
Antiviral Activity and Synergy of Herba Andrographidis and Radix Eleutherococci Preparations against SARS-CoV-2 Infected Vero E6 Human Primary Embryonic Kidney Epithelial Cells
*Corresponding Author(s):
Alexzander AseaUniversity Of Toledo College Of Medicine And Life Sciences, Arlington Avenue, Toledo, Ohio, United States
Email:draaasea@gmail.com
Karl Georg Wikman
Swedish Herbal Institute Research And Development Ab, Vallberga, Sweden
Email:karl.georg.wikman@gmail.com
Abstract
Background: Preparations from Herba Andrographidis (AP) and Radix Eleutherococci (ES) are known to exhibit antiviral, immunomodulatory and anti-inflammatory effects and are commonly used for prevention and treatment of viral respiratory diseases.
Aim of the study: The aim of our study was to assess antiviral activity of AP (SHA-10), ES (SHE-3) extracts and their fixed combination Kan Jang against coronavirus SARS-CoV-2 infected epithelial cells.
Methods: The antiviral activity of herbal extracts against coronavirus SARS-CoV-2 (MOI=0.05) was assessed in Vero E6 human primary embryonic kidney epithelial cells using the cytopathic effect (CPE) inhibition assay in which infection preventive, mitigating and curative models were applied.
Results: Significant antiviral activity was observed in the infection preventive in vitro model. Kan Jang combination at the concentration corresponding to the therapeutic daily dose used for the treatment of the common cold, effectively inhibited SARS-CoV-2 viral growth by 33.06±4% vs control (p<0.05). Kan Jang combination that contains higher content of ES (1 μM ES and 2 μM AP) resulted in a 42.87±4.5% inhibition in antiviral activity (p<0.05). The most potent antiviral effect (63.43±4.5% viral inhibition vs control) resulted from a 5-fold higher dose of the Kan Jang combination that corresponds to 5 μM ES and 10 μM AP, respectively.
Conclusion: Our study for the first time, demonstrates that ES, AP, and their combinations significantly inhibits SARS-CoV-2 viral growth in Vero E6 cells in a dose-dependent manner. The antiviral activity of the combinations of ES and AP are greater than expected.
Keywords
Andrographis; Coronavirus SARS-COV-2; Eleutherococcus; Epithelial Cells; Kan Jang; Synergy
Abbreviations
AP - Herba Andrographidis
ES - Radix Eleutherococci
Introduction
The COVID-19 pandemic has raised new challenges in the field of biomedical science, particularly in the development of effective therapeutics for the prevention and treatment of acute viral and stress-induced diseases, which were most severe in elderly people [1,2]. Conventional drugs including anti-viral (remdesivir), anti-inflammatory (dexamethasone) and anti-malarial (hydroxychloroquine) drugs, show only moderate benefit along with many adverse effects [3,4]. Several complex Traditional Chinese medicine (TCM) formulations were successfully used in seven original studies in which a total of 732 adults with COVID-19 in China participated. The meta-analysis shows that TCM adjunct treatment with standard care helps to improve treatment outcomes in patients with COVID-19 [5]. Using in silico modeling, several medicinal plants and natural compounds have been predicted to exert antiviral activity against SARS-Cov-2 [6-10].
Importantly, some herbal preparations used for the treatment of viral respiratory diseases have also been recommended for prevention [11,12], mitigation [11,13], as adjuvant therapy [11,14] and for the recovery of patients with COVID-19 [11]. Among these preparations, two adaptogenic plants, Andrographis paniculata (Burm. F.) Wall. ex. Nees, Acanthaceae (AP), Eleutherococcus senticosus (Rupr. & Maxim.) Maxim, Araliaceae (ES) and their fixed combination Kan Jang, are known to exhibit antiviral, immunomodulatory and anti-inflammatory effects [11] and clinical efficacy in the respiratory tract of patients with infectious diseases [15-26]. The aim of our study was to assess the antiviral activity of AP (SHA-10), ES (SHE-3) extracts and their fixed combination Kan Jang against SARS-CoV-2 infection of Vero E6 human primary embryonic kidney epithelial cells.
Materials and Methods
Herbal Extracts and Positive Control
Pharmaceutical grade standardized extracts of A. paniculata and E. senticosus genuine extracts and their fixed combination, Kan Jang, were manufactured in accordance to ICHQ7A and EMEA guidelines for Good Agricultural and Collecting Practice (GACP) and Good Manufacturing Practice (GMP) of active pharmaceutical ingredients [27].
