
Application of Ginsenoside-Rg1 Enhanced Glutathione Peroxidase Activity but could not Ameliorate Hyperoxia-Induced Vascular Endothelial Oxidative Stress and Apoptosis
*Corresponding Author(s):
Yen ChuDepartment Of Research And Development, Division Of Thoracic And Cardiovascular Surgery, Laboratory Thoracic And Cardiovascular Physiology, Chang Gung Memorial Hospital, Linkou Medical Center, Taoyuan City, Taiwan
Tel:+886 33281200,
Email:chuyen@cgmh.org.tw
Abstract
Objective: Oxygen therapy is necessary to preterm infants with respiratory distress. However, hyperoxia may cause bronchopulmonary dysplasia and retinopathy of prematurity due to suppression of vasogenesis and increase of cell death. Ginsenoside-Rg1, one of the active components of Ginseng, is shown as a proangiogenic factor of vascular endothelial cells. We evaluated whether application of ginsenoside-Rg1 is able to improve hyperoxic-induced vascular endothelium injury.
Methods: Human umbilical vein endothelial cells (HUVECs) were cultured under room air and 60% oxygen for 72 hours, respectively. Gensenoside-Rg1 was added to the medium at 0, 75, 150, 300nM. HUVECs proliferation, oxidative stress and apoptosis under normoxic and hyperoxic conditions were assayed by Western Blot.
Results: Under hyperoxia (60% O2), HUVECs proliferation and levels of vascular endothelial growth factor (VEGF) were significantly decreased after ginsenoside-Rg1 treatment. However, both levels of glucocorticoid receptor (GR) and glutathione peroxidase (GPx) were increased after 72 hours ginsenoside-Rg1 treatment, but no changed under room air control. The levels of oxidative stress-induced Bax and cytochrome c and apoptosis-related active caspase 3 and poly ADP-ribose polymerase were significantly increased after ginsenoside-Rg1 treatment under hyperoxic condition.
Conclusion: In HUVECs model, ginsenoside-Rg1 is unable to overcome the major hyperoxic-induced vascular endothelium injury. It might aggravate oxidative stress and endothelial apoptosis caused by oxygen toxicity. However, both elevated levels of GR and GPx indicate that ginsenoside-Rg1 could be involved in vascular signaling and the regulation of oxidative stress under hyperoxia. Further investigation of Rg1 effects under hyperoxia is required.
Keywords
Apoptosis; Ginsenoside-Rg1; Hyperoxic Injury; Oxidative Stress; Vascular Endothelial Cell
Abbreviations
BPD: Bronchopulmonary Dysplasia
Cyt c: Cytochrome c
ECGS: Endothelial Cell Growth Supplement
eNOS: Endothelial Nitric Oxide Synthease
FBS: Fetal Bovine Serum
GPx: Glutathione Peroxidase
GR: Glucocorticoid Receptor
HUVECs: Human Umbilical Vein Endothelial Cells
PARP: Poly ADP-Ribose Polymerase
ROP: Retinopathy of Prematurity
ROS: Reactive Oxygen Species
VEGF: Vascular Endothelial Growth Factor
Introduction
Oxygen administration is necessary to preterm infants with respiratory stress, but hyperoxia is shown to be correlated with several diseases in preterm infants [1], such as bronchopulmonary dysplasia (BPD) and Retinopathy of Prematurity (ROP) [2]. Under hyperoxia, the characteristic of injury includes inflammatory cell influx, endothelial and epithelial cell death, and vasogenesis suppression [3]. Vascular endothelial cells appear to be one of the major targets of hyperoxic injury. From the pathophysiology, hyperoxia disrupts the survival and functions of vascular endothelial cells, which contributes to the development of diseases [4]. Hyperoxia also alters Vascular Endothelial Growth Factor (VEGF) signaling pathway in both BPD and ROP [1], and high concentration oxygen administration releases Reactive Oxygen Species (ROS), which is believed to cause oxidative stress. Moreover, hyperoxic microenvironment is associated with multiple alternations in the extracellular and intracellular milieu of vascular endothelium that oxidative stress injury may act as inducers of apoptosis and cell death.
Ginseng, the root of Panax ginsengg, has been used for centuries as a component of traditional complementary medicine [5]. It could be used as a tonic agent to combat stress, or a medicine to improve cardio-pulmonary function. The molecular compositions of ginseng have been studied extensively. Ginsenosides are the constituents that responsible for the actions of ginseng, and Rg1 is among the most abundant and active components. Recent in vitro studies showed that ginsenoside-Rg1 is a proangiogenic factor of vascular endothelial cells [6]. In endothelial cell culture model, ginsenoside-Rg1 is capable of regulating VEGF through activation of Glucocorticoid Receptor (GR) and vascular modeling via endothelial nitric oxide synthease (eNOS) [5-10]. Whether ginsenoside-Rg1 could regulate vascular endothelial signaling under hyperoxic condition needs to be ascertained.
Literatures concerning the application of ginsenoside-Rg1 in protection of hyperoxia-induced vascular endothelium injury are limited. We hypothesize that the treatment of ginsengoid-Rg1 could involve in vascular endothelium signaling under hyperoxic condition. The vascular endothelial oxidative stress and cellular apoptosis were carefully evaluated.
