
Assessment of Balance in Individuals with and without Motion Sickness: A Comparative Study
*Corresponding Author(s):
Snehal JoshiProfessor, D.E.Society’s Brijlal Jindal College Of Physiotherapy, Pune , India
Tel:+91-9765592071,
Email:drsnehalmandke@gmail.com
Abstract
Introduction: This vestibular malfunction leads to symptoms from dizziness, orientation problems and postural disequilibrium to the distressing visual symptoms of vertigo (an illusion of rotatory motion) and nystagmus during activities that require head movement suggesting that vestibular disorders markedly disrupt balance control. Therefore, in this study an attempt was made to assess whether individuals with motion sickness are having impaired balance compared to the healthy counterparts.
Aim: To compare the balance of individuals with and without motion sickness.
Material and methods: There were two groups in this study. Each group was having 48 participants. Group A was individuals with motion sickness (Experimental group) and group B was individuals without motion sickness, (control group). Balance of both the groups was assessed using, Neuro-Com basic balance manager i.e. Basic Balance Master (Version 9.2) from Natus balance and mobility. The test administered were unilateral stance and CTSIB.
Results: There was statistically significant difference in sway velocity of both the groups in m CTSIB as well as unilateral stance. (p<0.001). Increased sway velocity was found in group with motion sickness.
Conclusion: Balance was affected in individuals with motion sickness.
Keywords
Balance; CTSIB; Motionsickness; Sway velocity; Unilateral stance
INTRODUCTION
Motion sickness is caused by non-adapted motion stimuli and due to inter-sensory perceptual incongruence between the visual, vestibular, and somatosensory system [1].
The exact cause of motion sickness is unknown, but motion sickness is generally thought to produce by environmental challenges such as repetitive linear and or angular accelerations and difference between perceived motion and visual stimuli of motion. Thus, ears and eyes are considered as the potent receptors of provocation of motion sickness [2].
Many studies have been done to establish the cause and objective criteria for diagnosis and evaluation motion sickness. The greatest incidence of motion sickness is seen at a heave frequency of about 0.2 Hz, from a threshold value of the 0.1 m.s2 [3].
According to recent evidences combination of roll and pitch with heave produce significant motion sickness i.e. a non-linear interaction between roll, pitch and heave. And horizontal translational oscillations in the surge i.e. linear longitudinal directional motion show peak potential for motion sickness at frequency of 0.2 Hz [4].
Along with this the break frequency between tilt and oscillation perception of otolith’s is around 0.2 Hz which in turn produces motion sickness [3]. According to the evolutionary theory, motion sickness is essentially a response to poisoning [5].
Here vestibular function has been proposed as a toxin detector. Thus, the evolutionary purpose of what we call ‘motion sickness’ is considered to be the same as for any emetic response, which is to protect the organism from the toxic effects of potentially harmful substances that it may have ingested [2].
This “toxin detector” hypothesis proposes that the brain provides a ‘backup’ to the main toxin detector system of chemoreceptors of the afferent vagal nerves and the chemoreceptor trigger zone of the brainstem [6].
According to this hypothesis, motion sickness occurs by activation of defence reflex by the sensory conflicts produced by altered visual and environmental stimuli [6]. The sensory conflict theory of motion sickness was proposed by Reason and Reason and Brand was developed into a quantitative model by Oman.
Sensory conflict theory states that conflict of motion stimuli alone is not sufficient to cause motion sickness. This sensory conflict can only lead to be nauseogenic when a perceived conflicting stimulus is different from what is expected from previous experience [7]. Therefore both the theories appear to be equivalent but the computation of conflict differs based on the involvement of vestibular and opto-kinetic signals [7].
A theory based on the vestibulo-occular reflex and on gravitational inertial vector orientation, links the theoretical and physiological framework of sensory conflict theory [7]. It states that the motion sickness is related to the difference between the yaw eigenvector, these are the orientation vectors which can be activated by velocity with specific direction, velocity storage etc. This mechanism integrates and co-ordinate signals for self-motion perception and the actual vestibular velocity signals [7].
Most sickness-provoking sensory conflicts can be classified into two different categories:
(1) Conflict between visual an vestibular/proprioceptive signals and
(2) Conflict between canal and otolith signals [8].
Riccio and Stoffregen put forward the ecological theory of motion sickness which is based on the hypothesis that motion sickness is caused by postural instability, a loss of postural control [9]. Various studies have been done to correlate motion sickness and postural stability in nauseogenic environment as well as postural sway in the absence of visual cues. And it explains connection between motion sickness and verticality perception [7,10].
