
Assessment of 35 Years Longitudinal Observations on Melanoma Patients in a Single Center of Hungary’s Central Region: Effect of Interferon Immunotherapy
*Corresponding Author(s):
Szilvia ZámolyiDepartment Of Dermatology, Central Hospital Of The Hungarian Defense Forces, Hungary
Tel:+36 203739754,
Email:szilvi.zamolyi@gmail.com
Abstract
Background: The incidence of melanoma malignum increases worldwide. Detailed objective analyses from central European region are scarcely available.
Objective: To obtain validated data on melanoma distribution and retrospective analysis in the central region of Hungary based on 35 years of experience in a single center.
Methods: The authors monitored the clinical course of their patients with melanoma between 1984 and 2018 based on the digitalized documentation of their institute. They analyzed the treated and non-treated cases with the leadership of a dermatologist-pathologist-oncologist.
Altogether 867 cases (450 female, 417 male) were examined from the removal of the primary tumor onwards in retrospective way.
Results: Distribution of gender, age, tumor stages and body regions by genders were examined. The authors also reviewed the asymptomatic period until tumor progression, morbidity data, the period and effect of the adjuvant low, intermediate and high dose interferon α-2b immunotherapy. Patients with lymph node metastasis who received low or intermediate dose immunotherapy experienced significantly longer tumor-free period than those who got high-dose immunotherapy. In both treated groups, the median value of the asymptomatic months was higher compared to the observation only” group.
Conclusion: The results of their long-term follow-up contribute to the accurate evaluation of melanoma and of the interferon therapy in Hungary and in the region.
Keywords
Melanoma in central-eastern europe; Interferon α- 2b therapy; Malignant melanoma; Melanoma prevalence in one centrum
Introduction
In the past decades, several analyses were published concerning the increase in the incidence and prevalence of malignant melanoma in industrially developed countries [1-4]. The comparison of European trends clearly showed years ago that the increase in incidence was highest in Northern and Northwestern countries such as Sweden, Netherlands, Great Britain, and Ireland and it was lowest in continental Europe on the Iberian Peninsula (Spain, Portugal) [1]. Detailed German data highlighted the important role of regional UV and sunshine irradiation differences as well as socioeconomic factors and education [3]. It was suggested as soon as in 2009 that the incidence of melanoma increases in Central and Eastern European countries as well. However, incidences were 1 order of magnitude lower than in “wealthy” Western European countries. Data were only provided for the Baltic States, Belarus and Serbia [5]. Similarly, 5-year melanoma survival statistics of 21 countries created on the basis of EUROCARE 3 and 4 include data only from the Czech Republic, Slovenia and Poland. Data from Hungary and other regions of the Carpathian Basin were missing [6]. On the other hand, the Hungarian National Cancer Registry provided accurate numbers on the development of the incidence of melanoma in the period from 2001 and 2015.These can also be obtained separately for the 19 counties and Budapest [7]. By selecting specific time points (2001, 2009, 2015), it shows that incidence increased continuously throughout the country (12.5, 20.4., and 28.3 per 100,000 population, respectively). When compared with the Czech data from the period between 1994 and 2004, close correlation was detected [6]. Our data represent the population living in the central Hungarian region that gives approximately 30% of our country’s population. It needs to be emphasized that in the above years, the incidence of melanoma was approximately 3.5 times higher than the national average (47, 73 and 103 cases per 100 000 population, respectively, ref 7). In the National Institute of Oncology, 2972 patients from the same region were registered between 2003 and 2015 [8]. Further domestic data regarding the period from 2000 to 2014 were published from the database of the Department of Dermatology, University of Debrecen. These publications summarize the data from Hajdú-Bihar County where UV radiation is the highest. Increase of incidence which was a few percentage points above the national average was observed between 2001 and 2007, and then the occurrence of new cases was stagnating at this level until 2014. In addition to increasing awareness within the population, early diagnosis of “thinner” primary tumors may have played a role in this. It was observed that in men over 60 years of age the increase in incidence and the occurrence of “thicker” tumors was still significant [9]. Our study aimed on one hand to present our melanoma prevalence data representing the central region of Hungary in the Carpathian Basin which area is deficiently documented according to the European professional general opinion and to draw important conclusions from these regarding the adjuvant interferon therapy that we used continuously.