Methods
Study design
In order to evaluate the effects of ginsenoside-Rg1 on HUVECs under hyperoxia, various concentrations of ginsenoside-Rg1 were added to endothelial cells cultured under normoxia and hyperoxia in the experiment period. Cell proliferation and protein production will be measured. In clinical condition, Fick’s first law is used to describe the exchange of oxygen in the lung capillaries, where oxygen passes from the alveoli through the alveolar membrane and entering the blood stream. Fick’s law can also be applied to cell culture in a similar manner if the oxygen concentration at the air-culture medium interface is considered similar to the oxygen concentration of gas at the surface of the alveolar membrane, and oxygen right above the cell monolayer is paralleled to the oxygen concentration in the blood stream [11]. Thus the culture atmosphere was set at 60% O2, which is similar to the oxygen percentage administrated to preterm infants with respiratory distress syndrome.
Cells
Primary human umbilical vein endothelial cells (HUVEC, CRL-1730TM) were used in our study. The cells were purchased from ATCC (Manassas, VA). Culture medium M199 and Endothelial Cell Growth Supplement (ECGS) were purchased from Sigma. Fetal Bovine Serum (FBS) was from Gibco.
Ginsenoside-Rg1
Experimental reagent ginsenoside-Rg1 is a reference compound, with purity around 97.7%, purchased from the Division of Chinese Material Medica and Natural Products, National Institute for the Control of Pharmaceutical and Biological Products, Ministry of Public Health, China. A stock solution of Ginsenoside-Rg1 50 mM was prepared in sterile double distilled H2O and stored at -80°C.
Antibodies
β-actin was used as a loading control. Anti-β-actin antibody was obtained from Millipore (Temecula, CA). Antibodies for VEGF, GR, GPx (Glutathione Peroxidase), Bax, Cyt c (cytochrome c), PARP (Poly ADP-ribose polymerase), caspase 3 were purchased from Cell Signaling Technology, Beverly, MA, USA. Cell proliferation was measured by MTT colorimetric assay (CytoSelect™ MTT Cell Proliferation Assay, CELL BIOLABS, INC.).
Cell Culture
HUVECs were cultured in medium M199 with 10% FBS, 25μg/ml ECGS, and 1% penicillin/streptomycin in an incubator at 37°C. After seeding, the cells were stabilized in incubator under room air over night before experiment. In the experiments, cells were divided into normoxia and hyperoxia groups. In normoxia group, cells remained in incubator supplied with 95% room air (approximately 20% O2) and 5% CO2. In hyperoxia group, cells were transferred to a chamber supplied with mixed air containing 60% O2, 35% N2, and 5% CO2. Culture medium was saturated with mixed air previously before experiments. The duration for experiments were 24, 48, and 72 hours after cells were exposed to normoxia or hyperoxia. In hyperoxia group, NexBiOxy Hypoxia/Hyperoxia System (NexBiOxy, Taiwan) was used to monitor and maintain the oxygen concentration in the culture chamber.
Ginsenoside-Rg1 administration
According to references, 0, 75, 150, 300nM of ginsenoside-Rg1 were used in our experiments [6]. Various concentrations of ginsenoside-Rg1 were prepared and added to the medium just before experiment. Medium was changed every day to keep ginsenoside-Rg1 within half-life period.
Western blot
HUVECs were washed in phosphate-buffered saline and then extracted with lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Triton, 0.1% SDS, and 0.5% sodium deoxycholate) and protease inhibitors. Lysates were centrifuged at 12,000 rpm for 5 min, and the resulting supernatant was collected. The extracted protein was quantized by protein assay. Aliquots (25 μg) of cellular lysate was separated by 8% sodium dodecyl sulphate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride (PVDF) membrane. After blocking with 5% BSA, blots were incubated with primary antibodies and then corresponding secondary antibodies. An enhanced chemiluminescence kit (Amersham, Piscataway, NJ) was used for immunodetection. The protein bands were quantified using the Image J software (NIH, Bethesda, MD).
Statistical analysis
Each experiment was repeated for at least three times. Data were expressed as means ± S.D. Statistical comparisons were carried out by one-way ANOVA for multiple groups or t test for two groups. Significance was accepted at the p ≤ 0.05 level and was marked with * in the figures.
Results
HUVECs proliferation under room air and 60% O2
We firstly assessed the impact of oxygen concentration on HUVEC proliferation in response to ginsenoside-Rg1. Under normoxic conditions (room air), HUVECs exhibited a time-dependent increase in cell number following Rg1 stimulation (Figure 1A), consistent with its reported pro-proliferative properties. In contrast, exposure to hyperoxia (60% O2) resulted in a significant reduction in HUVEC proliferation upon Rg1 treatment (Figure 1B). These findings suggest that ginsenoside-Rg1 may exacerbate oxygen-induced cytostatic effects, impairing endothelial cell expansion under hyperoxic stress, while exerting no inhibitory influence under physiological oxygen levels.
