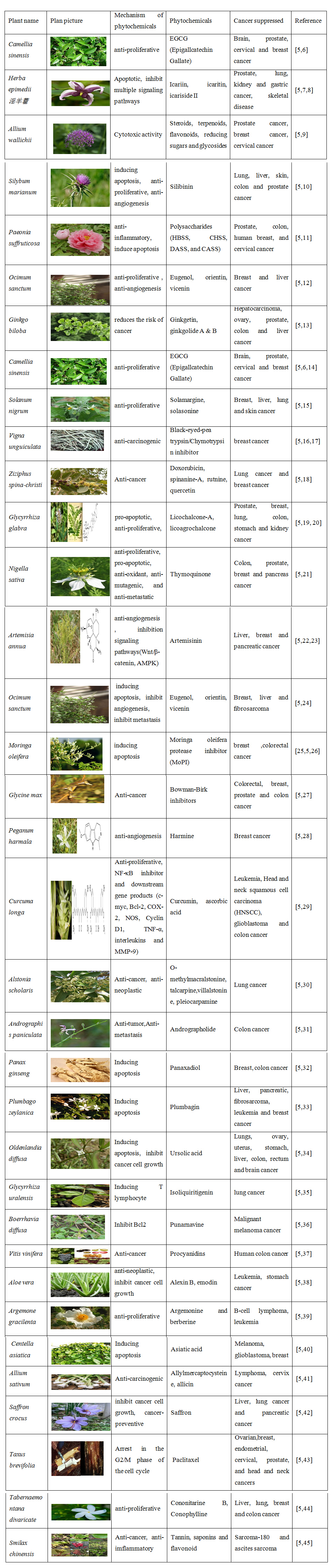 Table 1: Herbal bio-activity components of anti-cancer.
Table 1: Herbal bio-activity components of anti-cancer.
MECHANISM
Cell death
Apoptosis is a programmed cell death. During this highly regulated process unnecessary cells die off. Numerous conditions active the apoptotic pathway, including DNA damage and unregullated cellular division. Apoptotic process is activated by intracellular (intrinsic) and extracellular (extrinsic) signaling pathways. This is also known as mitochondrial pathway and death receptor pathway. Intrinsic pathway regulation includes DNA damage, growth factor deficiency, cytokine depletion, whereas extrinsic pathway regulation includes cytotoxic T cell damage of immune system response induced cell death stimulating signal. In order to proceed apoptosis through hundreds of proteins, the intrinsic and extrinsic signalling pathways use proteins called caspase. All these pathways unite in caspase protein [46].
Cancer cell death
Cancer cells go under unregullated cellular division and stimulate angiogenesis in order to evade from apoptosis. Apoptosis is the process that prevents from cancer and becoming the promising objective of target therapy against cancer. Loss of apoptotic control enables the longivity and mutation process of cancer cells. Moreover it also increases invasive feature of cancer cell and stimulate angiogenesis and regulate nutritional status of the cells. Cancer cells have many ways to evade the apoptosis. Including supressing caspase function and inactivating apoptotis stimaling factors [46].
Cell death is an essential for cancer treatment. The standard treatments for cancer which are being used in clinic (chemotherapy, radiotherapy, target therapy and immunotherapy) are directed to kill cancer cells [3,46]. Furthermore, plant bioactive compounds have effect against inflammation; virus and cancer induce cell death. In comparison with chemotherapy, natural phytochemical compounds are able to cell signaling pathways as target induce cell death factors such as transcriptional factor, growth factor, cancer cell survival factor, inflammation cytokines, protein kinases and angiogenesis. Due to simultaneous target signaling pathways , there is the ability to kill cancer cells selectively [47].
- • In the cell death cycle: <blebbing>, cell shrinkage and nuclear fragmentation occur [3]. Cell death is divided into inner and outer regulation.
- • Inner regulation: as DNA is injured, P53 protein activates, eliminate BAX protein, mitochondria makes a hole on its membrane, eliminate cytochrome C protein, activates apoptotic protease activating factor-1 (Apaf-1) and ATP, as a result, caspase 3 is activated by effect of caspase 9, breaks DNA and finally, cell death occur [46,3].