Also, motion sickness is thought to arise from conflicting information from different sensory modalities, this system works to identify ones motion with respect to his environment, this has been termed as neural mismatch theory [11].
The neural mismatch theory is given by Holst's and later modifications of this concept proposed by Groen and Held. Neural mismatch theory hypotheses that brain mostly evaluates the sensory information which gets stored in the form of memory and then updates on the basis of experiences interacting with environment. The key component for adaptation is the neural store [11].
If there is a discrepancy between the present inputs and these stored patterns, a mismatch signal is generated which triggers the various neural and neuro-humoral mechanism mediating the nausea syndrome and the allied perceptual disturbances [11].
The severity of the motion sickness reactions and the allied perceptual phenomena are presumed to be directly proportional to the strength of this signal [11]. Also evidence suggests that posture may play an important role in the development of disorientation and nauseous symptoms [12].
Hence in all the theories vestibular system is considered as a crucial to produce conflict with other system leading to motion sickness, thus to elicited motion sickness the conflict between the vestibular system and other sensory systems should occur. Or else it should occur in between the vestibular canals or otolith in relation to motion [13]. Vestibular system provides information about the position and the movement of head with respect to gravity and inertial forces forming gravito-inertial frame of reference [10].
Postural control
Postural control is an important factor of the motor system when performing activities of daily living. Human ability to maintain an upright stance and perform locomotion is guided by somatosensory, vestibular and visual information used in a complex regulatory feedback system - the postural control [14].
Information from the vestibular, visual, and somatosensory system is combined to produce appropriate postural adjustments for particular motor task. Each of these systems provides accurate kinematic feedback about position, velocity and acceleration variables of movement contributing to appropriate postural control [15].
The control of posture is dependent on the interaction between sensorimotor system, which obtains information from the visual, vestibular, and somatosensory system [14].
The somatosensory system
There are two types of somatosensory receptors. Proprioceptive receptors sense the position, movements and tension in muscles, tendons and joints thereby detecting the position and load of the different body segments and limbs. Exteroceptive receptors sense pressure and forces acting on the skin [16]. Postural control is dependent upon a certain amount of proprioceptive information to maintain balance when deprived of vision [17].
The vestibular system
Vestibular system works on two components for postural control:
- A peripheral sensory system and
- A central processing unit
A peripheral sensory system consists of semi-circular canal and otolith which works as motion sensors. These sensors send information to central nervous system about head angular velocity, linear acceleration and orientation of head with gravitational axis [18].
Then central processing unit receives and combines information from peripheral sensory organs of vestibular system and other sensory system to estimate head orientation. On the basis this information extra ocular muscles and the spinal cord generate two reflexes; the vestibulo-ocular reflex and vestibular-spinal reflex [19].
Vestibule-ocular reflex controls eye movements and enhances the quality of visual information by stabilizing the retinal image and improve visual perception.
And vestibular-spinal reflex controls body motion compensation, to maintain head and postural stability to prevent falls. The performance of both the reflexes is monitored by the central nervous system and when necessary they are adapted [19].
The visual system
The visual system uses the light-sensitive photoreceptor cells in the retina to detect movement in the visual field. Visual perception of the body motions depend on the three dimensional structure of the environment as well as on the visual conditions such as illumination and accommodation. A movement in the visual environment might evoke a sensation of self-motion and postural imbalance by the optokinetic effect. But visual information is important but not essential in postural control [20]
Vision is generally ascribed three important functions relevant to motion control.
- Vision detects fast and unexpected changes in the visual field and thereby activates appropriate anticipatory movements.
- Vision is used for determining the speed and distance to a target, providing information about the forces and direction of the movements required to reach the target.
- Vision is used for detecting the motion of one’s own body, thereby providing a frame of reference, which can be used for continuous correction of the motions needed to maintain balance or follow an object [21].
Also visual information can also compensate for vestibular lesions, suppress nystagmus and postural imbalances and to some extent also compensate for loss of proprioceptive information.
Visual information can provide a frame of reference, which reduces the high frequency motion necessary to withstand a postural disturbance and maintain balance [22].
Recently the integrity of the postural control system is typically evaluated using static and/or dynamic posturography [23]. Assessment of postural control using posturography gives sensitive measurements for early detection or pre-clinical changes in the postural control system [24].