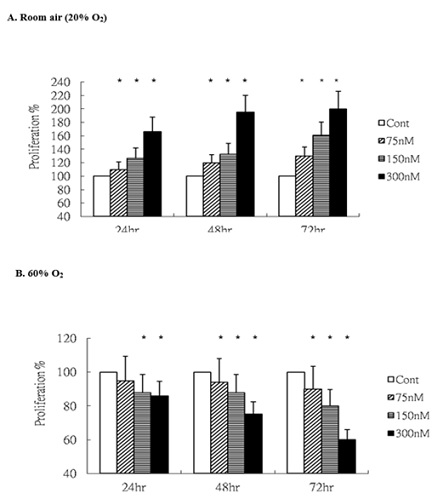 Figure 1: Rg1 downregulates HUVECs proliferation under 60% O2. HUVECs exposed to room air or 60% O2 in paired chambers and treated with various concentrations of Rg1 for 24, 48 and 72hr, respectively. Cell proliferation was determined by colorimetry based on the uptake of MTT. (A). Under room air condition, the total number of HUVECs increased gradually in a time dependent fashion in response to Rg1 stimulation. (B). Under 60% O2 condition, the total number of HUVECs was significantly decreased in response to increasing concentrations of Rg1 (75, 150, and 300nM) stimulation. *P < 0.05 vs. Control. n=5.
Figure 1: Rg1 downregulates HUVECs proliferation under 60% O2. HUVECs exposed to room air or 60% O2 in paired chambers and treated with various concentrations of Rg1 for 24, 48 and 72hr, respectively. Cell proliferation was determined by colorimetry based on the uptake of MTT. (A). Under room air condition, the total number of HUVECs increased gradually in a time dependent fashion in response to Rg1 stimulation. (B). Under 60% O2 condition, the total number of HUVECs was significantly decreased in response to increasing concentrations of Rg1 (75, 150, and 300nM) stimulation. *P < 0.05 vs. Control. n=5.
Levels of VEGF
Next, we evaluated VEGF expression to elucidate its regulatory role in vascular endothelial cell proliferation under both normoxic and hyperoxic conditions. Under room air, the levels of VEGF were significantly elevated in response to ginsenoside-Rg1 treatment in a time-dependent fashion (Figure 2A). Under 60% O2 condition, ginsenoside-Rg1 significantly decreased the levels of VEGF in a time-dependent fashion compared with control (Figure 2B). The inhibition of VEGF expressions indicating the ginsenoside-Rg1 could damper the potential vasogenesis under hyperoxic condition.
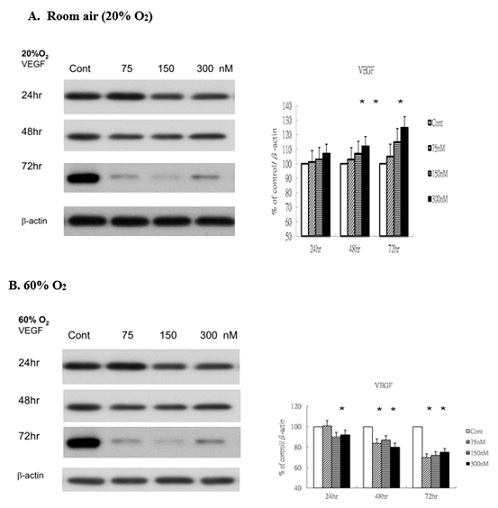 Figure 2: Rg1 does not induce VEGF production in HUVECs under 60% O2. HUVECs exposed to room air or 60% O2 in paired chambers and treated with various concentrations of Rg1 for 24, 48 and 72hr, respectively. The levels of VEGF were determined by Western blot. b-actin was used as the loading control. The signal intensities were determined by densitometry. Data are shown as mean ± SD of three independently experiments. (A). Under room air condition, the levels of VEGF were significantly elevated in response to Rg1 stimulation. Treatment with increasing concentrations of Rg1 (75, 150, and 300nM) increased the level of VEGF. The stimulatory effect of Rg1 on VEGF production on HUVECs was time-dependent. (B). Under 60% O2 condition, Rg1 significantly decreased the levels of VEGF compared with control. The inhibitory effect of Rg1 on decreased VEGF production was time-dependent. *P < 0.05 vs. Control.
Figure 2: Rg1 does not induce VEGF production in HUVECs under 60% O2. HUVECs exposed to room air or 60% O2 in paired chambers and treated with various concentrations of Rg1 for 24, 48 and 72hr, respectively. The levels of VEGF were determined by Western blot. b-actin was used as the loading control. The signal intensities were determined by densitometry. Data are shown as mean ± SD of three independently experiments. (A). Under room air condition, the levels of VEGF were significantly elevated in response to Rg1 stimulation. Treatment with increasing concentrations of Rg1 (75, 150, and 300nM) increased the level of VEGF. The stimulatory effect of Rg1 on VEGF production on HUVECs was time-dependent. (B). Under 60% O2 condition, Rg1 significantly decreased the levels of VEGF compared with control. The inhibitory effect of Rg1 on decreased VEGF production was time-dependent. *P < 0.05 vs. Control.
Levels of GR
To explore the potential involvement of glucocorticoid signaling in Rg1-mediated endothelial responses, we next examined the expression of the Glucocorticoid Receptor (GR) under both normoxic and hyperoxic conditions. Under room air, GR levels were significantly elevated following ginsenoside-Rg1 treatment (Figure 3A). Similarly, under 60% O2, Rg1 induced a marked increase in GR expression at 72 hours compared with control (Figure 3B). This upregulation suggests that ginsenoside-Rg1 may enhance vascular signal transduction pathways mediated by GR, particularly under hyperoxic stress.