The stock solution (SS-A) of herba Andrographidis extract in the concentration of 30 mg/ml was obtained by dilution of 1,000 mg of 30% Herba Andrographidis spissum (soft genuine extract SHA-10) in 10 ml of distilled water. It was used for further dilutions to obtain final concentrations shown in table 1. Similarly, the stock solution (SS-E) of Radix Eleutherococcici extract in the concentration of 30 mg/ml was obtained by dilution of 577 mg of Radix Eleutherococcici spissum (soft genuine extract SHE-3) in 10 ml of distilled water. It was used for further dilutions to obtain final concentrations shown in table 1. The concentration of Kan Jang in incubation media is based on the results of pharmacokinetic study of Kang Jang-derived and rographolide in human blood plasma, where it was detected in concentrations of ∼ 0.7 μg/ml = 2μM [28]. The concentrations of the total extracts of both herbal ingredients and their active constituents were compatible in all test samples. The concentrations of genuine extracts have been calculated using specifications of Kan Jang to ensure that they correspond to therapeutically effective doses.
The antiviral agent, remdesivir (GS-5734; Gilead Science Inc., Foster City, CA), an inhibitor of the viral RNA-dependent, RNA polymerase with in vitro inhibitory activity against SARS-CoV-1 and the Middle East respiratory syndrome (MERS-CoV) [29], was used as a positive control and PBS used as a negative control.
Cell Line
Vero E6 human primary embryonic kidney epithelial cell line (CRL-1586TM, American Type Cell Culture; Rockville, MD) was obtained from the ATCC (Rockville, MD), which routinely performs cell line characterization. Cells were passaged in our lab for not more than 3 months after receiving them from ATCC and maintained and grown in Dulbecco’s Modified Eagle’s Medium (DMEM, Invitrogen, Carlsbad, CA) containing 100 U/ml penicillin, 100 mg/ml streptomycin, and 10% heat-inactivated fetal calf serum (FCS) (Gibco, Gaithersburg, MD). Cells were maintained in an incubator adjusted to 37°C with humidified atmosphere and 5% CO2.
Cytopathic Effect (CPE) Inhibition Assay and Herbal Antiviral Model Systems
In Vitro Preventative Model: Vero E6 human primary embryonic kidney epithelial cells (CRL-1586™) were grown as monolayers in 96-well plates. Herbal extracts were added to Vero cells and incubated for 24 h at 37°C with 5% CO2. Remdesivir was added as a positive control. The plates were then washed three times with PBS. SARS-CoV-2 (MOI=0.05) was then added to the plates and incubated for a further 2 h at 37°C with 5% CO2. After incubation, the inoculum was removed by washing three times with PBS. Cells were subsequently incubated for an additional 72 h at 37°C with 5% CO2, as which time the infected cells shown 100% CPE under the microscope. The percentage (%) inhibition of CPE in herbal extract-treated cells was calculated by the GraphPad Prism 7.0 software.
In Vitro Mitigating Model: Vero E6 human primary embryonic kidney epithelial cells (CRL-1586™) were grown as monolayers in 96-well plates. SARS-CoV-2 (MOI=0.05) was then added to the plates and incubated for 2 h at 37 °C with 5% CO2. After incubation, the inoculum was removed by washing three times with PBS. Herbal extracts were then added to Vero cells for 0 h, 1 h and 3 h at 37°C with 5% CO2. The cell monolayers were then washed three times with PBS and incubated for an additional 72 h at 37°C with 5% CO2. The percentage (%) inhibition of CPE in herbal extract-treated cells was calculated by the GraphPad Prism 7.0 software.
In Vitro Curative Model: Vero E6 human primary embryonic kidney epithelial cells (CRL-1586™) were grown as monolayers in 6-well plates. SARS-CoV-2 (MOI=0.05) was then added to the plates and incubated for 2 h at 37°C with 5% CO2. After incubation, the inoculum was removed by washing three times with PBS. Herbal extracts were then added to Vero cells for 24 h, 48 h and 72 h at 37°C with 5% CO2. The cell monolayers were then washed three times with PBS and incubated for an additional 72 h at 37°C with 5% CO2. The percentage (%) inhibition of CPE in herbal extract-treated cells was calculated by the GraphPad Prism 7.0 software.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 7.0 software on the comparison between two groups, the SARS-CoV-2-infected or SARS-CoV-2-non-infected groups, under equivalent conditions. Therefore, significant differences in viral titer were determined by an unpaired two-tailed Student’s t-test and two-way analysis of variance (ANOVA) throughout this study (p values <0.05 were considered significant).
