
Antioxidant Activities of Aqueous Bitter Kola (Garcinia Kola) Seed Extract on Wistar Rats
*Corresponding Author(s):
Odigie Mike OsagieDepartment Of Public And Community Health, College Of Health Sciences, Novena University, Delta State, Nigeria
Tel:+234 7036844454,
Email:osgiedeprof@yahoo.com
Abstract
Notwithstanding the advances in modern medicine, the multiple effects and medical health implications of Bitter kola (Garcinia kola) intake have made medical plants an alternative with some hope in health care delivery. Current study investigated the durational and dose related effects of aqueous extract of bitter kola (Garcinia kola) on body weights and serum antioxidant activities of Wistar rats. Thirty adult wistar rats of between 150 - 200 kg body weights were procured, acclimatized and randomly selected into five groups of six rats each. Group A (Control) received standard rat diet and water ad libitum, while group B rats were fed with 100 mg/kg body weight of aqueous extract of Garcinia kola. Groups C, D and E received 200 mg/kg, 300 mg/kg and 400 mg/kg body weight of aqueous extracts of Garcinia kola respectively. After twenty one days of administration of test substance, the rats were sacrificed via cervical dislocation and blood samples collected and assayed for selected antioxidant marker enzyme activities [Superoxide Dismutase SOD, Catalase, CAT and Malonyldialdehyde, MDH]. Study also weighed the animals before and after administration of test substance, to ascertain the comparative changes in body weights across groups. Using the Statistical Package for Social Sciences (SPSS, version 20), study carefully conducted statistical test (student t-test) on obtained data; while setting p-values ≤ 0.05 as statistically significant. Upon careful observation of results, the study observed a marked increase in serum antioxidant enzyme activities with notable increase in body weights over a period of time. This proved the effect of Garcinia kola on body weight to be duration and dose dependent. For improved serum antioxidant activity, administration of aqueous extract of Garcinia kola is therefore recommended. Further studies aimed at corroborating these results should be carried out.
Keywords
Antioxidant; Body weight; Garcinia kola
INTRODUCTION
Garcinia kola (Heckel Gultiferae) is a largely cultivated forest tree indigenous to sub-Saharan Africa. It has been referred to as a ‘wonder plant’ because almost every part of it has been found to be of medicinal importance [1]. The seed (commonly known as bitter kola, male kola or false kola) is a masticatory used in traditional hospitality, cultural and social ceremonies [1,2].
In Africa, Garcinia kola seed is commonly recommended in folklore medicine for the treatment of diabetes and its associated complications [3]. Garcinia kola seed administration significantly ameliorate hyperglycemia mediated damage by decreasing the blood glucose level, enhancement of the antioxidant system, inhibition of lipid peroxidation, and improving the architecture of the kidney, liver and testes in STZ-induced diabetic rats [3]. In addition, G. kola seed intervention has been found to restore the kidney and liver function biomarkers, the sperm characteristics as well as the plasma levels of Luteinizing Hormone (LH), Follicle-Stimulating Hormone (FSH), testosterone, Triiodothyronine (T3), and Thyroxine (T4) to normal in STZ-induced diabetic rats [4].
Extracts of the plant have been traditionally used for ailments such as laryngitis, liver diseases and cough [5]. The seeds are used to prevent or relieve colic, cure head or chest colds and relieve cough [6]. The seed also has anti-inflammatory, antimicrobial, antidiabetic and antiviral as well as antiulcer properties [6]. Various extracts of Garcinia kola have been found to elicit a number of biochemical properties including hepatoprotection, antidiabetic properties and antigenotoxic potentials.
The proximate composition of Garcinia kola includes the following parameters (in g/100g); moisture content, crude protein, crude fibre, ash, crude fat, and carbohydrate. The values represent the mean triplicates ± standard deviation. The qualitative anti-nutrient screening of Garcinia kola has revealed the following parameters (%) to be present in abundant amount: tannin, oxalate, and phytate and trypsin inhibitor. The quantitative anti-nutrient screening has also revealed Garcinia kola to contain tannin, oxalate, phytate, and trypsin inhibitor. Its mineral compositions (ppm) include but unlimited to magnesium zinc, iron, manganese, copper, lead, phosphorus, sodium, potassium and calcium [7].