Materials and Methods
As a clinician since 1984, pathologist since 1988 and clinical oncologist since 1997, the head of our team (A.V.) has been diagnosing and treating out- and in-patients in our progression level 3 university teaching hospital with special regard to malignant melanoma (ICD 10; C43). Data have been recorded in a computer system and followed in MedSolution™ from 1998 to 2003 and in the “MedWorks” program of GlobeNet® Computer Technology Inc. (Budapest, Hungary) since 2003. Computing medical records from between 1984 and 1998 in the system was performed retrospectively. After query by diagnosis, the variables that we considered as most important were individually refined in a data mask for the 867 patients [10]. The date of primary tumor removal was considered as starting point. The extracted data were recorded on Excel tables and were statistically analyzed. For this end, MedCalc program package was used. Significance threshold was 0.05. Uncertain data were also indicated (unk=unknown). Sentinel lymph node removal and its histological testing started in 2007 in our Institute. For the above research institutional Ethics Committee approval was obtained. Patients receiving low to intermediate dose adjuvant IFN therapy were given 5-10 IU Interferon a-2b (Intron A®) 3 times a week for 18 months [11]. For patients receiving high-dose adjuvant IFN therapy the SmPC of Intron A was followed [12].
Results
Demographic data:
Total number of patients: 867. Women = 450 (mean age 52 ± 16.9 SD; range 17 to 94). Men = 417 (mean age 57.2 ± 17.4 SD; range 14 to 100). Women aged 17 to 40 = 124 (1.59 times the number of men): Men aged 61 to 100 = 339 (2.07 times the number of women). Both differences are meant within the same age groups. Median observation time for all patients was 72.5 months.
Trends of melanoma prevalence
The observation time equaling 34 years was divided to 3 phases. Phase 1. 1984-2001; phase 2. 2002-2009; phase 3. 2010-2018. In the entire Phase 1, and the first half of Phase 2, our patients consisted of employees of the Hungarian Army and their families with nationwide coverage. In the second half of Phase 2, in addition to the above scope of patients, several other patients presented from some districts in Budapest and the north-western region of Hungary due to a reorganization as well. This expansion made our Institute accessible for 1/6th to 1/5th of the population of Hungary in Phase 3. Before 2008 the annual rates of new patients had been almost constant but after 2008, the annual increase became almost linear. The number of newly diagnosed patients in the year of primary tumor excision varied between min. 45 (2008) and max. 80 (2018) in this period. The real large-scale increase in the prevalence of melanoma took place in the 3rd period (2010 to 2018) as in this period the total number of attending novel dermatology patients increased significantly less than between 2008 and 2010.
It can be seen that besides the in situ group being identical, the proportion of stage 1a-b is 21% higher in women as well as stage 4a is 2.5 times and 4b is 20% more common in men than is in women, respectively. The other differences between genders are not significant. In 26 patients in total, the classification according to primary tumor stage was not feasible (Figure 1).
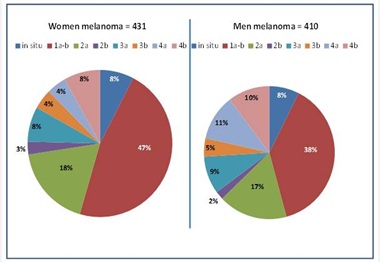 Figure 1: Primary tumor stages by gender.
Figure 1: Primary tumor stages by gender.
Figure 2 shows that the most common localization in men was the trunk. The percentage of lower limb localization was less than half of trunk localization. In women, although lower limb was affected 11% more often than in men, this body region represents a similar ratio as trunk.
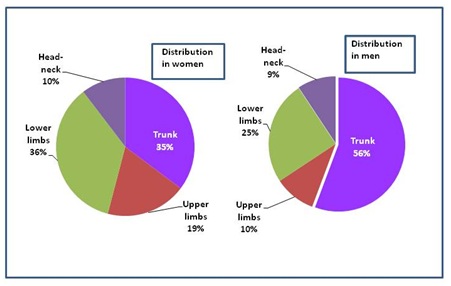 Figure 2: Primary tumor localization on the body surface.
Figure 2: Primary tumor localization on the body surface.
In women, upper limb localization showed a decreasing trend in the entire observation period (from 25% to 14%), while it was not typical in men; the initial 13% frequency decreased by half in the second phase and returned to baseline value in the third phase of the observation period. The incidence of head and neck melanoma was continuously low in both genders. The percentage of trunk localization decreased from the initial 70% by half between 2002 and 2010 and increased to almost the initial percentage in Phase 3. In women, the fluctuation of trunk localization was mild and in total, lain below the levels seen in men. The share of lower limbs in women was nearly identical, while in men, it displayed counter-fluctuation as compared with the trunk and upper limbs.