The Balance Master®, is one of these key measurement system. Balance master software gives three measurements of COG:
- The sway velocity (degrees/s) which is mean for each test condition.
- The composite Limits of Stability across all four test conditions, and
- The COG alignment.
Static posturography, analyses the performance of the postural control system in a static position and environment during quiet standing. The evaluation is based on both eyes-open and eyes-closed conditions to determine the role of the visual system in static postural control.
Standing balance depends on the ability of an individual to control movement of the body’s Centre of Mass (COM) within the base of support. There are several factors that influence the COM movement. These include the location or position of the Centre of Gravity (COG) within the BOS, the velocity of COM (i.e., both the speed and direction of movement), and the size and configuration of the BOS [25].
Other evidence suggested that absolute angular velocity was the best variable in controlling the vertical position of the body, modelled as an inverted pendulum. Therefore, it was suggested that the CNS adopts a postural control strategy that depends on velocity information provided through multisensory integration.
Therefore, velocity information seems to be highly useful for the CNS to anticipate COM position and produce compensatory adjustments through COP displacement [15]. The most commonly used are i.e. unilateral stance and Modified Clinical Test of Sensory Interaction on Balance [25].
Therefore, vestibular system provides information about angular velocity along with information about linear acceleration and gravity to central nervous system. The central nervous system processes this information and tells about orientation of a person in space and how far he/she is tilted away from gravity vertical. This in turn, helps individual to remain upright while standing or walking.
If vestibular system is not working at its best fall may occur if the person cannot sense his orientation to vertical, or if an individual lacks muscle control for balance reactions necessary to maintain posture and locomotion. Therefore the continuous feedback from vestibular system is essentially for maintaining balance in any scenario [26].
Hence in this study, an attempt will be made to find out if there is any difference in the balance of individuals with motion sickness using posturography. Now as the time spent on transport system has increased and occupies a considerable part of individual’s daily life travellers tend to perform a variety of activities during transportation; leading to various active head movements during passive motion causing motion sickness thus considerably affecting the quality of travel [7].
Along with this in recent decades illusion of passive motion is also provided with video games on large screens, 3D movies, and virtual reality etc. leading to motion sickness [27]. Therefore motion sickness has a major influence on modern traveling activities and the emerging virtual reality exposure and indirectly increasing the incidence of motion sickness.
This vestibular malfunction leads to symptoms from dizziness, orientation problems and postural disequilibrium to the distressing visual symptoms of vertigo (an illusion of rotatory motion) and nystagmus during activities that require head movement suggesting that vestibular disorders markedly disrupt balance control [27,28].
Therefore in this study an attempt was made to assess whether individuals with motion sickness are having impaired balance compared to the healthy counterparts [28].
Hypothesis
- • Null Hypothesis: There is no difference between the balance of individuals with and without motion sickness.
- • Experimental Hypothesis: There is difference between the balance of individuals with and without motion sickness.
Aim and objectives of the study
- • Aim: To compare the balance of individuals with and without motion sickness.
- • Objectives
- To assess the balance in individuals with motion sickness.
- To assess the balance in individuals without motion sickness.
- To compare the balance in individuals with and without motion sickness.
MATERIALS AND METHODOLOGY
- • Study setting: Outpatient department.
- • Study duration: 12 months.
- • Type of study: Cross sectional (analytical) study.
- • Study Population: individuals with and without motion sickness.
- • Materials: Basic Balance Master (Version 9.2) from Natus balance and mobility.
- • Inclusion Criteria: Healthy participants age 20- 40 years.Both males and females.
- • Exclusion Criteria: Participants with balance disorders due to other pathologies. Participants with any neurological, visual, auditory conditions, and musculoskeletal condition of lower limb.
OUTCOME MEASURES
- • Motion sickness questionnaire: It is reliable and validated tool for assessing susceptibility of motion sickness with values of r= 0.8 and test re-test reliability =0.8 [29].
- • Modified Clinical Test of Sensory Interaction on Balance: The MCTSIB condition 4, standing on foam with the eyes closed has sensitivity of 95%, and specificity of 90% compared to SOT condition 5 in identifying vestibular dysfunction [27].
- • Unilateral stance: ICC for sway velocity = 0.70 to 0.98 [30].
- • Sampling technique: Purposive sampling
- • Sample size: Sample size was 96 participants from which 48 will be with motion sickness and rest 48 will be without motion sickness. Pilot study was conducted to calculate sample size.