- • Outer regulation: Fas ligand of CTL cell (transmembrane protein of TNF) interacts with Fas molecule (Apo-1 or CD95) of target cell and activates caspase 8. As a result, caspase 8 activate caspase 3 and cell death occur [46,3].
Cell signaling pathway
There are 12 main pathways and in some type of cancer, these pathways have abnormal function [4]. Hedgehog signaling pathway leads to cell differentiation, growth and determines embryonic normal function [48]. On the other hand, due to an abnormal activation of the Hedgehog (Hh) pathway, cancer of brain, lung, prostate, leukemia, gastric and skin develop [3,49]. Moreover, transforming adult stem cells to cancer stem cell, as a result, cancer [3] and drug resistance can be developed. The Hedgehog signaling pathway is three ligand (Sonic hedgehog-Shh, Indian hedgehog-Ihh ,Desert hedgehog-Dhh), has two of receptors (PTCH1, PTCH2), key signaling converter smoothened (SMO) and 3 of transcription factors (Gli1, Gli2, Gli3) [49]. The sonic hedgehog (SHH) contains ~20 kDa N-terminal signaling domain (SHH-N) and ~25 kDa C-terminal signaling domain (role of its is unknown). As SHH is attached to Patched-1 (PTCH1) receptor, then Smoothened (inhibited by PTCH1) is activated and GLI transcriptional factor is activated. The activated GLI is accumulated in nucleus and hedgehog target gene transcription, as a result hedgehog signaling pathway is activated, Fas and apoptotic gene decrease and angiogenesis increase [3].
The Wnt signaling pathway does not involve in normal physical functions of adult human and animals and it only involves in embryogenesis and cancer development [3,50]. Wnt signaling pathway regulates embryonic cell migration, cell polarization, neuronal differentiation and organogenesis and affects renewing of stem cell. Due to loss of normal Wnt signaling pathway regulation, embryogenesis interrupt, cancer of breast, colon, gastric, leukemia, melanoma, dermal cancer and bone damage are develop [50,51]. The Wnt signaling pathway is divided into β-catenin (canonical) and non β-catenin (non-canonical) [50]
- • β-catenin (canonical): Wnt protein connect to Frizzle receptor of cell surface at that time Dishevelled (DSH) complex is activated, GSK is inhibited, β-catenin enters to cell nucleus freely which activates TCF, as a result, gene expression gets improved [50,51].
- • Non β-catenin (non-canonical): due to deficiency of Wnt protein , DSH complex is not activated, therefore, secondary protein complex which are GSK, axin and APC are activated and break β-catenin [3,50,51].
NF-κB signaling pathway NF-κB (Nuclear factor kappa-light-chain-enhancer of activated B cells) controls cell inflammation, change, proliferation, angiogenesis, metastasis, resistance of chemotherapy and radiotherapy. NF-κB is active in most of cancer cells and the cell activation induces cancer cell growth , inhibit cell apoptosis and induces angiogenesis [52,53]. TNFα induces cell death and inflammation mechanism. Although the process of inflammation is innate immunological protective mechanism, if it continues (chronic) for a long period of time, it leads to tissue damage, autoimmune disorder, excess in cell loading, collect inflammation factors is makes DNA damage, as a result various types of cancer develop and support the growth of cancer by changing the genetic sequense, damaged tissue epigenetic, its micro environmental [53]. TNFα is connected to cell surface receptor TNFR-1, as a result TRADD (TNF receptor type 1- associated death domain protein), ?RAF-2 (TNF receptor-associated factor 2), IAP (inhibitor of apoptosis protein), RIP/RIP K (receptor-interacting protein kinases) proteins are activated, as a result IKK (inhibitor of nuclear factor kappa-B kinase subunit alpha) complex is activated and due to that IkBa (inhibitor of kappa-B) deficiency occur. Due to the deficiency of IkBa, NF-κB enters to cell nucleus, pre inflammation gene, anti-apoptotic gene and cytokines are released.