In patients with in situ melanoma, no metastases were detected during the observation following excision. The incidence of late metastases (lymph node, other visceral and remote cutaneous ones together) in stage 1a was infinitesimally small in both genders (1.7% in women and 2.1% in men).Our only metastatic patient with stage 1b was a man. He received low to intermediate dose IFN treatment. In men, as the stage progressed, the occurrence of metastases became more and more frequent. In women, the ulcerated nature of the tumors has significantly increased metastatic ability. In stage 4b, the development of metastases affected approximately 50% of patients in both genders, taking into consideration the median 72 months of observation.
The number of men and women dying in our Institute was 30 and 15, respectively. There was no mortality among patients with in situ melanoma. In the other stages, the mortality increased with the progression of stages and in the case of stage 3 and stage 4 tumors, mortality was higher in patients with ulcerated tumors. It was especially true for women. Mortality rates followed the ratio of late metastases albeit with significantly lower case numbers.
After deducting the number of in situ melanoma and uncertain (unk) cases, 1/4th of the 652 patients who could be classified according to stages 1-4 received adjuvant treatment. Most of our patients (19%) received low to intermediate dose treatment and a small proportion of them (6%) was given high dose. The duration of treatment was preferably 12 months. IFN therapy has been given since autumn 1997. Table 1 shows the median values of symptom-free months per stage in the treated and non-treated groups. The median values of tumor-free months in treated patients had always been higher than those of patients who were observed only.
|
Stages |
Male-female obs. only (month) |
Male-female low to intermed. IFN-2b (month) |
|
1a |
54 |
117.5 |
|
1b |
50* |
63** |
|
2a |
30 |
102 |
|
2b |
30 |
84 |
|
3a |
32 |
80 |
|
3b |
13 |
61 |
|
4a |
20 |
63 |
|
4b |
12.5 |
36 |
Table 1: Median values of tumor-free months as a function of melanoma stages.
*mean of 11 patients
**one treated patient
The figures clearly demonstrated that comparisons showed significantly higher mean number of symptom-free months in each stage, except for stages 3a and 4b. In stage 3a, the difference was marked but not significant in favor for the treated patients (Figures 3&4). The same could be seen in the course of median values of symptom-free months as well.
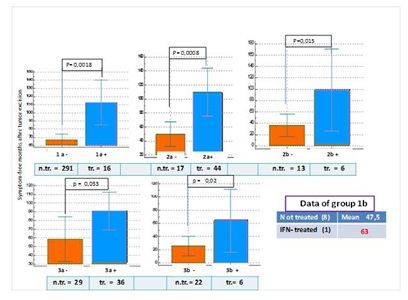 Figure 3: Changes in the average number of symptom-free months in patients receiving low to intermediate dose IFN treatment and in those undergoing observation only after excision-1.
Figure 3: Changes in the average number of symptom-free months in patients receiving low to intermediate dose IFN treatment and in those undergoing observation only after excision-1.
n.tr.= not treated; tr.= treated
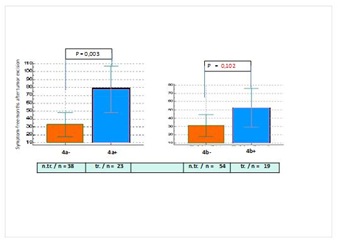 Figure 4: Changes in the average number of symptom-free months in patients receiving low to intermediate dose IFN treatment and in those undergoing observation only after excision-2.
Figure 4: Changes in the average number of symptom-free months in patients receiving low to intermediate dose IFN treatment and in those undergoing observation only after excision-2.
n.tr. = not treated; tr. = treated.
Of the 331 “observation only” patients with stage 1a, progression was seen in 6 patients; and those who received low to intermediate dose were tumor free in the entire observation period. Stage 1a patients did not receive high-dose IFN treatment.
No progression was observed among our stage 1b patients except for one man with metastasis and intermediate IFN treatment (Figure 3). The shorter symptom-free period may be explained by the early discontinuation of 2 patients. Their further fate is unknown. In one of them a lymph node metastasis was already present when the primary tumor was excised. This patient received HDI treatment then for 4 months and disappeared thereafter.