Over time, the antioxidant and scavenging activity of Garcinia kola has been investigated in a range of established in vitro assays involving reactive oxygen species. This showed that G. Kola elicited significant reducing power and a dose-dependent inhibition of oxidation of linoleic acid. G. Kola inhibited H2O2, and was more effective than the standard antioxidants BHA and β-carotene and equivalent in power to α-tocopherol. Kolaviron also significantly scavenged superoxide generated by phenazine methosulfate NADH. Furthermore, G. Kola scavenged hydroxyl radicals as revealed by significant inhibition of the oxidation of deoxyribose. The inhibitory activity of G. Kola in deoxyribose assay may relate directly to prevention of the propagation of the process of lipid per oxidation and modulation of other biomarkers of oxidative stress in animal model [8,9]. Furthermore, G. Kola dose dependently inhibited the intracellular ROS production induced by H2O2 in HepG2 cells detected as 2‘7’-Dichlorofluorescein Diacetate (DCF) fluorescence. Thus the ability of G. Kola to act as antioxidant in this cell underscores its role as an antioxidant and its potential role in the chemoprevention of chemically-induced genotoxicity [10].
The acclaimed health effects of G. kola on blood and its proven ability to suppress oxidative stress in different experimental models has elicited curiosity into its antioxidant potentials. These affirm the fact that it could have effect on the scavenging of free radicals due to tissue damages. Considering the anti-oxidant capacity of G. Kola, it becomes necessary to determine its effects on the serum levels of selected oxidative stress markers; hitherto was this study designed.
Aim of Study
This study aimed at investigating the serum antioxidant activities of the aqueous leaf extract of Garcinia Kola, using wistar rats as experimental model. Specifically, study;
• Examined the effects of different doses of Kola on the body weights.
• Assessed the changes in selected serum oxidative stress markers at different doses of Kola administration to Wistar rats.
MATERIALS AND METHODS
Care and management of animals
Prior to actual commencement of the experiment, a pilot study was conducted to ascertain the feasibility of the work to cost and time. Thereafter, thirty adult female wistar rats of an average weight of about between 160g - 200g were procured and acclimatized for two weeks. The rats were then maintained in the Animal Holding of the Department of Public and Community Health, College of Health Sciences, Novena University, Ogume and randomly selected into five groups of six rats each as follows;
• Group A (Control): Received standard rat diet and water ad libitum
• Group B: Received 100 mg/kg body weight of aqueous seed extract of Gacinia Kola
• Group C: Received 200 mg/kg body weight of aqueous seed extract of Gacinia Kola (Olaleye et al., 2006, 2014)
• Group D: Received 300 mg/kg body weight of aqueous seed extract of Gacinia Kola (Obi and Nwoha, 2014)
• Group E: Received 400 mg/kg body weight of aqueous seed extract of Gacinia Kola (Onasanwo and Rotu, 2014)
The treatment materials were administered twice daily for a period of two-week via oral gavage. The weights of animals were measured weekly and administration lasted for 14 days.
Preparation of kola extract
About 8.2kg of powdered samples (blended Garcinia kola) was weighed into a glass container and 5 liters of solvent (pure n-hexane) was added stirred at intervals of 2 hours and was left to stand for 72 hours. The defatting process was repeated by adding another 2 liters of pure n-hexane to the plant shaft for another 72hours. This was done to properly remove the fat present in the Garcinia kola. The solvent (n-hexane) containing the crude fat was collected. The solvent (n-hexane) containing the crude fat collected after 72 hours was added together and concentrated using a rotary evaporator after being filtered, it was then set at 40?C and was further concentrated in a vacuum oven at temperature of 40?C and pressure of 600mm Hg.
The Garcinia kola shaft (that is, defatted seeds) was spread and air-dried for 5 hours so as to remove the traces of n-hexane used. The defatted, dried marc was then repacked into a glass container and 5liters of solvent (water) was added stirred at intervals of 2 hours and was left to stand for 72 hours. The process was repeated by adding another 5 liters of distilled water to the plant shaft for another 72hours. The solvent (pure water) containing the crude aqueous extract was collected after 72 hours was concentrated using a rotary evaporator after being filtered it was set at 40?c and was further concentrated in a vacuum oven at temperature of 40?c and pressure of 600mm Hg.