ANGIOGENESIS
Angiogenesis is the new vascular formation physiological process and also is the cancer cell prognostic general marker that indicates cancer prognosis, invasion, metastasis, growth, nutritional status and oxygen supply. Thus inhibiting angiogenesis has a great importance. Angiogenesis inhibitors are classified into direct inhibitors and indirect inhibitors [54,3].
Direct inhibitors of angiogenesis
Angiogenesis direct endogen inhibitors include angiostatin, endostatin, arrestin, canstatin and tumstatin etc.., These are certain parts are from extracellular matrix protein proteolysing process. Endogens inhibitors prevents from endothelial cell division. Direct inhibition process against angiogenesis uses cerrtain intracellular signalling pathways that uses endothelial surface integrin proteins. These includes, PI3K, Akt, mammalian target of rapamycin (mTOR) etc.., [54].
Indirect inhibitors of angiogenesis
Angiogenesis indirect inhibitors are used in common chemo therapuetic substances, target therapy against oncogene, micro environment of cancer and other target therapuetic pathway. These inhibtors prevent from formation and expression of angiogenesis and inhibits the pro-angiogenesis protein function [54]. VEGF (vascular endothelial growth factor) induce angiogenesis of cancer. Due to VEGF is connected to VEGFR (VEGF receptor), MAP kinase signaling pathway is activated and MMP, uPA,uPAR, Plasminogen activator proteins are released. By influence of VEGF, signaling cascade in epithelial cell occur. VEGFR-2 (VEGF receptor-2) activates cascade of tyrosine kinase signal and makes chance to create maturely developed vessel, maintain transcription, growth and survival [3]. Using angiogensis inhibitors along with chemotherapy and targeted anti cancer therapy is showing a great promise. Depending on the cancer type, anti angiogenis drug, and chemothreapy, the angiogensis inhibitors can be used before chemotherapy or after chemothreapy. These combined therapy has showed some result in numerous precilincal and clinical study.
IMMUNE SYSTEM AGAINST CANCER
The main goal of immunotherapy is eliminate cancer and reduce cancer mass [2]. Currently, the immunotherapy is intended to inhibit cancer cell surface marker, induce non specific immune cells such as dendritic cell,? cell, NK cell, CTL, NKT cells, and cytokines IFNα, IL12, IFNγ, IL2, IL7 function. As a result, immune reaction rate increases. Although the standard treatment of cancer is able to destroy cancer cell, the mainstem cell of cancer is not destroyed. Therefore, post treatment resistance, recurrence and metastasis occur. Cancer stem cells are the small part of total cancer cells and it is potential to renew itself and grow unlimitedly in an accumulation of gene population. Furthermore, they lack of nutrients, oxygen content and they take nutrients from adjacent cells and they have high flexibility, and are potential to cope with strain [54-56]. One of the protecting and survival methods of cancer cell is cell surface marker (Table 2). Currently, It has not yet studied comprehensively that cancer cell surface markers and their activities.
|
Tumor types |
Surface marker on the CSCs |
References |
|
Breast |
CD44+/CD24-, CD133+, EpCAM+, CD29, |
[57,58,14] |
|
Colon |
CD133+, EpCAM+, CD44+, CD166+, CD24+ |
[57,58,59] |
|
Glioma (brain) |
CD133+, CD15+, CD49f+, CD90+ |
[57,59] |
|
Leukemia (AML) |
CD34+, CD38-, CD123, CD117, CD90, CD71 |
[57,59] |
|
Lung |
ABCG2, CD133+, CD147+, CD117+, ATP+, CD34+/CD44+, ALDH1+, OCT4+, CD24-, CD45-, CD166+CD44+, CD166+EPCAM+, CXCR4+ |
[60,14,59] |
|
Melanoma |
ABCB5, CD133+, CD20+, CD271+, CD166, Nestin+ |
[57,59] |
|
Ovarian |
CD44+, CD117+, CD133+, CD24+, ALDH1A1+ |
[57,59] |
|
Pancrease |
EPCAM+, CD44+, CD24+, CD133+, ESA |
[60,14,59] |
|
Cervical |
CD133+ |
[61] |
Table 2: Cancer stem cell surface markers in human cancer.