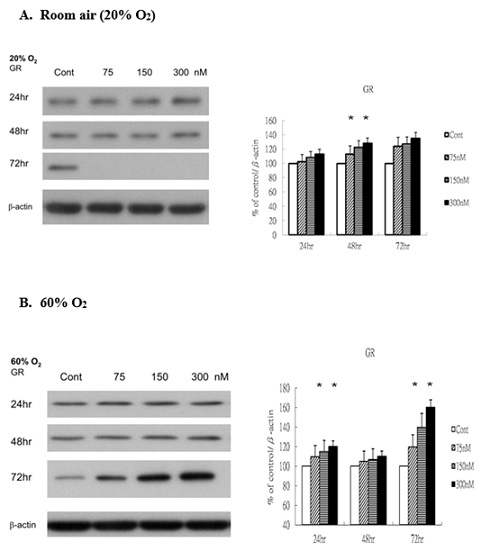 Figure 3: Rg1 induces GR production in HUVECs under 60% O2. HUVECs exposed to room air or 60% O2 in paired chambers and treated with various concentrations of Rg1 for 24, 48 and 72hr, respectively. The levels of GR (glucocoticoid receptor) were determined by Western blot. b-actin was used as the loading control. The signal intensities were determined by densitometry. Data are shown as mean ± SD of three independently experiments. (A). Under room air condition, the levels of GR were significantly elevated in response to Rg1 stimulation. Treatment with increasing concentrations of Rg1 (75, 150, and 300nM) increased the levels of GR. (B). Under 60% O2 condition, Rg1 elevated the levels of GR compared with control. Treatment with increasing concentrations of Rg1 (75, 150, and 300nM) elevated significantly the level of GR at 72hr. *P < 0.05 vs. Control.
Figure 3: Rg1 induces GR production in HUVECs under 60% O2. HUVECs exposed to room air or 60% O2 in paired chambers and treated with various concentrations of Rg1 for 24, 48 and 72hr, respectively. The levels of GR (glucocoticoid receptor) were determined by Western blot. b-actin was used as the loading control. The signal intensities were determined by densitometry. Data are shown as mean ± SD of three independently experiments. (A). Under room air condition, the levels of GR were significantly elevated in response to Rg1 stimulation. Treatment with increasing concentrations of Rg1 (75, 150, and 300nM) increased the levels of GR. (B). Under 60% O2 condition, Rg1 elevated the levels of GR compared with control. Treatment with increasing concentrations of Rg1 (75, 150, and 300nM) elevated significantly the level of GR at 72hr. *P < 0.05 vs. Control.
Levels of GPx
To investigate the potential antioxidant response elicited by ginsenoside-Rg1, we analyzed cytosolic glutathione peroxidase (GPx) levels in HUVECs following treatment. Under normoxic conditions, GPx expression remained unchanged in response to Rg1 (Figure 4A). In contrast, under hyperoxic exposure (60% O2), GPx levels were significantly upregulated following Rg1 treatment (Figure 4B). Notably, increasing concentrations of Rg1 (75, 150, and 300 nM) induced a dose- and time-dependent elevation in GPx expression. These findings suggest that ginsenoside-Rg1 may enhance antioxidant defense mechanisms under hyperoxic stress, potentially contributing to redox regulation in vascular endothelial cells.
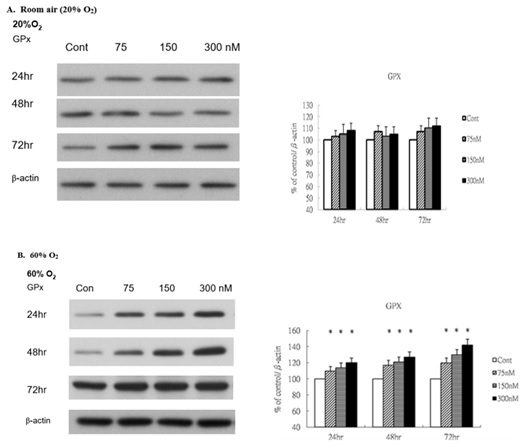 Figure 4: Rg1 induces glutathione peroxidase production in HUVECs under 60% O2. HUVECs exposed to room air or 60% O2 in paired chambers and treated with various concentrations of Rg1 for 24, 48 and 72hr, respectively. The levels of glutathione peroxidase (GPx) were determined by Western blot. b-actin was used as the loading control. The signal intensities were determined by densitometry. Data are shown as mean ± SD of three independently experiments. (A). Under room air condition, the levels of GPx did not change in response to Rg1 stimulation. (B). Under 60% O2 condition, the levels of GPx were significantly elevated in response to Rg1 stimulation. Treatment with increasing concentrations of Rg1 (75, 150, and 300nM) increased the level of GPx. The stimulatory effect of Rg1 on GPx production was time-dependent. *P < 0.05 vs. Control.