When comparing the further stages, it becomes obvious that the factor of hazard ratio was >1 in stages 2a, 2b, 3a, and 3b as compared with “observation only” patients, In stages 4a and 4b, the factor is < 1 and approaches 0 in this order (Table 2). Each patient receiving HDI IFNa-2b and each patient selected for comparison receiving low to intermediate dose IFN also had lymph node metastasis at the time of primary tumor removal. The “observation only” group was also narrowed down based on similar principles, that is, we observed how many months after primary tumor removal had lymph node metastases occur. The difference between patients receiving HDI and low to intermediate dose IFN was marked as the symptom-free period was significantly longer in patients receiving low to intermediate dose IFN. When comparing low to intermediate dose IFN treatment and “observation only”, interferon treatment has resulted in longer tumor-free period with the exception of primary tumor stage 4b but the result is not significant. Distribution by stages was compared by similar numbers of patients receiving HDI (25 patients) and low to intermediate dose IFN (29 patients). The percentages in stages 2a and 4b were similar while the difference between the ratios of stages 2b, 3a 3b, and 4b are marked, and it is much higher in patients receiving HDI treatment than in those receiving low to intermediate doses of IFN. The median value of symptom-free months was the lowest in the “observation only” group (19) while it was the highest in the low to intermediate dose IFNα-2b treatment group (63.5). The difference between median months and the mean of symptom-free months was the greatest among non-treated patients (Figure 5).
|
Melanoma Stages |
Observation Only |
Progression |
IFN-2b Treated |
Progression |
Total |
Hazard ratio* |
|
2a |
97 |
8 |
45 |
4 |
142 |
1.1 |
|
2b |
12 |
1 |
6 |
0 |
18 |
N/A ** |
|
3a |
32 |
10 |
37 |
11 |
69 |
1.05 |
|
3b |
30 |
13 |
5 |
1 |
35 |
2.16 |
|
4a |
38 |
15 |
22 |
11 |
60 |
0.79 |
|
4b |
53 |
20 |
13 |
12 |
66 |
0.41 |
Table 2: The effect of low to intermediate dose interferon treatment on melanoma progression.
The median duration of observation was 72.5 months in our cases
*: hazard ratio: The proportion of progression in treated vs. untreated patients
**: not applicable (since the divider in this group was 0)
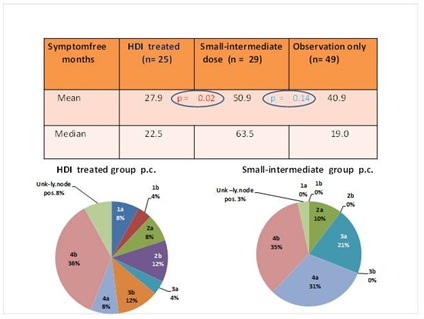 Figure 5: The effect of low to intermediate dose IFNα and of HDI treatment on the number of symptom-free months as compared with patients undergoing observation only Lower panels. p.c. = percentages of compared melanoma tumor stages.
Figure 5: The effect of low to intermediate dose IFNα and of HDI treatment on the number of symptom-free months as compared with patients undergoing observation only Lower panels. p.c. = percentages of compared melanoma tumor stages.
Patients receiving HDI treatment were closely followed. Of the 25 evaluable patients, 5 died (their average survival was 40.6 months) and the treatment had to be discontinued due to tumor progression in 4 patients, elevated liver enzymes in 2 patients and mental issues in 3 patients (=9 patients). In addition, temporary dose reduction was necessary in almost all patients. In contrary, low to moderate dose was well tolerated by patients.
Discussion
In the study period of 34 years (1984 to 2018) in the scope of our study, a total of 867 patients (450 women and 417 men) were processed. Very similarly to literature data, in the age group below 40 years, female dominance of almost 60% was observed while over 60 years of age, the proportion of male patients was more than twice as many as of female patients [1]. According to more recent data, although female gender is considered a predisposition for melanoma development in younger age, it may also provide protection against the carcinogenic effect of UV radiation [13]. It has been suggested that the prevalence of melanoma among the population of Central-Eastern Europe is under documented and that an Italian survey suggested that melanoma cases among employees from this region were more severe and had worse prognosis than among the local population [14]. Although the published case numbers are difficult to compare between the above study and our own followed patient data, we aimed to fill the void regarding our region including Hungary.
Gradual annual increase of melanoma cases was observed, and this increase has been almost linear since 2008. It must be noted that the increase of annual case numbers can only be supposedly deducted from our prevalence data as the total number of (dermatology) patients also increased continuously. The actual large-scale increase of melanoma prevalence in our study took place in Phase 3 (2010-2018).