From surface markers above, the uppermost in the mind is CD133 and CD44. CD133 /Prominin 1/ is in group of Prominin and with 5 of transmembrane and 2 glycosylation capsule in outer environment. Excess growth of CD133 protein in cancer cell surface lead to drug resistance and ability of cancer development [62]. This protein is detected in numerous types of cancer, but currently, it is not studied comprehensively and having debate [62,60] CD133 is commonly detected in stem cell surface of neuronal, prostate, brain, colon, lung, liver and ovarian cancer [60]. CD44 is in group of non kinase and it is cell surface transmembrane glycoprotein. It is contained a bit or it does not contained in healthy individual. The excess detection of CD44 induce cancer stem cell growth and negatively affect the patient’s prognosis [63]. If CD44 is detected in cancer stem cell surface commonly, which reduces glutathione synthesis, increase an active oxygen capability, as a result various types of treatment resistance occur. The main role of CD44 is pump to put cystine into chancer stem cell. Therefore, due to inhibition of glutamatcystine transportation, CD44 dependent cancer growth is decreased and sensitiveness for treatment increases [59]. CD44 has an essential role to start MAPK, Hippo, β-catenin, AKT, TGF-β, Emmprin, MMPs and STAT3 signaling pathways [63].
It is proved that plant bioactive compounds have less toxicity, high effect against cancer by several studies. For instance, paclitaxel which is extracted from barks of yews in Pacific ocean [64] in the experimental model MDA-MB-231 xenograft, it inhibits CD133 and has effectiveness to inhibit cancer growth. But after stopping treatment, cancer develops faster [55]. In APCMin+/− mouse model, dasatinib and curcumin reduced cancer stem cell surface markers (LDH, CD44, CD133, CD166 ) by 80-90 % [65]. Although ?urcumin was proved having able to kill [66] cancer stem cell by clinical trial, it has low bioavailability. Therefore, it was hard to use in treatment [67,68].
RESULT AND DISCUSSION
Long ago, it was known that cancer cells continuously proliferate, and metastasize however recently researchers have proven that cancer cells have stem cell population responsible for drug resistance and metastasis. In worldwide, currently we are using gene therapy, anti angiogenic therapy for patients with cancer. These have multiple effects such as maintaining remission without relapse after standardized treatment of cancer, reducing tumor size etc. However, expense of chemotherapy is high (unable to be used in poor countries), high toxicity (damages other healthy organs) and does not have effect on cancer stem cells. Plant based bioactive compounds decrease risk of cancer development risk, and increase drug effects through synergetic manner, suppress progress of cancer, and able to be used with solely or with other drugs and thus is possible low cost variation. To eradicate cancer completely, it is necessary to eradicate cancer stem cells, and by eradicating cancer stem cells there is possibility to stop progression of cancer, and maintain remission without relapse. In recent years research on cancer stem cell have been performed intensively, and by 2015 there are up to 5000 research studies have been publish on this topic. From several pathways to treat cancer stem cells, most effective methods are signal pathway and suppression of surface markers.
Through many studies, CD44 a cancer stem cell surface marker has been proven that it is possible target treatment. Current treatment strategy is directed to reducing CD44 detection. Thus current treatment strategy is directed to reducing CD44 detection load. Furthermore, signal transduction pathways of cancer stem cells play major role for cancer cells and microscopic environment of tumor, therefore this topic have been studied for last 10 years. Through many epidemiology and in vitro studies, it was proved that green tea has effect on cancer cells and support tumor radiation treatment. Currently international research centers are testing tumor effecting ability of plants based bio-active compound are often used in in vitro, in vivo cancer prevention, treatment studies. Some of these clinical trials have been successive. Plant based bio-active compound research are interesting and thus we should understand their potential, pharmacokinetic indicators, pharmacokinetic interaction, metabolism, toxicity, drug interaction and polymorphism. Thus to study importance of plant based bio-active compounds, there are several issues we are confronting. These include: It is necessary to develop model for cancer stem cells and cell, and test plant based bioactive compound mechanism and dose. Through these, we can clarify important mechanism of treatment, and has responsibility to develop better treatment methods. Signal transduction pathways of cancer cells play important role of biologic activity, thus to develop treatment we should thoroughly assess. Recently, clinical trials of the signal transduction pathway during pancreas, colon, and ovary cancer have been unsuccessful. Furthermore, information of detailed biochemical understanding of signal transduction is insufficient. Inhibition of signal transduction pathways has poor results if used solely, and interaction suitfulness of chemotherapy drug or other treatment targets should be determined. In recent years, possible plant based bioactive compound that can suppress self-renewal, survival of cancer stem cells are being performed. Starting from now, we face need for new studies on complex effect of CSC and performing testing of bioactive compounds for treating for metastatic cancer. We assume that in future through clinical trial studies, an additional treatment strategy of using signal transduction inhibitors will be developed.