Figure 4: Rg1 induces glutathione peroxidase production in HUVECs under 60% O2. HUVECs exposed to room air or 60% O2 in paired chambers and treated with various concentrations of Rg1 for 24, 48 and 72hr, respectively. The levels of glutathione peroxidase (GPx) were determined by Western blot. b-actin was used as the loading control. The signal intensities were determined by densitometry. Data are shown as mean ± SD of three independently experiments. (A). Under room air condition, the levels of GPx did not change in response to Rg1 stimulation. (B). Under 60% O2 condition, the levels of GPx were significantly elevated in response to Rg1 stimulation. Treatment with increasing concentrations of Rg1 (75, 150, and 300nM) increased the level of GPx. The stimulatory effect of Rg1 on GPx production was time-dependent. *P < 0.05 vs. Control.
Levels of Bax and Cytochrome c
Because GPx levels remained unchanged under normoxic conditions, implying minimal oxidative stress encounted, we subsequently examined the oxidative stress markers Bax and cyt c under hyperoxic conditions to evaluate whether ginsenoside-Rg1 exacerbates oxidative stress signaling. Under 60% O2 condition, the levels of Bax (Figure 5A) and Cyt c (Figure 5B) increased significantlyin a dose- and time- dependent manner when compared with those in control.
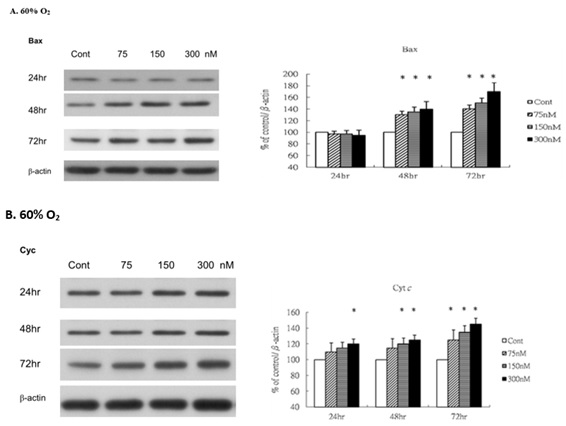 Figure 5: Rg1 increases Bax and cytochrome c (Cyt c) production in HUVECs under 60% O2. HUVECs exposed to room air or 60% O2 in paired chambers and treated with various concentrations of Rg1 for 24, 48 and 72hr, respectively. The levels of Bax and cytochrome c (Cyt c) were determined by Western blot with b-actin used as the loading control. The signal intensities were determined by densitometry. Data are shown as mean ± SD of three independently experiments. (A). Under 60% O2 condition, the levels of Bax were significantly elevated in response to Rg1 stimulation. Treatment with increasing concentrations of Rg1 (75, 150, and 300nM) increased the level of Bax. The stimulatory effect of Rg1 on Bax production was time-dependent. (B). Under 60% O2 condition, the levels of Cyt c were significantly elevated in response to Rg1 stimulation. Treatment with 300nM Rg1 increased the level of Cyt c. The stimulatory effect of Rg1 on Cyt c production was time-dependent. *P < 0.05 vs. Control.
Figure 5: Rg1 increases Bax and cytochrome c (Cyt c) production in HUVECs under 60% O2. HUVECs exposed to room air or 60% O2 in paired chambers and treated with various concentrations of Rg1 for 24, 48 and 72hr, respectively. The levels of Bax and cytochrome c (Cyt c) were determined by Western blot with b-actin used as the loading control. The signal intensities were determined by densitometry. Data are shown as mean ± SD of three independently experiments. (A). Under 60% O2 condition, the levels of Bax were significantly elevated in response to Rg1 stimulation. Treatment with increasing concentrations of Rg1 (75, 150, and 300nM) increased the level of Bax. The stimulatory effect of Rg1 on Bax production was time-dependent. (B). Under 60% O2 condition, the levels of Cyt c were significantly elevated in response to Rg1 stimulation. Treatment with 300nM Rg1 increased the level of Cyt c. The stimulatory effect of Rg1 on Cyt c production was time-dependent. *P < 0.05 vs. Control.
Levels of active Caspase 3 and cleaved Poly ADP-Ribose Polymerase (PARP)
We next sought to determine whether vascular endothelium apoptosis could be influenced after ginsenoside-Rg1 treatment, the Western blot was performed. Under 60% O2 condition, the levels of active Caspase 3 (Figure 6A) and cleaved PARP (Figure 6B) increased significantly when compared with those in control. The levels of active caspase 3 (cleaved 17 and 19 kDa) were significantly increased under 60% O2. Similar to active caspase 3, immunoblotting analysis of cleaved PARP showed that the C-terminal 85 kDa PARP apoptotic fragment was significantly generated under 60% O2, indicating vascular endothelium apoptosis has upregulated after ginsenoside-Rg1 treatment.