METHODOLOGY
Data collection was done in following manner:
- • Clearance was obtained from the institutional ethics committee
- • Individuals from age 20-40 years from community were approached for the study, both males and females were included.
- • Interested individuals were asked to fill the motion sickness questionnaire for the information about the symptom of motion sickness.
- • Written consent was taken from these individuals.
- • There were two groups in this study. Each group was having 48 participants.
- • Group A was individuals with motion sickness (Experimental group) and group B was individuals without motion sickness, (control group).
- • Balance was assessed in both the groups using, Neuro-Com basic balance manager i.e. Basic Balance Master (Version 9.2) from Natus balance and mobility.
- • Following two tests were used to assess balance:
- • Modified Clinical Test of Sensory Interaction on Balance.
- • Unilateral stance.
- • The age and gender of each participant from both the groups was matched.
- • Data was recorded and then analyzed with unpaired t-test.
All the testes were done on The BASIC Balance Master® (9.2) version.Mctsib and unilateral stance were administered 3 trial were given..
Test is terminated when a subject's arm or feet change position.
Time is stopped during a trial and recorded if:
- a) Patient deviates from initial crossed arm position,
- b) Patient opens eyes during an “eyes closed” trial condition, or
- c) Patient moves feet (takes a step) or requires manual assistance to prevent loss of balance.
The best performance of 3 trials was used for data analysis.
Postural sway in unilateral stance was tested on a force plate.
The centre of gravity (COG) sway velocity (/s), which is the ratio of the distance travelled by the COG to the time of the trial (10 s), was calculated for each trial.
The best performance of 3 trials were given was used for data analysis
RESULTS
Data was recorded and analyzed with unpaired t-test using the Statistical Package for the Social Sciences (SPSS), results are given in the form of charts and tables. The sample size estimated for the study was 96 i.e. 48 individuals with motion sickness and 48 individuals without motion sickness (control). For the study individuals in the community were approached, interested individuals were asked to fill the motion sickness questionnaire for the information about the symptom. Out of the entire sample 6 were males and rest 90 were females in the age group 20-40 years. Balance was assessed in both the groups using, Neuro-Com basic balance manager i.e. Basic Balance Master (Version 9.2) from Natus balance and mobility.
Following two tests were used
- • Modified Clinical Test of Sensory Interaction on Balance. (MCTSIB)
- • Unilateral stance.
DISCUSSION
Figure 1 give representation of age distribution of participants in the study where maximum participants are of age group between 20 to 30 years.
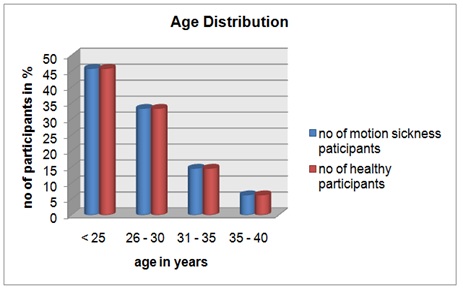 Figure 1: Age Distribution.
Figure 1: Age Distribution.
Inference: Figure 1 represents age distribution among the participants.
Figure 2 describes higher susceptibility of females to motion sickness than men. This higher susceptibility to females can be due to hormonal influences. Also the overall gender differences across all ages may reflect a ‘hard-wired’ greater emetic and nausea susceptibility in females. Along with this psychological variable such as anxiety was found play a role in motion sickness susceptibility variability [6,31].
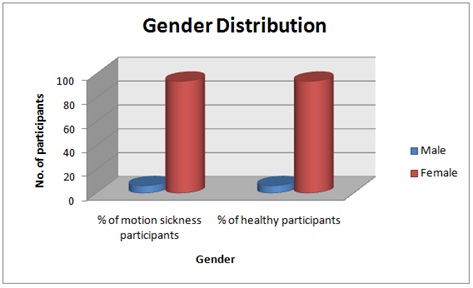 Figure 2: Gender distribution.
Figure 2: Gender distribution.
Inference: Figure 2 show no of cases according to gender distribution. (Females=94%; males=6%).
Tables 1 and 2 shows there is no significant difference between the age and gender of distribution of study population.
|
Groups |
N |
Mean |
SD |
Paired t test |
P |
|
Motion sickness |
48 |
25.79 |
4.60 |
0.000 |
>0.9999 |
|
Control |
48 |
25.79 |
4.60 |
Table 1: Age distribution.