Results
Dose dependence of ginseng induced excitatory neurotransmission in rat hippocampal slice preparations ex vivo
To determine the antiviral activity of herbal extracts on SARS-CoV-2, we initially applied the in vitro preventative model system in which various concentrations of herbal extracts were added to Vero cells 24 h prior to infestation by SARS-CoV-2 (MOI=0.05), table 1.
|
Eleutherococcus ES (SHE-3) |
Eleutherosides |
Andrographis AP (SHA-10) |
Andrographolides |
Kan Jang combination |
Active markers |
|
µg/ml |
μM (ES) |
µg/ml |
μM (AP) |
µg/ml (AP+ES) |
μM (AP+ES) |
|
3 |
0.1 |
6 |
0.4 |
9 (6+3) |
0.4 (AP) + 0.1 (ES) |
|
30 |
1 |
30 |
2 |
33 (30+3) |
2.0 (AP) + 0.1 (ES) |
|
150 |
5 |
150 |
10 |
60 (30+30) |
2.0 (AP) + 1.0 (ES) |
|
|
|
|
|
180 (150+30) |
10 (AP) + 1 (ES) |
|
|
|
|
|
300 (150 + 150) |
10 (AP) + 5 (ES) |
Table 1: Final concentration of the AP (SHA-10), ES (SHE-3) extracts (µg/ml) and active markers (μM) in incubation media.
We demonstrate that the Kan Jang combination at a concentration of 0.1 μM ES and 2 μM AP, which corresponds to the therapeutic daily dose used for the treatment of the common cold, effectively inhibited the viral load of SARS-CoV-2 in Vero E6 human primary embryonic kidney epithelial cells by 33.06±4% vs control (p<0.05), as determined by the CPE inhibition assay in which remdesivir was used as a positive control (Figure 1).
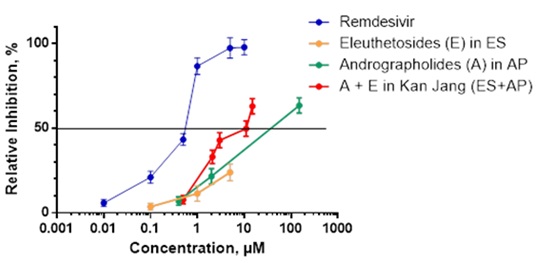 Figure 1: Antiviral Activity of Herbal Extracts against SARS-CoV-2 Measured in an In Vitro Preventative Model using the Cytopathic Effect (CPE) Inhibition Assay. Vero cell monolayers were incubated without or in the presence of different dilutions (expressed in μM concentrations of Eleutherosides (E) and Adrofrapholides (A), ordinate), of ES (SHE-3), AP (SHA-10) extracts, their combination Kan jang or Remdesivir (positive control) for 24 h and infected with coronavirus SARS-CoV-2 (MOI of 0.05). The antiviral activity of test samples against the virus was determined using the CPE inhibition assays. Infected cells showed 100% CPE under the microscope. The percentage of CPE in herbal extract-treated cells was recorded. Data are mean ± SD of four independently performed experiments. Data represent six replicates derived from four independently performed experiments.
Figure 1: Antiviral Activity of Herbal Extracts against SARS-CoV-2 Measured in an In Vitro Preventative Model using the Cytopathic Effect (CPE) Inhibition Assay. Vero cell monolayers were incubated without or in the presence of different dilutions (expressed in μM concentrations of Eleutherosides (E) and Adrofrapholides (A), ordinate), of ES (SHE-3), AP (SHA-10) extracts, their combination Kan jang or Remdesivir (positive control) for 24 h and infected with coronavirus SARS-CoV-2 (MOI of 0.05). The antiviral activity of test samples against the virus was determined using the CPE inhibition assays. Infected cells showed 100% CPE under the microscope. The percentage of CPE in herbal extract-treated cells was recorded. Data are mean ± SD of four independently performed experiments. Data represent six replicates derived from four independently performed experiments.