The crude (aqueous) extract was made into solution with methanol and equal volume of water was added. It was done in batches 200ml of this mixture (methanol/water) was added 200ml of chloroform and transferred into a separating funnel of 500ml and was carefully shaken and allowed to stay for 30 minutes for proper partitioning of the chloroform and mixture (methanol/water) layer. This process was repeated four times for proper extraction of kolaviron (active component of G. Kola) with the aid of chloroform. The chloroform fraction was collected and concentrated using a rotary evaporator, it was set at 40?C. The crude chloroform fraction was further concentrated in a vacuum oven at 40?c in the pressure of 600mmHg as to properly remove any trace of solvent (chloroform).
Percentage yield was calculated as follows:
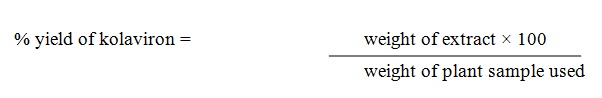
Preparation of stock solutions from G.Kola extract
After weighing 2g of G. Kola with electronic balance, the substance was then homogenized in pestle and mortar using 10ml of distilled water, and then filtered with Wattmann filter paper. This gave a 200mg/ml stock solution. Graded doses of G. Kola [high, medium, low and very low] were estimated from previously established lethal dose (192mg/kg). About 1g, 2g, 3g and 0.4g were dissolved in 100ml, 200ml, 300ml and 400ml of distilled water to make the aforementioned stock solutions respectively. The body weights of the animals were then taken and the dose of test drugs in millilitre to be administered was calculated.
G.Kola administration
The rats in the treatment groups received calculated doses of G. kola stocks (aforementioned) per kilogram body weight per day for a duration of two weeks. This was achieved through the use of orogastric tube, while the control rats received equal volume of distilled water through the same route and for the same period.
Blood sample collection
At the end of the twenty first day, the animals were euthanized by cervical decapitation and blood samples were collected from the superior vena cava. The sample was centrifuged at 3000rpm for 15minutes and the sera collected were stored frozen. The animals were dissected and the ovaries were removed, cleared of adherent tissues and weighed immediately using an electronic weighing balance. Of each animal euthanized, one Ovary was used for histological studies while the other Ovary was homogenized in 100mM Phosphate buffer (pH 7.4), centrifuged at 3000rpm for 15minutes.
Biochemical techniques
Preparation of sample
The different serum samples from the experimental animals were collected using a 2ml syringe. For each group, the obtained serum was centrifuged at 3500rpm for 5 minutes with the aid of a centrifuge. The clear supernatants were collected using a micropipette and transferred into an empty specimen container and refrigerated till needed for the assays.
Superoxide Dismutase (SOD) assay
Serum SOD activities were determined by the method of Drury et al., (1976) [10]. An aliquot (0.4ml) of the supernatant was added to 5ml of 0.05M carbonate buffer (pH 10.2) equilibrated in the spectrophotometer for 2-3 minutes. The reaction was then initiated by the addition of 0.6ml of freshly prepared 0.3mM adrenaline as substrate to the buffered-supernatant mixture which was quickly mixed by inversion and the absorbance taken. The reference cuvette contained 5ml of the carbonate buffer, 0.6ml of the substrate and 0.4ml of distilled water. The increase in absorbance at 420nm due to the adenochrome formed was monitored every 30 seconds for l20 seconds. 1 unit of SOD activity was given as the amount of SOD necessary to cause 50% inhibition of the auto-oxidation of adrenaline to adenochrome during 120 seconds. It was allowed to equilibrate before adding adrenaline solution and read immediately after addition of 0.3ml of adrenaline. Take absorbance at 420nm.
Protocol:
|
Tubes |
Blank |
Test |
Reference |
|
Distilled H2O |
3.0ml |
- |
0.2ml |
|
Sample |
- |
0.2ml |
- |
|
Carbonate buffer |
- |
2.5ml |
2.5ml |
|
Adrenaline |
- |
0.3ml |
0.3ml |
|
Solution |
|
|
|
Catalase (CAT) assay
The method of Cohen et al., (1970) was adopted. Aliquots of the homogenate supernatant (0.5ml) were added into ice cold test tubes while the blank contained 0.5ml distilled water. The reaction was initiated by adding sequentially, at fixed interval, 5ml of cold 30mM hydrogen peroxide and was mixed thoroughly by inversion. The test samples and the blank were taken one at a time, and 7ml of 0.01M potassium permanganate was added which was mixed twice by inversion and absorbance at 480nm. It was read within 30-60 seconds. The spectrophotometer standard was prepared by adding 7ml of 0.01M potassium permanganate to a mixture of 5.5ml of 0.05M phosphate buffer with pH 7.0 and 1ml of 6M-tetraoxosulphate (VI) acid solution. The spectrophotometer was zeroed with distilled water and the activity of the enzyme was estimated.