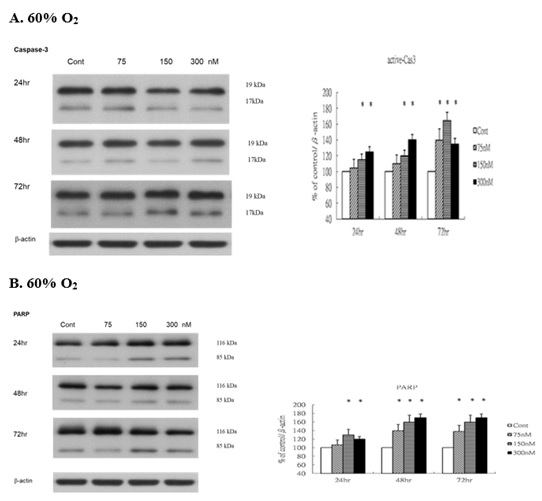 Figure 6: Rg1 increases proapoptotic active caspase 3 and Poly (ADP-ribose) polymerase (PARP) production in HUVECs under 60% O2. HUVECs exposed to room air or 60% O2 in paired chambers and treated with various concentrations of Rg1 for 24, 48 and 72hr, respectively. The levels of active caspase 3 and PARP were determined by Western blot with b-actin used as the loading control. The signal intensities were determined by densitometry. Data are shown as mean ± SD of three independently experiments. (A). Under 60% O2 condition, the levels of caspase 3 were significantly elevated in response to Rg1 stimulation. Treatment with increasing concentrations of Rg1 (75, 150, and 300nM) increased the level of active caspase 3. The stimulatory effect of Rg1 production was time-dependent. (B). Under 60% O2 condition, the levels of PARP were significantly elevated in response to Rg1 stimulation. Treatment with increasing concentrations of Rg1 (75, 150, and 300nM) increased the level of PARP. The stimulatory effect of Rg1 on PARP production was time-dependent. *P < 0.05 vs. Control.
Figure 6: Rg1 increases proapoptotic active caspase 3 and Poly (ADP-ribose) polymerase (PARP) production in HUVECs under 60% O2. HUVECs exposed to room air or 60% O2 in paired chambers and treated with various concentrations of Rg1 for 24, 48 and 72hr, respectively. The levels of active caspase 3 and PARP were determined by Western blot with b-actin used as the loading control. The signal intensities were determined by densitometry. Data are shown as mean ± SD of three independently experiments. (A). Under 60% O2 condition, the levels of caspase 3 were significantly elevated in response to Rg1 stimulation. Treatment with increasing concentrations of Rg1 (75, 150, and 300nM) increased the level of active caspase 3. The stimulatory effect of Rg1 production was time-dependent. (B). Under 60% O2 condition, the levels of PARP were significantly elevated in response to Rg1 stimulation. Treatment with increasing concentrations of Rg1 (75, 150, and 300nM) increased the level of PARP. The stimulatory effect of Rg1 on PARP production was time-dependent. *P < 0.05 vs. Control.
Discussion
Ginseng has been used as a tonic remedy in traditional complementary medicine for over 2000 years. In recent years, many beneficial effects of ginsenoside-Rg1, one of the major active ingredients of ginseng, have been reported. The effects include increasing glucose uptake, relieving oxidative stress, suppressing adipocyte development and possible neuroprotective role [12]. It is a potent proangiogenic factor of vascular endothelial cells and stimulator of VEGF expression in human umbilical vein endothelial cells [6]. We hypothesize that treatment with ginsenoside Rg1 may participate in modulating vascular endothelial signaling under hyperoxia.
In this study, Human Umbilical Vein Endothelial Cells (HUVECs) were cultured under normoxic (room air) and hyperoxic (60% O2) conditions, followed by administration of ginsenoside Rg1. Endothelial proliferation, oxidative stress, and apoptosis were systematically assessed. Under normoxia, Rg1 significantly enhanced HUVEC proliferation and VEGF expression in a time- and concentration-dependent manner, whereas these effects were markedly attenuated under hyperoxic exposure. Ginsenoside Rg1 has been reported to activate the Glucocorticoid Receptor (GR), a pivotal regulator involved in initiating angiogenic signaling pathways [5,13]. Consistent with these findings, our data demonstrate that Rg1 treatment upregulated GR expression in HUVECs under both normoxic and hyperoxic conditions, with the highest GR levels observed following 72-hour exposure to hyperoxia. Previous studies have shown that the proangiogenic effects of ginsenoside Rg1 are mediated through VEGF production via glucocorticoid receptor (GR)-dependent signaling pathways in HUVECs [5,7,13]. In contrast, our findings under hyperoxic conditions revealed increased GR expression accompanied by reduced VEGF levels following Rg1 treatment, suggesting activation of GR-dominant signaling independent of VEGF-mediated angiogenesis. Notably, Rg1 has been identified as a GR agonist capable of inducing nitric oxide production via endothelial nitric oxide synthase [9], and exerting anti-inflammatory effects through GR-mediated pathways [14,15]. These observations imply that under hyperoxia, GR-dominant signaling triggered by Rg1 may contribute primarily to vascular anti-inflammatory responses rather than angiogenic activity [16,17].
Glutathione peroxidase (GPx), a vital antioxidant enzyme within the selenoprotein family, mitigates oxidative damage by catalyzing the reduction of lipid hydroperoxides to alcohols and converting hydrogen peroxide into water. In our study, HUVECs exposed to hyperoxia and treated with ginsenoside Rg1 exhibited a significant, time- and dose-dependent increase in GPx expression. This upregulation suggests that Rg1 may activate intrinsic antioxidant defense mechanisms to counteract hyperoxia-induced oxidative stress. Supporting this interpretation, previous studies have also demonstrated that Rg1 confers cytoprotection against oxidative injury through modulation of the glutathione system [18-20].