We further observed that the Kan Jang combination that contains a higher content of ES (1 μM ES and 2 μM AP) resulted in a 42.87±4.5% inhibition in antiviral activity against SARS-CoV-2, as compared to control (p<0.05) (Figure 1 and Table 2). Interestingly, we demonstrated that the most potent antiviral activity (63.43±4.5% viral inhibition vs control) resulted from a 5-fold higher dose of the Kan Jang combination that corresponds to 5 μM ES and 10 μM AP, respectively (Figure 1 and Table 2). However, similar concentrations of herbal extracts did not significantly inhibit SARS-CoV-2 using the in vitro mitigation and in vitro curative models (Table 3).
|
Remdesivir |
Eleutherococcus |
Andrographis |
Kan Jang combination |
||||
|
|
Eleutherosides (ES) |
Andrographolides (AP) |
AP + ES |
||||
|
Conc. μM |
Inhibition, % |
Conc. μM |
Inhibition, % |
Conc. μM |
Inhibition, % |
Conc. μM |
Inhibition, % |
|
0 |
4.37±2.25 |
0 |
4.37±2.25 |
0 |
4.37±2.25 |
0 |
4.37±2.25 |
|
0.01 |
5.85±2 |
0.1 |
3.6±2 |
0.4 |
7.05±2.5 |
0.4 (AP) + 0.1 (ES) |
7.76±2.5 |
|
0.1 |
21.08±3.5 |
1 |
11.39±4.5 |
2 |
21.57±4.5 |
2.0 (AP) + 0.1 (ES) |
33.06 ±4 |
|
0.5 |
43.31±3.5 |
5 |
23.9±5 |
150 |
63.43±4.5 |
2.0 (AP) + 1.0 (ES) |
42.87±4.5 |
|
1 |
86.59±5 |
|
|
|
|
10 (AP) + 1 (ES) |
49.7±4.5 |
|
5 |
97.26±6 |
|
|
|
|
10 (AP) + 5 (ES) |
62.93±4.5 |
|
10 |
97.78±4.5 |
|
|
|
|
|
|
Table 2: Antiviral Activity of Herbal Extracts Against SARS-CoV-2 as Determined in an In Vitro Preventative Model1 using the CPE inhibition assay.
1Agents were added to Vero cells and incubated for 24 h at 37°C. SARS-CoV-2 (MOI=0.05) was then added to the cells and incubated for 2 h at 37°C. Remdesivir was used as a positive control. The CPE inhibition assay was performed 48 h post infection as described in detail in the Materials and Methods section.
|
|
Eleutherococcus |
Andrographis |
Kan Jang combination |
|
Time, h |
Eleutherosides (ES) 5 μM |
Andrographolides (AP) 150 μM |
AP (10 μM) + ES (5 μM) |
|
0 |
17.59±3.0 |
41.62±4.5 |
83.32±6.5 |
|
1 |
11.26±5.5 |
26.08±5.0 |
64.08±6.0 |
|
3 |
6.34±1.75 |
11.09±3.5 |
16.75±3.5 |
|
24 |
3.32±1.5 |
6.98±2.25 |
20.44±4.0 |
|
48 |
3.0±1.5 |
2.37±2.0 |
9.12±1.75 |
|
72 |
4.89±1.5 |
1.99±1.35 |
2.34±1.75 |
Table 3: Antiviral Activity of Herbal Extracts against SARS-CoV-2 as Determined in an In Vitro Mitigating and Curative Models1 using the CPE Inhibition Assay.
1SARS-CoV-2 (MOI=0.05) was added to Vero cells and incubated for 48 h at 37°C with 5% CO2. Agents were then added to the cells for 0 h, 1 h, 3 h (mitigating model), and 24 h, 48 h and 72 h (curative model) at 37°C with 5% CO2. The CPE inhibition assay was performed 48 h after the end of each incubation cycle as described in detail in the Materials and Methods section.
Discussion
The earliest evidence for the clinical efficacy of ES against respiratory infections was reported in the 1970-80s during the influenza virus epidemic in the Soviet Union [30-33]. It was demonstrated that the prophylactic treatment with the ES extract reduced a number of complications associated with the influenza infection, including pneumonia, bronchitis, otitis, as well as morbidity and mortality rates [34-36].
Antiviral effect of Eleutherococcus extracts were further demonstrated in experimental model systems in which rodents were infected with H1N1 influenza A virus [37-41], human rhinovirus and respiratory syncytial virus [38]. Recently, in silico studies have predicted the antiviral actions of ES against SARS-COV-2 virus docking and replication by targeting Nsp5 (3-chymotrypsin-like protease 3Clpro) and Nsp3 (papain like protease Plpro) structural proteins [41,42].