Protocol:
|
Tubes |
Test |
Blank |
|
Sample aliquots |
0.5ml |
- |
|
Distilled H2O |
- |
0.5ml |
|
Cold 30mM H2O2 |
5ml |
5ml |
|
0.01m KMnO4 |
7ml |
7ml |
Malonyldialdehyde (MDA) assay
Lipid peroxidation was estimated in terms of Thiobarbituric Acid Reactive Species (TBARS), using Malonyldialdehyde (MDA) as standard by the method of Beuge and Aust, (1978). 1.0ml of the sample extract was added with 2.0ml of the TCA-TBA-HCL reagent (15% (w/v) TCA, 0.375% (w/v) TBA and 0.25N HCL). The contents were boiled for 15 minutes, cooled and centrifuged at 10,000g to remove precipitate. The absorbance was read at 535nm and the malonyldialdehyde concentration of the sample was calculated using extinction coefficient of 1.56 x 105 M-1Cm-1.
Morphometric techniques
For each group, animals’ body weights were measured before and after administration of test substance using the Mettler Toledo weighing balance before and after (21st day) administration of test substances.
Statistical Analysis
The results were calculated using mean and Standard Error of Means (SEM) respectively. The results from the various assays were analysed using one way anova test and for significant level of (p < 0.05) as taken below variables. The graphical summary of the observation on the selected oxidative stress parameters on the serum were presented.
DISCUSSION
Many people, mostly from developing countries now depend on herbal medicine for health care, possibly because standard treatments modalities are becoming more expensive and often carry severe side effects. It is now urgent that options in folklore medicine for the management of diseases should be investigated. Garcinia kola is one of such natural products involved in the treatment of several diseases. Some pharmacological tests carried out on the plant for its antimalarial, anti-trypanosomal, anti-asthmatic, antihypertensive, antioxidant, antimicrobial, anti-diabetic, anti-inflammatory and analgesic, anti-candida infections, cardio-protective, gastro-protective, hepatoprotective and nephroprotective activities; its clinical effects in knee osteoarthritis and on the reproductive function, against oral cavity infections and intraocular pressure, revealed positive results without significant adverse side effects. Current study investigated the effects of G. Kola extract on the Female reproductive system, using Wistar rats as experimental model.
Effects of G. kola on ovary and body weight
Results from this study reveals a reduction in the ovary weight of female wistar rats fed with aqueous extract of G. Kola when compared to control group. In figure 1, the body weight showed a relative body weight loss but not statistically significant when compared to initial body weight and final body weight of the control group A (9.11%), Group B; -4.53%, Group C; -0.13% and Groups D and E; -3.40% and -7.26% respectively. This is indicative that prolonged administration of aqueous extract of G. kola on wistar rats can mildly reduce and alter body weights. Similar effect was observed with the body weight, relative organ weight of the ovary (Figures 2-4) were control group had 1.41% increment, Group B; 1.25%, Group C; 1.34% and Groups D and E; 1.47% and 1.44% respectively. There were also mild alterations in relative organ and body weights with increase doses; but not significant (p < 0.05) when compared in between groups. Both acute and chronic G. kola administration have been found to be intricately associated with changes in sleep patterns and cognitive deficits both in preclinical as well clinical studies, resulting in weight loss [11]. This finding concurs with the results from figure I of the current study.
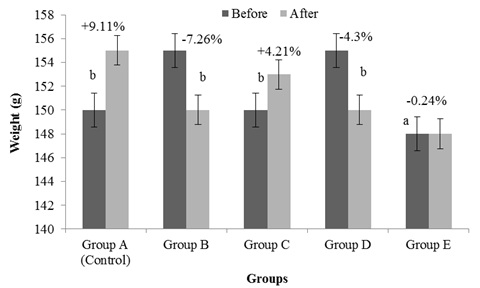
Figure 1: Effects of Garcinia Kola extract on body weights.