Hyperoxia has been shown to induce cell death in vascular endothelial cells, primarily via apoptotic pathways driven by the accumulation of reactive oxygen species and inflammatory mediators [21-25]. In our study, ginsenoside Rg1 treatment under hyperoxic conditions resulted in a concentration-dependent increase in the expression of Bax, active caspase-3, cytochrome c (Cyt c), and PARP, indicating activation of intrinsic apoptotic signaling.
Bax, a pro-apoptotic member of the Bcl-2 protein family localized to the outer mitochondrial membrane, plays a pivotal role in initiating apoptosis through the release of cytochrome c (Cyt c) and subsequent caspase activation [26,27]. Our findings suggest that higher concentrations of ginsenoside Rg1 under hyperoxic conditions may exacerbate apoptosis in HUVECs, as evidenced by elevated levels of Bax and Cyt c. To further assess whether Cyt c release reflects hyperoxia-induced apoptotic progression, we examined the cleavage of poly (ADP-ribose) Polymerase (PARP), a hallmark of programmed cell death. Notably, increased levels of the cleaved 85 kDa PARP fragment were observed, consistent with activation of a PARP-dependent apoptotic pathway involving apoptosis-inducing factor translocation [28]. The dose-dependent activation of PARP in response to Rg1 under hyperoxia underscores its potential to aggravate vascular endothelial cell death.
Under hyperoxic conditions (60% O2), ginsenoside Rg1 did not promote vascular endothelial cell proliferation or VEGF expression, indicating its limited capacity to counteract hyperoxia-induced endothelial injury. Additionally, the dose-dependent upregulation of pro-apoptotic markers including Bax, cytochrome c, active caspase-3, and cleaved PARP suggests that Rg1 may exacerbate apoptotic signaling under oxidative stress. Conversely, the observed elevation of GR and GPx points to a potential involvement of Rg1 in modulating vascular redox signaling and antioxidant defense mechanisms.
Conclusion
These findings highlight the dualistic nature of ginsenoside Rg1’s bioactivity in hyperoxic environments and underscore the need for further mechanistic studies to clarify its role in endothelial survival and stress adaptation.
Ethics Approval and Consent to Participate
Not applicable. Since there is no patient involvement, no ethics approval and consent required.
Funding Declaration
Chang Gung Memorial Hospital, Linkou Branch, research project CMRPG490021 and CMRP3D0971.
Acknowledgement
This research was supported by Chang Gung Memorial Hospital, Linkou Branch, with research projects (CMRPG490021, CMRP3D0971).
Consent for Publication
Not applicable.
Availability of Data and Materials
All datasets generated and analyzed during the current study are not publicly available because not yet published, but are available from the corresponding author on reasonable request.
Competing Interests
There are no financial or non-financial competing interests.
Authorship Contribution Statement
Ren-Huei Fu: research idea, literature review, protocol design, experiment processing, data analysis and discussion, manuscript writing. Chi-Nan Tseng: literature review, protocol design, data analysis and manuscript discussion. Cih-Yi Yen & Yu-Hsueh Cho: protocol design, experiment processing, data collecting. Yen Chu: research idea, literature review, protocol design and improvement, experiment guiding and processing, data analysis and discussion, manuscript writing and refinement.
References
- Fujinaga H, Baker CD, Ryan SL, Markham NE, Seedorf GJ, et al. (2009) Hyperoxia disrupts vascular endothelial growth factor-nitric oxide signaling and decreases growth of endothelial colony-forming cells from preterm infants. Am J Physiol Lung Cell Mol Physiol 297: 1160-1169.
- Wacker J, Beghetti M (2025) Pulmonary hypertension in pediatrics: from clinical suspicion to management. Eur J Pediatr 184: 288.
- Lahm T, Crisostomo PR, Markel TA, Wang M, Lillemoe KD, et al. (2007) The critical role of vascular endothelial growth factor in pulmonary vascular remodeling after lung injury. Shock 28: 4-14.
- Hosford GE, Olson DM (2003) Effects of hyperoxia on VEGF, its receptors, and HIF-2α in the newborn rat lung. Am J Physiol Lung Cell Mol Physiol 285: 161-168.
- Leung KW, Pon YL, Wong RNS, Wong AST (2006) Ginsenoside-Rg1 induces vascular endothelial growth factor expression through the glucocorticoid receptor-related phosphatidylinositol 3-kinase/Akt and β-catenin/T-cell factor-dependent pathway in human endothelial cells. J Biol Chem 281: 36280-36288.
- Shi AW, Wang XB, Lu FX, Zhu MM, Kong XQ, et al. (2009) Ginsenoside Rg1 promotes endothelial progenitor cell migration and proliferation. Acta Pharmacol Sin 30: 299-306.
- Cheung LWT, Leung KW, Wong CKC, Wong RNS, Wong AST (2011) Ginsenoside-Rg1 induces angiogenesis via non-genomic crosstalk of glucocorticoid receptor and fibroblast growth factor receptor-1. Cardiovasc Res 89: 419-425.
- Du J, Cheng B, Zhu X, Ling C (2011) Ginsenoside Rg1, a novel glucocorticoid receptor agonist of plant origin, maintains glucocorticoid efficacy with reduced side effects. J Immunol 187: 942-950.