The antiviral activity of AP was demonstrated against H1N1 influenza A [43-45], H5N1 avian influenza [46], Chikungunya [47] and Dengue [48,49] viruses. The predicted antiviral effect of AP against SARS-COV-2 virus docking and replication by targeting Nsp5 (3-chymotrypsin-like protease 3Clpro), Nsp3 (papain like protease Plpro), Nsp12 (RNA-dependent RNA polymerase RdRp), Nsp1 (the most N-terminal gene 1 protein) structural proteins and S2 Spike glycoprotein receptor to type-II Transmembrane Serine Protease Enzymes (TMPRSS2) of host cells was also demonstrated by in silico modeling [50,51].
In this study, our group was the first to demonstrate potent antiviral effects of ES and AP extracts as well as their fixed combination Kan Jang against SARS-CoV-2 infection of Vero E6 human primary embryonic kidney epithelial cells.
The highest antiviral activity was observed in the in vitro infection preventive model. These results, however, do not exclude the possibility that preparations are effective in vivo models, e.g., when orally applied after viral exposure of subjects. We hypothesize that this could be due to activation of innate immunity and other immune defense mechanisms described in numerous publications, for review see [11].
In this study, we demonstrated that Kan Jan combination is significantly more effective than its ingredients (ES or AP) in the prevention of growth of SARS-COV-2 in isolated Vero E6 human primary embryonic kidney epithelial cells (Figure 1). These results are intriguing. Although the exact reason is currently unknown. We hypothesize that ES and AP’s mutual potentiation and synergistic interactions within target cells could be a cause. A similar effect was recently observed in isolated neuroglia cells [27,52-55]. This suggestion is in line with recent publications where synergistic interaction of ES and AP were demonstrated in isolated neuroglia cells [27,52-55]. The synergistic effect is hypothesized to be associated with their effects on tumor cell proliferation [53], expression on genes involved in inflammatory and immune responses [27], neuroprotection [52], regulation of Nrf2-mediated signaling proteins [55] and on enzymes associated with antioxidants, metabolization and detoxification [54,56].
Table 2 demonstrates that the Kan Jang combination at concentrations of ES (0.1 μM) and AP (2 μM), that corresponds to the therapeutic daily dose used for the treatment of common cold, significantly inhibits the viral load by 33.06±4% vs control (p<0.05). On the other hand, the Kan Jang combination with a higher content of ES (1 μM ES and 2 μM AP) significantly inhibits antiviral activity by 42.87±4.5% vs control (p<0.05). Importantly, the Kan Jang combination with 5-fold higher concentrations of ES and AP (5 μM ES and 10 μM AP) exhibited the highest level of SARS-CoV-2 antiviral inhibition, 63.43±4.5% vs control (p<0.05).
Interestingly, the effective concentrations of Kan Jang are higher than effective concentrations of positive control Remdesivir in our experiments, Table 2 and Figure 1. Importantly, it should be noted that Kan Jang has at least two important advantages compared to Remdesivir: (i) beneficial effects on immune, antioxidant and detoxifying systems [11], which are of importance on all phases of infection and recovery of COVID-19 patients, and (ii) lack of serious adverse events [11].
Further experimental studies on animals and clinical studies in COVID-19 are required to confirm efficacy of Kan Jang for prevention and amelioration of symptoms of COVID-19.
Conclusion
ES, AP, and their combinations inhibit SARS-CoV-2 replication in vitro infected Vero E6 human primary embryonic kidney epithelial cells in a dose-dependent manner. The antiviral activity of the combinations of ES and AP are greater than expected.
Declarations
Ethics approval
No ethic approvals are required for in vitro studies of commercially available Vero E6 human primary embryonic kidney epithelial cell line (CRL-1586TM).
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author A.A. upon reasonable request.
Conflict of Interest Statement
The authors AA and PK declare no conflicts of interest. KGW is the founder of Swedish Herbal Institute AB and Swedish Herbal Institute Research and Development AB. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Authors' contributions
Conceptualization AA and GW; methodology AA, PK; software AA and PK; validation AA and PK; formal analysis AA and PK; investigation AA and PK; resources AA, GW; data curation AA and PK; writing original draft preparation AA and PK; writing review and editing AA and PK; visualization AA GW and PK; supervision AA and PK; project administration AA; funding acquisition GW. All authors have read and agreed to the submitted version of the manuscript.
Funding
This research was funded by Swedish Herbal Institute AB, and the University of Toledo.
Acknowledgement
The authors acknowledge the support of Swedish Herbal Institute AB for the supply of investigational products, their characterization, and material support. The authors thank Hakan Lejon and Torben Larsson for technical assistance. The authors thank Daniel Torn brink for management of production of investigational products and Susanna Panosyan for their quality assurance. The authors are grateful to Alexander Panossian for reviewing the manuscript, valuable comments, and recommendations.