CONCLUSION
Signal transduction pathways are associated to multiple types of cancer, thus it shows it signal transduction has high importance. Even though there is advancement in studies on signal transduction, we should throughly study signaling pathway mechanisms.From the information above, It can be concluded that the use of plant bioavailability in cancer treatment can be effective in patients who unable to cope with chemotherapy in the last stage of cancer, there is lack of clinical trial. Therefore, it is necessary to carry out further research in this field.
CONFLICTS OF INTEREST
There are no conflicts of interest.
REFERENCES
- IARC Press Release 263. latest global cancer data: cuncer burden rises to 18.1 milion new cases 9.6 milion cancer deaths in 2018. International agency for research on cancer.
- Dashtsoodol N, Shigeura T, Tashiro T, Aihara M, Chikanishi T, et al. (2017) Natural Killer T Cell-Targeted Immunotherapy Mediating Long-term Memory Responses and Strong Antitumor Activity. Immunol 25: 8-1206.
- Wang H, Khor TO, Shu L, Su ZY, Fuentes F, et al. (2012) Plants vs. cancer: a review on natural phytochemicals in preventing and treating cancers and their druggability.Anticancer Agents Med Chem 12: 1281-1305.
- Gullett NP, Ruhul Amin AR, Bayraktar S, Pezzuto JM, Shin DM,et al. (2010) Cancer prevention with natural compounds. Semin Oncol 37: 258-281.
- Iqbal J, Abbasi BA, Mahmood T, Kanwal S, Ali B,et al. (2017) AT Plant-derived anticancer agents: A green anticancer approach. Asian Pacific Journal of Tropical Biomedicine 7: 1129-1150.
- Rafieian-Kopaei M, Movahedi M (2017) Breast cancer chemopreventive and chemotherapeutic effects of Camellia Sinensis (green tea): an updated review. Electron Physician 9: 3838-3844.
- Wang L, Li Y, Guo Y, Ma R, Fu M, et al. (2016) Herba Epimedii: An Ancient Chinese Herbal Medicine in the Prevention and Treatment of Osteoporosis. Curr Pharm Des 22: 328-349.
- Chen M, Wu J, Luo Q, Mo S, Lyu Y, et al. (2016) The Anticancer Properties of Herba Epimedii and Its Main Bioactive Componentsicariin and Icariside II. Nutrients 8: 563-573.
- Bhandari J1, Muhammad B2, Thapa P1, Shrestha BG3 (2017) Study of phytochemical, anti-microbial, anti-oxidant, and anti-cancer properties of Allium wallichii. BMC Complement Altern Med 17: 1-10.
- Ramasamy K1, Agarwal R (2008) Multitargeted therapy of cancer by silymarin. Cancer Lett 269: 352-362.
- Kim D, Radin D, Leonardi D (2017) Probing the Molecular Mechanisms Governing the Oncolytic Activity of Paeonia suffruticosa on Triple-negative Breast Cancer Cells In Vitro. Anticancer Res 37: 4813-4819.
- Pratima NM, Larry T, Victor H, Fred Miller, Avraham Raz (2006) Inhibition of breast cancer progression by a medicinal herb Ocimum sanctum. Cancer Res 66: 513-520.