- Leung KW, Cheng YK, Mak NK, Chan KKC, Fan TPD, et al. (2006) Signaling pathway of ginsenoside-Rg1 leading to nitric oxide production in endothelial cells. FEBS Lett 580: 3211-3216.
- Mohanan P, Subramaniyam S, Mathiyalagan R, Yang DC (2018) Molecular signaling of ginsenoside Rb1, Rg1, and Rg3 and their mode of actions. J Ginseng Res 42: 123-132.
- Place TL, Domann FE, Case AJ (2017) Limitations of oxygen delivery to cells in culture: An underappreciated problem in basic and translational research. Free Radic Biol Med 113: 311-322.
- Xie W, Zhou P, Sun Y, Meng X, Dai Z, et al. (2018) Protective effects and target network analysis of Ginsenoside Rg1 in cerebral ischemia and reperfusion injury: A comprehensive overview of experimental studies. Cells 7: 270.
- Sung WN, Kwok HH, Rhee MH, Yue PYK (2017) Korean Red Ginsengg extract induces angiogenesis through activation of glucocorticoid receptor. J Ginsengg Res 41: 477-486.
- Garatti G, Matthews L, Poolman T, Kershaw S, Baxter M, et al. (2015) Glucocorticoid receptor function in health and disease. Clin Endocrinol (Oxf) 83: 441-448.
- Gao Y, Chu S, Li J, Li J, Zhang Z, et al. (2015) Anti-inflammatory function of ginsenoside Rg1 on alcoholic hepatitis through glucocorticoid receptor related nuclear factor-kappa B pathway. J Ethnopharmacol 173: 231-240.
- Fan X, Zhang Y, Li X, Ding J, Huang J, et al. (2025) Unraveling Ginsenoside Rg1’s osteoprotective pathways in zebrafish models of glucocorticoid-induced osteoporosis via transcriptomics. Sci Rep 15: 15284-15296.
- Leung KW, Leung FP, Huang Y, Mak NK, Wong RNS (2007) Non-genomic effects of ginsenoside-Re in endothelial cells via glucocorticoid receptor. FEBS Lett 581: 2423-2428.
- Liu Q, Kou JP, Yu BY (2011) Ginsenoside Rg1 protects against hydrogen peroxide-induced cell death in PC12 cells via inhibiting NF-κB activation. Neurochem Int 58: 119-125.
- Fernández-Moriano C, González-Burgos E, Iglesias I, Lozano R, Gómez-Serranillos MP (2017) Evaluation of the adaptogenic potential exerted by ginsenosides Rb1 and Rg1 against oxidative stress-mediated neurotoxicity in an in vitro neuronal model. PLoS ONE 12: 0182933.
- Bi S, Ma X, Wang Y, Chi X, Zhang Y, et al. (2019) Protective effect of Ginsenoside Rg1 on oxidative damage induced by hydrogen peroxide in chicken splenic lymphocytes. Oxid Med Cell Longev 18: 8465030.
- Hafner C, Wu J, Soto-Gonzalez L, Kaun C, Stojkovic S, et al. (2017) Moderate hyperoxia induces inflammation, apoptosis and necrosis in human umbilical vein endothelial cells. Eur J Anaesthesiol 34: 141-149.
- Hurskainen M, Mižíková I, Cook DP, Schittny JC, Haider T, et al. (2021) Single-cell transcriptomic analysis of murine lung development on hyperoxia-induced damage. Nat Commun 12: 1565-1578.
- Hanidziar D, Nakahori Y, Cahill LA, Gallo D, Keegan JW, et al. (2020) Characterization of pulmonary immune responses to hyperoxia by high-dimensional mass cytometry analyses. Sci Rep 10: 61489-61498..
- Wu J, Zhang G, Xiong H, Li Y, Zhao Y, et al. (2021) miR-181c-5p mediates apoptosis of vascular endothelial cells induced by hyperoxemia via ceRNA crosstalk. Sci Rep 11: 16440-16452.
- Hafner C, Wu J, Soto-Gonzalez L, Kaun C, Stojkovic S, et al. (2017) Moderate hyperoxia induces inflammation, apoptosis, and necrosis in human umbilical vein endothelial cells. Eur J Anaesthesiol 34: 141-149.
- Yamazaki T, Galluzzi L (2022) BAX and BAK dynamics control mitochondrial DNA release during apoptosis. Cell Death Differ 29: 1296-1298.
- Cosentino K, Hertlein V, Jenner A, Dellmann T, Gojkovic M, et al. (2022) The interplay between BAX and BCL-XL tunes apoptotic pore growth to control mitochondrial-DNA-mediated inflammation. Mol Cell 82: 933-949.
- Morales JC, Li L, Fattah FJ, Dong Y, Bey EA, et al. (2014) Review of Poly (ADP-ribose) Polymerase (PARP) mechanisms of action and rationale for targeting in cancer and other diseases. Crit Rev Eukaryot Gene Expr 24: 15-28.
Citation: Fu R-H, Tseng C-N, Yen C-Y, Cho Y-H, Chu Y (2025) Application of Ginsenoside-Rg1 Enhanced Glutathione Peroxidase Activity but could not Ameliorate Hyperoxia-Induced Vascular Endothelial Oxidative Stress and Apoptosis. HSOA J Altern Complement Integr Med 11: 643.
Copyright: © 2025 Ren-Huei Fu, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