References
- Hamid S, Mir MY, Rohela GK (2020) Novel coronavirus disease (COVID-19): a pandemic (epidemiology, pathogenesis and potential therapeutics). New Microbes and New Infections 35: 100679.
- Yi Y, Lagniton PNP, Ye S, Li E, Xu R-H (2020) COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci 16: 1753-1766.
- Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, et al. (2020) ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19 - Final Report. The New England journal of medicine 383: 1813-1826.
- Recovery Collaborative Group, Horby P, LimWS, Emberson JR, Mafham M, et al. (2020) Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. The New England journal of medicine 384: 693-704.
- Fan AY, Gu S, Alemi SF (2020) Research Group for Evidence-based Chinese Medicine: Chinese herbal medicine for COVID-19: Current evidence with systematic review and meta-analysis. Journal of integrative medicine 18: 385-394.
- Fuzimoto AD, Isidoro C (2020) The antiviral and coronavirus-host protein pathways inhibiting properties of herbs and natural compounds - Additional weapons in the fight against the COVID-19 pandemic? Journal of traditional and complementary medicine 10: 405-419.
- Zhang DH, Wu KL, Zhang X, Deng SQ, Peng B (2020) In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus. J Integr Med 18: 152-158.
- Wu C, Liu Y, Yang Y, Zhang P, Zhong W, et al. (2020) Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B 10: 766-788.
- Enmozhi SK, Raja K, Sebastine I, Joseph J (2020) Andrographolide as a potential inhibitor of SARS-CoV-2 main protease: an in silico approach. J Biomol Struct Dyn 1-7.
- Yang R, Liu H, Bai C, Wang Y, Zhang X, et al. (2020) Chemical composition and pharmacological mechanism of Qingfei Paidu Decoction and Ma Xing Shi Gan Decoction against Coronavirus Disease 2019 (COVID-19): In silico and experimental study. Pharmacological Research 157: 104820.
- Panossian A, Brendler T (2020) The Role of Adaptogens in Prophylaxis and Treatment of Viral Respiratory Infections. Pharmaceuticals (Basel) 13: 236.
- Boozari M, Hosseinzadeh H (2020) Natural products for COVID-19 prevention and treatment regarding to previous coronavirus infections and novel studies. Phytother Res 35: 864-876.
- Ang L, Lee HW, Kim A, Lee MS (2020) Herbal medicine for the management of COVID-19 during the medical observation period: A review of guidelines. Integr Med Res 9: 100465.
- Silveira D, Prieto-Garcia JM, Boylan F, Estrada O, Fonseca-BazzoYM, et al. (2020) COVID-19: Is There Evidence for the Use of Herbal Medicines as Adjuvant Symptomatic Therapy? Frontiers in pharmacology 11: 581840.
- Panossian A, Wikman G (2012) Efficacy of Andrographis paniculata in upper respiratory tract (URT) infectious diseases and the mechanism of action. In: Wagner EH, Merzenich GU (Eds.). Evidence and rational based research on Chinese Drugs. Springer Publ Comp 137-179.
- Coon JT, Ernst E (2004) Andrographis paniculata in the treatment of upper respiratory tract infections: a systematic review of safety and efficacy. Planta Med 70: 293-298.
- Jayakumar T, Hsieh CY, Lee JJ, Sheu JR (2013) Experimental and Clinical Pharmacology of Andrographis paniculata and Its Major Bioactive Phytoconstituent Andrographolide. Evid. Based Complement. Alternat Med 2013: 846740.
- Puri A, Saxena R, Saxena RP, Saxena KC, Srivastava V, et al. (1993) Immunostimulant agents from Andrographis paniculata. J Nat Prod 56: 995-999.
- Cáceres DD, Hancke JL, Burgos RA, Wikman GK (1997) Prevention of common colds with Andrographis paniculata dried extract. A Pilot double blind trial. Phytomedicine 4: 101-104.
- Gabrielian ES, Shukarian AK, Goukasova GI, Chandanian GL, Panossian AG, et al. (2002) A double blind, placebo-controlled study of Andrographis paniculata fixed combination Kan Jang in the treatment of acute upper respiratory tract infections including sinusitis. Phytomedicine 9: 589-597.
- Hancke JBR, Cáceres D, Wikman G (1995) A double-blind study with a new monodrug Kan Jang®: decreases of symptoms and improvement in the recovery from common colds. Phytotherapy Research 9: 559-562.