Figure 1 above shows the changes body weight of female wistar rats administered with graded doses of aqueous extract of G. Kola. Here, was observed that decreased doses of G. Kola administration caused a statistically insignificant decrease (p < 0.05) in the body weight of the rats. This decrease in body weight was however reversed in a dose dependent manner following administration of graded dose of G. Kola extract. These changes in body weight change were statistically significant. a = statistically insignificant, b = statistically significant
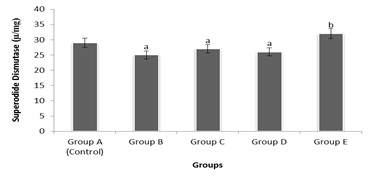
Figure 2: Comparative effects of graded doses of G. Kola on Serum Superoxide Dismutase (SOD) activity.
Figure 2 above shows changes in Serum Superoxide Dismutase (SOD) activities, following administration of graded doses of G. Kola on female wistar rats over a period of time. Findings from this experiment showed a statistically significant decrease (p < 0.05) in serum activities of groups B, C and D rats treated with graded aqueous G. Kola extract. a = significand decrease, b = significant increase compared to control.
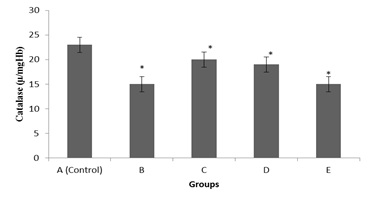
Figure 3: Comparative effects of graded doses of G. Kola on Serum Catalase (CAT) activity.
Figure 3 shows changes in serum catalase (CAT) activity of G. Kola treated rats at graded doses. Findings from this experiment revealed a statistically significant decrease in serum CAT activities across groups as compared with control.* = Significant decrease.
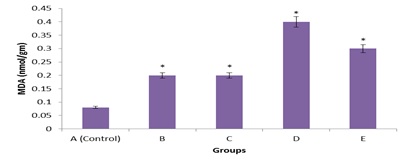
Figure 4: Comparative effects of graded doses of G. Kola on Serum Malonyldialdehyde (MDH) activity.
Figure 4 shows the changes in serum Malonyldialdehyde (MDA) level of G. Kola rats treated with graded dose. The effects on MDA level was not pronounced as minimal increase in serum MDA activities was observed. Subsequent administration of G. Kola also induced minimal effect in the MDA level of rats. * = Significant increase (p < 0.05) compared with control.
Effects of G. kola on selected oxidative stress markers
Another independent study had suggested that in humans, oxidative stress linked with diabetes mellitus can be possibly reduced by the administration of G. kola and thus improved metabolic activities in the disease [9]. Iranloye et al., (2013) reported that G. kola causes a hypoglycaemic action by enhancing insulin secretion, thus ameliorating oxidative stress. In this study, lipid peroxidation as designated by significant increase in MDA levels of treated animals. MDA, a lipid peroxidation product and marker of oxidative stress significantly decreased to show the efficacy of G. kola in combating oxidative stress. Also, the activities of serum SOD, an enzymatic oxidant significantly (p < 0.05) increased with an accompanying insignificant increase in that of Serum Catalase (CAT) activity. The results of this study are similar to the significantly decreased fasting blood glucose, serum MDA, CAT, and SOD observe by Akatugba (2010). Other researchers have reported that G. kola significantly reduced serum MDA in patients with type II diabetes mellitus, suggesting that G. kola is as effective in diabetes management as in conventional exercise in substantially improving biochemical, oxidative stress profile and the antioxidant status in type II diabetic patients over six months duration [9].
Diabetic patients who generally have been described as having excessive high levels of oxidative stress. Oxidative stress induces damage to cellular membrane polyunsaturated fatty acids leading to the generation of malonyldialdehyde (MDA), a Thiobarbituric Acid Reacting Substance (TBARS). It has also been reported that there are increase in lipid peroxidation products in diabetic subjects with vascular complications [9]. Some authors have shown that high levels of glucose in blood may be linked with the presence of oxidative stress which is reflected by the increase of intracellular lipoperoxide [11]. Serum MDA activities are higher in patients newly diagnosed type 2 diabetes mellitus and its concentration is raised in poorly regulated type 2 diabetic patients. The serum MDA concentrations in controls, altered over the two weeks period was significantly lower than those of the G. kola treated groups in this study, irrespective of dosage. However, administration of increased doses of G. kola improved the antioxidant status by decreasing the levels of MDA and lipid Peroxidation. Findings of the present study therefore indicate that G. kola has some antioxidant activities. This is consistent with reports by Dosumu et al., (2012). Oxidative stress has been discovered to be involved in the progression of diabetes mellitus [10].