- Biggs ML, Sorkin BC, Nahin RL, Kuller LH, Fitzpatrick AL (2010) Ginkgo biloba and risk of cancer: secondary analysis of the Ginkgo Evaluation of Memory (GEM) Study. Pharmacoepidemiol Drug Saf 19: 694-698.
- Boehm K, Borrelli F, Ernst E, Habacher G, Hung SK, et al. (2009) Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database Syst Rev 8:CD005004.
- Lai YJ, Tai CJ, Wang CW, Choong CY, Lee BH, et al. (2016) Anti-Cancer Activity of Solanum nigrum (AESN) through Suppression of Mitochondrial Function and Epithelial-Mesenchymal Transition (EMT) in Breast Cancer Cells. Molecules 21: 553-564.
- Joanitti GA, Azevedo RB, Freitas SM (2010) Apoptosis and lysosome membrane permeabilization induction on breast cancer cells by an anticarcinogenic Bowman-Birk protease inhibitor from Vigna unguiculata Cancer Lett 293: 73-81.
- Mehdad A, Brumana G, Souza AA, Barbosa JARG, Ventura MM, et al. (2016) A Bowman-Birk inhibitor induces apoptosis in human breast adenocarcinoma through mitochondrial impairment and oxidative damage following proteasome 20S inhibition. Cell Death Discovery 2: 15067.
- Jafarian A, Zolfaghari B, Shirani K (2014) Cytotoxicity of different extracts of arial parts of Ziziphus spina-christi on Hela and MDA-MB-468 tumor cells. Adv Biomed Res 24: 38.
- 1Shiva AN, Alireza N, Reza B, Fatemeh K(2018) The anticancer Effect of Arctium lappa and Glycyrrhiza glabra on HT-29 Colon Cancer and MCF-7 Breast Cancer Cell Lines. Crescent Journal of Medical and Biological Sciences 5: 133-137.
- Nourazarian SM, Nourazarian A, Majidinia M, Roshaniasl E (2015) Effect of Root Extracts of Medicinal Herb Glycyrrhiza glabra on HSP90 Gene Expression and Apoptosis in the HT-29 Colon Cancer Cell Line. Asian Pac J Cancer Prev 16: 8563-6.
- Majdalawieh AF, Fayyad MW (2016) Recent advances on the anti-cancer properties of Nigella sativa, a widely used food additive. J Ayurveda Integr Med 7: 173-180.
- Konstat-Korzenny E, Ascencio-Aragón JA, Niezen-Lugo S, Vázquez-López R (2018) Artemisinin and Its Synthetic Derivatives as a Possible Therapy for Cancer. Med Sci (Basel) 6: 19.
- Efferth T (2017) From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin Cancer Biol 46: 65-83.
- Baliga MS, Jimmy R, Thilakchand KR, Sunitha V, Bhat NR, et al. (2013) Ocimum sanctum L (Holy Basil or Tulsi) and its phytochemicals in the prevention and treatment of cancer. Nutr Cancer 65 Suppl 1: 26-35.
- Al-Asmari AK, Albalawi SM, Athar MT, Khan AQ, Al-Shahrani H, et al. (2015) Moringa oleifera as an Anti-Cancer Agent against Breast and Colorectal Cancer Cell Lines. PLoS One 10: e0135814.
- Abd-Rabou AA, Abdalla AM, Ali NA, Zoheir KM (2017) Moringa oleifera Root Induces Cancer Apoptosis more Effectively than Leave Nanocomposites and Its Free Counterpart. Asian Pac J Cancer Prev 18: 2141-2149.
- Wang Y, Liu L, Ji F, Jiang J, Yu Y, et al. (2018) Soybean (Glycine max) prevents the progression of breast cancer cells by downregulating the level of histone demethylase JMJD5. J Cancer Res Ther 14(Supplement): S609-S615.
- Moloudizargari M, Mikaili P, Aghajanshakeri S, Asghari MH, Shayegh J (2013) Pharmacological and therapeutic effects of Peganum harmala and its main alkaloids. Pharmacogn Rev 7: 199-212.
- Wilken R, Veena MS, Wang MB, Srivatsan ES (2011) Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer 10: 12.