By analyzing the occurrence rate of the different tumor stages, the most common stages of primary tumors excised, in accordance with global trends, were 1a and 1b in both genders. Stage 3 and 4 primary tumors were present in the same proportion in women while the prevalence of stage 4 was more common than the number of stage 3 cases.
As for the relationship of gender and localization, in accordance with literature data, trunk localization was the most common in men while in women, lower limb localization is by 11% more common than in men but only slightly more common than trunk localization. On the whole, these data are well comparable with the surveys from Western Europe, especially some regions of Germany. However, data from Istanbul that quite specifically represented the Mediterranean area and containing longitudinal observations from a 19-year period, showed the dominance of the head-neck region. In our material, head-neck region was the least affected in both genders.
In women, tumor localization showed mild differences between the different periods, except for the upper limb localization which decreased markedly. In men, striking fluctuation of the trunk and lower limb localization was seen. The fluctuations of the trunk and upper limb were similar within the same periods.
There is no unified standpoint regarding the follow-up of patients [15,16]. We continuously followed-up our melanoma patients up to stage 3a. From stage 3b, the patients were treated by our Department of Oncology and when the national centers were established, the patients were referred to the National Institute of Oncology or the Department of Dermatology, Semmelweis University, Budapest. From stage 1a to 2b, clinical examination was performed every 3 months for 2 years, followed by every 6 months for 3 years and then once per year. Since 2007, sentinel lymph node examination was also performed for each tumor of at least 1.0 mm thickness. If it revealed positivity a block-dissection was performed. The sentinel positive patients were periodically followed-up by imaging scans and laboratory tests.
In case of primary tumor stages of 1a and 1b, the risk of metastases was infinitesimally small. In men, as stages progressed, metastasis development became more and more common while in women, the metastatic ability of tumors was most expressly increased by tumor ulceration (Figure 6).
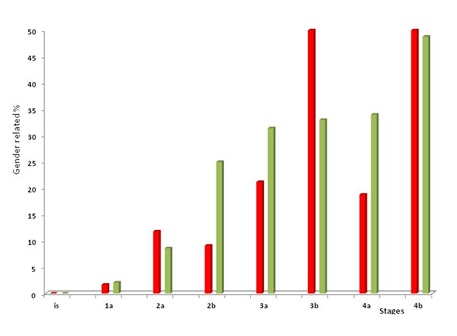 Figure 6: Metastasis development as a function of melanoma stages.
Figure 6: Metastasis development as a function of melanoma stages.
Regarding melanoma mortality in our institute, twice as many men died as women. However, as compared to the large number of followed-up patients, the mortality of melanoma appears to be low in both genders. This may be because our computer system does not show patients who died elsewhere. Also, in several cases the immediate cause of death could not be decided.
The efficacy of Interferons (IFN) was verified in several malignant diseases including melanoma. Due to their antiproliferative, antiangiogenic and proapoptotic effects, interferons are primarily effective in eliminating micrometastases that may remain following the removal of primary tumors [17]. Three different types of interferons are known and IFN-α2b is widely used in the treatment of melanoma [18]. Therapeutic regimens are different in duration, the method of administration: (SC, IV) and in the mortality risk of the treated groups, At the end of the first decade of this century, the already used low to intermediate dose interferon therapy was replaced by High-Dose Interferon (HDI) treatment.
In our practice, adjuvant IFN-α2b treatment was used in 25% of our patients.
Low to intermediate dose was used between 1995 and 2014 and our patients tolerated this treatment with excellent compliance. HDI therapy was started in 2012 and our last patient finished the treatment at the end of 2018.Due to reasons discussed in the “Results” section, the compliance of patient with HDI treatment was worse than that of patients with low to intermediate dose treatment.
It is emphasized that with the exception of stages 3a and 4b, low and intermediate dose treatment resulted in significantly longer tumor-free period and usually longer survival as compared with observation only. For low and intermediate dose treatment, patient groups were selected in such a way that each patient had lymph node metastasis in addition to any primary tumor stage and the observation period was nearly identical. The tumor-free period of patients receiving low to intermediate dose was significantly longer than in patients receiving HDI (Figure 5). Obviously, the most up-to-date immune therapy with the so-called check-point inhibitors is not present in our study. Data regarding these drugs are increasing by the day, however, the basis of comparison is still interferon therapy which is now considered as classic. This was what we could perform and taking into regard the number of patients, it can be considered useful until a rational step forward becomes necessary [19-21].
Acknowledgement
Authors express their gratitude to Drs. Katalin Schweitzer from Dept. of Pathophysiology, Hungarian Defense Forces and to Erzsébet Pintér from Synlab Hungary Kft for their valuable help in statistical analyses and calculations.