- Kulichenko LL, Kireyeva L, Malyshkina EN, Wikman G (2003) A randomised, controlled study of Kan Jang® versus amantadine in the treatment of influenza in Volgograd. J Herb Pharmacother 173: 188-194.
- Melchior J, Palm S, Wikman G (1997) Controlled clinical study of standardized Andrographis paniculata extract in common cold - a pilot trial. Phytomedicine 3: 315-318.
- Melchior J, Spasov AA, Ostrovskij OV, Bulanov AE, Wikman G (2000) Double-blind, placebo-controlled pilot and phase III study of activity of standardized Andrographis paniculata Herba Nees extract fixed combination (Kan Jang®) in the treatment of uncomplicated upper-respiratory tract infection. Phytomedicine 7: 341-350.
- Saxena RC, Singh R, Kumar P, Yadav SC, Negi MP, et al. (2010) A randomised double blind placebo controlled clinical evaluation of extract of Andrographis paniculata (KalmCold) in patients with uncomplicated upper respiratory tract infection. Phytomedicine 17: 178-185.
- Spasov AA, Ostrovskij OV, Chernikov MV, Wikman G (2004) Comparative controlled study of Andrographis paniculata fixed combination, Kan Jang and an Echinacea preparation as adjuvant, in the treatment of uncomplicated respiratory disease in children. Phytother Res 18: 47-53.
- Panossian A, Seo EJ, Wikman G, Efferth T (2015) Synergy assessment of fixed combinations of Herba Andrographidis and Radix Eleutherococci extracts by transcriptome-wide microarray profiling. Phytomedicine 22: 981-992.
- Panossian A, Hovhannisyan A, Mamikonyan G, Abrahamian H, Hambardzumyan E, et al. (2000) Pharmacokinetic and oral bioavailability of andrographolide from Andrographis paniculata fixed combination Kan Jang in rats and human. Phytomedicine 7: 351-364.
- Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, et al. (2020). Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nature communications 11: 222.
- European Medicines Agency (2014) Assessment report on Eleutherococcus senticosus (Rupr. et Maxim.) Maxim., radix. European Medicines Agency, Netherlands.
- Gagarin IA (1977) Eleutherococcus in the Prophylaxis of the disease incidence in the Arctic. In: Brekhman II (ed.). Adaptation and Adaptogens. Proceedings of the 2nd Symposium. Far East Scientific Center of the Academy Science of the USSR, Vladivostok,
- Galanova LK (1977) Eleutherococcus in preventive maintenance of a flu and relapses of hypertonic illness. In: Brekhman II (ed.). Adaptation and Adaptogens: Proceedings of the 2nd Symposium. Far East Scientific Center of the Academy Science of the USSR, Vladivostok, USSR, Russia.
- Schezin AK, Zinkovich VI, Matsuk VS (1977) Tentative data on the mass Eleutherococcus prophylaxis of influenza at the main assembly line and metallurgic plant of the Volga Automobile Plant. In: Abstracts of the reports delivered at the 2nd All-Union conference on human adaptation to different conditions. Novosibirsk 2: 44-46.
- Barkan AI, Gaiduchenya LI, Makarenko IA (1980) Effect of Eleutherococcus on respiratory viral infectious morbidity in children in organized collectives. Pediatriya 4: 65-66.
- Shadrin AS, Kustikova YG, Belogolovkina NA, Baranov NI, Oleinikova EV, et al. (1984) Estimation of prophylactic and immunostimulating effects of Eleutherococcus and Schizandra chinensis preparations. In: New Data on Eleutherococcus: Proceedings of the 2nd International Symposium on Eleutherococcus, Moscow, Russia.
- Sheparev AA, Zvereva LA, Kozlenko IY, Agapova TM, Chernysheva NM, et al (1986) Effect of preventive administration of Eleutherococcus extract on health of children under school age. In: New Data on Eleutherococcus: Proceedings of the 2nd International Symposium on Eleutherococcus, Moscow, Vladivostok, Russia.
- Protasova SF, Zykov MP (1986) Antiviral effect of Eleutherococcus in experimental influenza infection. In: New Data on Eleutherococcus: Proceedings of the 2nd International Symposium on Eleutherococcus, Moscow, Vladivostok, Russia.
- Glatthaar-Saalmüller B, Sacher F, Esperester A (2001) Antiviral activity of an extract derived from roots of Eleutherococcus senticosus. Antiviral Res 50: 223-228.