- Ahmad MS, Ahmad S, Ali A, Afzal M (2016) Anticarcinogenic and antimutagenic activity of Alstonia scholaris on the albino mice bone marrow cells and peripheral human lymphocyte culture against methyl methane sulfonate induced genotoxicity. Adv Biomed Res 5: 92.
- Li L, Yue GG, Lee JK, Wong EC, Fung KP, et al. (2017) The adjuvant value of Andrographis paniculata in metastatic esophageal cancer treatment - from preclinical perspectives. Sci Rep 7: 854.
- Kim SJ, Kim AK (2015) Anti-breast cancer activity of fine black ginseng (Panax ginseng Meyer) and ginsenoside Rg5. Journal of Ginseng Research 39: 125-134.
- Chen CA, Chang HH, Kao CY, Tsai TH, Chen YJ (2009) Plumbagin, isolated from Plumbago zeylanica, induces cell death through apoptosis in human pancreatic cancer cells. Pancreatology 9: 797-809.
- Gupta S, Zhang D, Yi J, Shao J (2004) Anticancer activities of Oldenlandia diffusa. J Herb Pharmacother 4: 21-33.
- Ayeka PA, Bian Y, Mwitari PG, Chu X, Zhang Y, et al. (2016) Immunomodulatory and anticancer potential of Gan cao (Glycyrrhiza uralensis Fisch.) polysaccharides by CT-26 colon carcinoma cell growth inhibition and cytokine IL-7 upregulation in vitro. BMC Complementary and Alternative Medicine 16: 206.
- Priya Antony P, Nazeem PA (2014) Boerhaavia Diffusa In Cancer Therapy-An Insilico Analysis. International Journal of Pharma Sciences and Research 5: 391-396.
- Zhou K, Raffoul JJ (2012) Potential anticancer properties of grape antioxidants. J Oncol 2012: 803294.
- Hussain A, Sharma C, Khan S, Shah K, Haque S (2015) Aloe vera inhibits proliferation of human breast and cervical cancer cells and acts synergistically with cisplatin. Asian Pac J Cancer Prev 16: 2939-2946.
- Leyva-Peralta MA, Robles-Zepeda RE, Garibay-Escobar A, Ruiz-Bustos E, Alvarez-Berber LP, et al. (2015) In vitro anti-proliferative activity of Argemone gracilenta and identification of some active components. BMC Complement Altern Med 15: 13.
- Babykutty S, Padikkala J, Sathiadevan PP, Vijayakurup V, Azis TK, et al. (2008) Apoptosis induction of Centella asiatica on human breast cancer cells. Afr J Tradit Complement Altern Med 6: 9-16.
- Thejasenuo Julia Kirha, Tsipila Thonger, Sanjay Kumar. A Review on the Benefits of Allium sativum on Cancer Prevention. Journal of Cancer Treatment and Research 4: 34-37.
- Milajerdi A, Djafarian K, Hosseini B (2016) The toxicity of saffron (Crocus sativus L.) and its constituents against normal and cancer cells. Journal of Nutrition & Intermediary Metabolism 3: 23-32
- Barbuti AM, Chen ZS (2015) Paclitaxel Through the Ages of Anticancer Therapy: Exploring Its Role in Chemoresistance and Radiation Therapy. Cancers (Basel) 7: 2360-2371.
- Kumar A, Selvakumar S (2015) Antiproliferative efficacy of Tabernaemontana divaricata against HEP2 cell line and Vero cell line. Pharmacogn Mag 11(Suppl 1): S46-52.
- Nho KJ, Chun JM, Kim HK (2015) Anti-metastatic effect of Smilax china L. extract on MDA-MB-231 cells. Mol Med Rep 11: 499-502.
- Pfeffer CM, Singh ATK (2018) Apoptosis: A Target for Anticancer Therapy. Internatuinal Journal of Molecular Sciences. Int J Mol Sci 19: 448.
- Fulda S, Debatin K-M (2006) Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 25: 4798-4811.
- Millimouno FM, Dong J, Yang L, Li J, Li X (2014) Targeting apoptosis pathways in cancer and perspectives with natural compounds from mother nature. Cancer Prev Res (Phila) 7: 1081-1107.