In several situations, Superoxide dismutase, catalase and lipid peroxidation (malonyldialdehyde) activities can be used to understand the biochemical events of free radical damages and scavenging in the studied animal. However little is known whether stress is an important consequence of G. kola continuous intake. In line with the administration of G. kola to animals in the present study over a period of 21 days not only depicted increased lipid peroxidation and nitrite production, but also diminished MDA production and antioxidant enzymes activities of Superoxide dismutase as well as catalase in serum. Also from the results of current study, G. kola aqueous extracts posed a dose dependent effect on estrogen levels on the female Wistar rats. Also, Sleep debt generated as a consequence of daily administration of G. kola within 21 days of experimental protocol, a significant decrease in serum SOD and catalase activities were observed in experimental compared to control group. Subsequent treatment with the extract (100mg/kg and 200mg/kg) reversed the depleted reduced Superoxide dismutase and catalase activity. Malonyldialdehyde activity in the serum increased when compared to control group. Treatment with the extract (100mg/kg and 200mg/kg) moderately decreased the MDA activity.
CONCLUSION
The present study demonstrated that prolonged administration of aqueous extract of G. kola on wistar rats at variable doses greatly increased oxidative stress marker activities, posing it as a good source of antioxidants to reduce the possible damage due to increased free radical damage to the blood.
REFERENCES
- Ajayi TO, Moody JO, Fukush Y, Adeyemi TA, Fakeye TO (2014) Antimicrobial Activity of Garcinia kola (Heckel) Seed Extracts and Isolated Constituents against Caries causing Microorganisms. African Journal of Biochemistry Research 17: 165-171.
- Ralebona N, Sewani-Rusike CR, Nkeh-Chungag, BN (2012) Effects of ethanolic extract of Garcinia kola on sexual behaviour and sperm parameters in male Wistar rats. Afr J Pharm Pharmacol 6:1077-1082.
- Adedara IA, Farombi EO (2012) Chemoprotection of ethylene glycol monoethyl ether induced reproductive toxicity in male rats by kolaviron, isolated biflavonoid from Garcinia kola Hum Exp Toxicol 31: 506-517.
- Adedara IA, Farombi EO (2013) Influence of kolaviron and vitamin E on ethylene glycol monoethyl ether-induced haematotoxicity and renal apoptosis in rats. Cell Biochem Funct 32: 31-38.
- Ayensu ES (1978) Medicinal Plants of West Africa. Reference Publications, Algonac, Pg No: 330.Beardsley A, O’Donnell L (2003) Characterization of normal spermiation and spermiation failure induced by hormone suppression in adult rats. Biol Reprod 68: 1299-1307.
- Iwu MM, Igboko OA, Okunji CO, Tempesta MS (1990) Antidiabetic and aldose reductase activities of Biflavanones of Garcinia kola. J Pharm Pharmacol 42: 290-292.
- Adesuyi AO, Elumm IK, Adaramola FB, Nwokocha AGM (2012) Nutritional and phytochemical screening of Garcinia kola. Adv J Food Sci Tech 4: 9-14.
- Farombi EO, Adedara IA, Oyenihi AB, Ekakitie E, Kehinde S (2013) Hepatic, testicular and spermatozoa antioxidant status in rats chronically treated with Garcinia kola J Ethnopharmacol 146: 536-542.
- Farombi EO, Tahnteng JG, Agboola AO, Nwankwo JO, Emerole GO (2000) Chemoprevention of 2-acetylaminofluorene-induced hepatotoxicity and lipid peroxidation in rats by kolaviron-a Garcinia kola seed extract. Food Chem Toxicol 38: 535-541.
- Drury RAB, Wallington EA, Cameron RC (1976) Carlton’s Histological techniques: 4th ed, oxford University Press NY. USA, Pg No: 279-280.
- Kushida CA (2005) Sleep Deprivation: Basic Science, Physiology, and Behavior. Taylor & Francis, New York, USA, American Academy of Sleep Medicine (2005) The International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine, Pg No: 297.
Citation: Emily O, Obaghwarhievwo AJ, Osagie OM (2020) Antioxidant Activities of Aqueous Bitter Kola (Garcinia Kola) Seed Extract on Wistar Rats. J Med Stud Res 3: 013.
Copyright: © 2020 Okorodudu Emily, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