References
- Arnold M, Holterhues C, Hollestein LM, Coebergh JWW, Nijsten T, et al. (2015) Trends in incidence and predictions of cutaneous melanoma across Europe up to 2015. J Eur Dermatol Vener 28: 1170-1178.
- Lyth J, Falk M, Maroti M, Eriksson H, Ingvar C (2017) Prognostic risk factors of first recurrence in patients with primary stages I-II cutaneous malignant melanoma - from the population-based Swedish melanoma register. J Eur Dermatol Vener 31: 1468-1474.
- Augustin J, Kis A, Sorbe C, Schäfer I, Augustin M (2018) Epidemiology of skin cancer in the German population: impact of socioeconomic and geographic factors. J Eur Acad Dermatol Vener 32: 1906-1913.
- Baykal C, Atci T, Polat Ekinci A, Buyukbabani N (2017) An update on cutaneous melanoma in Turkey: evaluation of 19-year data in a single tertiary centre and review of the literature. J Eur Acad Dermatol Venerol 31: 236-240.
- MacKie RM, Hauschild A, Eggermont AM (2009) Epidemiology of invasive cutaneous melanoma. Ann Oncol 20: 1-7.
- Karim-Kos HE, de Vries E, Soerjomataram I, Lemmens V, Siesling S, et al. (208) Recent trends of cancer in Europe: a combined approach of incidence, survival and mortality for 17 cancer sites since the 1990s.
Eur J Cancer 44: 1345-1389. - http://www.onkol.hu/hu/rakregiszter-statisztika (Hungarian cancer registry)
- Gorka E, Fabó D, Gézsi A, Czirbesz K, Liszkay G (2016) Distance from Primary Tumor Is the Strongest Predictor for Early Onset of Brain Metastases in Melanoma. Anticancer Res 36: 3065-3069.
- Janka EA, Kékedi K, Várvölgyi T, Gellén E, Kiss B, et al. (2019) Increasing melanoma incidence in the elderly in North-East Hungary: is this a more serious problem than we thought? Eur J Cancer Prev 28: 544-550.
- Medworks® an integrated hospital management system by GlobeNet.
- Hauschild A, Gogas H, Tarhini A, Middleton MR, Testori A, et al. (2008) Practical guidelines for the management of interferon-alpha-2b side effects in patients receiving adjuvant treatment for melanoma: expert opinion. Cancer 112: 982-994.
- Intron A® Summary of Product Characteristics.
- 13. Liu-Smith F, Ziogas A (2020) Age-dependent interaction between sex and geographic ultraviolet index in melanoma risk. J Am Acad Dermatol 82: 1102-1108.
- Astrua C, Fava P, Brizio M, Savoia P (2017) A study of melanoma in Eastern European migrants in Italy Eur J Dermatol 27: 139-143.
- Ribero S, Stucci LS, Marra E, Marconcini R, Spagnolo F, et al. (2018) Effect of Age on Melanoma Risk, Prognosis and Treatment Response. Acta DermVenereol 98: 624-629.
- Turner RM, Bell KJ, Morton RL, Hayen A, Francken AB, et al. (2011) Optimizing the frequency of follow-up visits for patients treated for localized primary cutaneous melanoma. J Clin Oncol 29: 4641-4646.
- Tarhini AA, Gogas H, Kirkwood JM (2012) IFN-α in the treatment of melanoma. J Immunol 189: 3789-3793.
- Rafique I, Kirkwood JM, Tarhini AA (2015) Immune checkpoint blockade and interferon-α in melanoma. Semin Oncol 42: 436-447.
- Larkin J, Asciert PA, Dréno B, Atkinson V, Liszkay G, et al. (2014) Combined vemurafenib and cobimetinib in BRAF- mutated melanoma. New Eng J Med 371: 1867-1876.
- Balatoni T, Ladányi A, Fröhlich G, Czirbesz K, Kovács P, et al. (2020) Biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Pathol Oncol Res 26: 317-325.
- Oláh J (2017) Advance in mmune Therapy of Metastatic Melanoma. Magy Onkol 61: 132-136.
Citation: Vajda A, Zámolyi S, Baló-Banga JM (2021) Assessment of 35 Years Longitudinal Observations on Melanoma Patients in a Single Center of Hungary’s Central Region: Effect of Interferon Immunotherapy. J Clin Dermatol Ther 7: 075.
Copyright: © 2021 Adrienne Vajda, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