- Chen JK, Taipale J, Young KE, Maiti T, Beachy PA (2002) Small molecule modulation of Smoothened activity. Proc Natl Acad Sci USA 99: 14071-14076.
- Jia Y, Wang Y, Xie J (2015) The Hedgehog pathway: role in cell differentiation, polarity and proliferation. Arch Toxicol 89: 179-191.
- Zhan T, Rindtorff N, Boutros M (2017) Wnt signaling in cancer. Oncogene 36: 1461-1473.
- Komiya Y, Habas R (2008) Wnt signal transduction pathways. Organogenesis 4: 68-75.
- Chaturvedi MM, Sung B, Yadav VR, Kannappan R, Aggarwal BB (2011) NF-κB addiction and its role in cancer: 'one size does not fit all'. Oncogene 30: 1615-1630.
- Asmaa E EI-Kenawi, Azza B EI-Remessy (2013) Angiogenesis inhibitors in cancer therapy: mechanistic perspective on classification and treatment rationales. British Journal of Pharmacology 170: 712-729.
- Xia Y, Shen S, Verma IM (2014) NF-κB, an active player in human cancers. Cancer Immunol Res 2: 823-830.
- Li L, Neaves WB (2006) Normal stem cells and cancer stem cells: the niche matters. Cancer Res 66: 4553-4557.
- Aponte PM, Caicedo A (2017) Stemness in Cancer: Stem Cells, Cancer Stem Cells, and Their Microenvironment. Stem Cells Int 2017: 5619472.
- Shackleton M (2010) Normal stem cells and cancer stem cells: similar and different. Semin Cancer Biol 20: 85-92.
- Kim YJ, Siegler EL, Siriwon N, Wang P (2016) Therapeutic strategies for targeting cancer stem cells. Journal of Cancer Metastasis and Treatment 2: 233-242.
- Halim NHA, Zakaria N, Satar NA, Yahaya BH (2016) Isolation and Characterization of Cancer Stem Cells of the Non-Small-Cell Lung Cancer (A549) Cell Line. Methods Mol Biol 1516: 371-388.
- Santamaria S, Delgado M, Kremer L, Garcia-Sanz JA (2017) Will a mAb-Based Immunotherapy Directed against Cancer Stem Cells Be Feasible? Front. Immunol 8: 1509.
- Glumac PM, LeBeau AM (2018) The role of CD133 in cancer: a concise review. Clin Transl Med 7: 18.
- Javed S, Sharma BK, Sood S, Sharma S, Bagga R, et al. (2018) Significance of CD133 positive cells in four novel HPV-16 positive cervical cancer-derived cell lines and biopsies of invasive cervical cancer. BMC Cancer 18: 357.
- Swaminathan SK, Roger E, Toti U, Niu L, Ohlfest JR, et al. (2013) D133-targeted paclitaxel delivery inhibits local tumor recurrence in a mouse model of breast cancer. J Control Release 171: 280-287.
- Chen C, Zhao S, Karnad A, Freeman JW (2018) The biology and role of CD44 in cancer progression: therapeutic implications. Journal of Hematology & Oncology 11: 64.
- Weaver BA (2014) How Taxol/paclitaxel kills cancer cells. Mol Biol Cell 25: 2677-2681.
- Dandawate P, Padhye S, Ahmad A, Sarkar FH (2013) Novel strategies targeting cancer stem cells through phytochemicals and their analogs. Drug Deliv Transl Res 3: 165-82.
- Oh J, Hlatky L, Jeong YS, Kim D (2016) Therapeutic Effectiveness of Anticancer Phytochemicals on Cancer Stem Cells. Toxins (Basel) 8: 199.
Citation: Nyamdavaa B, Burenbatu B, Wuritunashun W (2020) Regeneration Abilities of Vertebrates and Invertebrates and Relationship with Pharmacological Research: Hypothesis of Genetic Evolution Work and Micro-environment Inhibition Role. J Cell Biol Cell Metab 7: 024
Copyright: © 2020 Buyandelger Nyamdavaa, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