- Yan W, Chen J, Wei Z, Wang X, Zeng Z, et al. (2020) Effect of eleutheroside B1 on non-coding RNAs and protein profiles of influenza A virus-infected A549 cells. Int J Mol Med 45: 753-768.
- Yan W, Zheng C, He J, Zhang W, Huang XA, et al. (2018) Eleutheroside B1 mediates its anti-influenza activity through POLR2A and N-glycosylation. Int J Mol Med 42: 2776-2792.
- Zhang DH, Wu KL, Zhang X, Deng SQ, Peng B (2020) In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus. J Integr Med 18: 152-158.
- Mani JS, Johnson JB, Steel JC, Broszczak DA, Neilsen PM, et al. (2020) Natural product-derived phytochemicals as potential agents against coronaviruses: A review. Virus Res 284: 197989.
- Ding Y, Chen L, Wu W, Yang J, Yang Z, et al. (2017) Andrographolide inhibits influenza A virus-induced inflammation in a murine model through NF-κB and JAK-STAT signaling pathway. Microbes Infect. 19: 605-615.
- Ko HC, Wei BL, Chiou WF (2006) The effect of medicinal plants used in Chinese folk medicine on RANTES secretion by virus-infected human epithelial cells. J Ethnopharmacol 107: 205-210.
- Yu B, Dai CQ, Jiang ZY, Li EQ, Chen C, et al. (2014) Andrographolide as an Anti-H1N1 drug and the mechanism related to retinoic acid-inducible gene-I-like receptors signaling pathway. Chin J Integr Med 20: 540-545.
- Sornpet B, Potha T, Tragoolpua Y, Pringproa K (2017) Antiviral activity of five Asian medicinal pant crude extracts against highly pathogenic H5N1 avian influenza virus. Asian Pac J Trop Med 10: 871-876.
- Wintachai P, Kaur P, Lee RC, Ramphan S, Kuadkitkan A, et al. (2015) Activity of andrographolide against chikungunya virus infection. Sci Rep 5: 14179.
- Panraksa P, Ramphan S, Khongwichit S, Smith DR (2017) Activity of andrographolide against dengue virus. Antiviral Res 139: 69-78.
- Ramalingam S, Karupannan S, Padmanaban P, Vijayan S, Sheriff K, et al. (2018) Anti-dengue activity of Andrographis paniculata extracts and quantification of dengue viral inhibition by SYBR green reverse transcription polymerase chain reaction. Ayurveda 39: 87-91.
- Enmozhi SK, Raja K, Sebastine I, Joseph J (2020) Andrographolide as a potential inhibitor of SARS-CoV-2 main protease: an in silico approach. J Biomol Struct Dyn 1-7.
- Wu C, Liu Y, Yang Y, Zhang P, Zhong W, et al. (2020) Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B 10: 766-788.
- Seo EJ, Klauck SM, Efferth T, Panossian A (2019). Adaptogens in chemobrain (Part I): Plant extracts attenuate cancer chemotherapy-induced cognitive impairment - Transcriptome-wide microarray profiles of neuroglia cells. Phytomedicine 55: 80-91.
- Seo EJ, Klauck SM, Efferth T, Panossian A (2019) Adaptogens in chemobrain (Part II): Effect of plant extracts on chemotherapy-induced cytotoxicity in neuroglia cells. Phytomedicine 58: 152743.
- Seo EJ, Klauck SM, Efferth T, Panossian A (2019) Adaptogens in chemobrain (Part III): Antitoxic effects of plant extracts towards cancer chemotherapy-induced toxicity - transcriptome-wide microarray analysis of neuroglia cells. Phytomedicine 56: 246-260.
- Panossian A, Seo EJ, Klauck SM, Efferth T (2020). Adaptogens in chemobrain (part IV): adaptogenic plants prevent the chemotherapeutics-induced imbalance of redox homeostasis by modulation of expression of genes encoding Nrf2-mediated signaling proteins and antioxidant, metabolizing, detoxifying enzymes in neuroglia cells. Longhua Chin Med 3:4.
- Runfeng L, Yunlong H, Jicheng H, Weiqi P, Qinhai M, et al. (2020) Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacological research 156: 104761.
Citation: Asea A, Kaur P, Wikman KG (2021) Antiviral Activity and Synergy of Herba Andrographidis and Radix Eleutherococci Preparations against SARS-CoV-2 Infected Vero E6 Human Primary Embryonic Kidney Epithelial Cells. J Altern Complement Integr Med 6: 148.
Copyright: © 2021 Alexzander Asea, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