Inference: Table 1 shows there is no significant difference between the age of both the groups.
|
Groups |
N |
Mean |
SD |
Mann-Whitney U test |
P |
|
Motion sickness |
48 |
0.937 |
0.244 |
11152.0 |
0.9969 |
|
Control |
48 |
0.937 |
0.244 |
Table 2: Gender Distribution.
Inference: Table 2 shows no significant difference between the gender of both the groups.
For assessing balance, basic Balance Master (Version 9.2) from Natus balance and mobility was used. The tests used were modified CTSIB, Unilateral stance as both modified CTSIB and unilateral stance provides accurate information about postural control through calculation of sway velocity [32].
In various theories put forward to explain aetiology of motion sickness has pointed towards miss communication between various sensory systems mainly the vestibular system. Therefore these tests were used to challenge sensory systems and assess balance in various conditions [23].
Modified Clinical Test of Sensory Interaction in Balance (MCTSIB) is designed to assess how well an individual is using sensory inputs when one or more sensory systems are compromised [33] table 3.
|
Groups |
N |
Firm eyes Open |
Unpaired t |
P |
|
|
Mean ( Deg/ sec) |
SD |
||||
|
Motion sickness |
48 |
0.314 |
0.141 |
4.674 |
<0.0001 |
|
Control |
48 |
0.204 |
0.082 |
||
Table 3: Modified CTSIB Scores Firm surface eyes open.
Inference: Table 3 represent significant difference between mean sway velocity of healthy and motion sickness individuals on modified CTSIB on firm surface with eyes open condition.
In condition one, i.e. standing on firm surface with eyes open, all sensory systems (i.e., vision, somatosensory, and vestibular) are available for maintaining balance; the mean postural sway velocity was more in motion sickness individuals compared to healthy one. This can be due to inter- sensory system conflict or conflict due to any one sensory system [34].
In condition two, i.e. standing on firm surface with eyes close, vision has been removed and the individuals must rely on the somatosensory and vestibular systems to balance. But as shown in table 4, the mean postural sway velocity is more in motion sickness participants suggesting either somatosensory or vestibular system causing imbalance [35].
|
Groups |
N |
Firm eyes close |
Unpaired t |
P |
|
|
Mean ( Deg/ sec) |
SD |
||||
|
Motion sickness |
48 |
0.297 |
0.117 |
5.133 |
<0.0001 |
|
Control |
48 |
0.191 |
0.082 |
||
Table 4: Modified CTSIB Scores Firm surface eyes closed.
Inference: Table 4 represent significant difference between mean sway velocity of healthy and motion sickness individuals on modified CTSIB on firm surface with eyes closed condition.
In condition three i.e. standing on foam surface with eyes open, the somatosensory system has been compromised and the individuals must use vision and the vestibular system to balance. Again according to table 5, the mean postural sway velocity is higher in MS individuals than the healthy one. Suggesting, either visual or vestibular system is contributing to postural imbalance [35].
|
Groups |
N |
Foam eyes open |
Unpaired t |
P |
|
|
Mean ( Deg/ sec) |
SD |
||||
|
Motion sickness |
48 |
0.668 |
0.218 |
4.708 |
<0.0001 |
|
Control |
48 |
0.489 |
0.147 |
||
Table 5: Modified CTSIB scores Foam eyes open.
Inference: Table 5 represent significant difference between mean sway velocity of healthy and motion sickness individuals on modified CTSIB on foam surface with eyes open condition.
And in condition four i.e. standing on foam surface eyes close, vision has been removed and the somatosensory system has been compromised. The individuals must rely primarily on the vestibular inputs to balance. As shown in table 6 there is increased mean postural sway velocity in motion sickness participants has been observed thus pointing towards affection of vestibular system in motion sickness leading to balance impairment [35].
|
Groups |
N |
Foam eyes close |
Unpaired t |
P |
|
|
Mean ( Deg/ sec) |
SD |
||||
|
Motion sickness |
48 |
1.168 |
0.311 |
4.091 |
<0.0001 |
|
Control |
48 |
0.939 |
0.231 |
||
Table 6: Modified CTSIB scores Foam eyes closed.
Inference: Table 6 represent significant difference between mean sway velocity of healthy and motion sickness individuals on modified CTSIB on foam surface with eyes closed condition.
In all modified CTSIB conditions maximum increase in mean postural sway velocity was observed in forth condition i.e. standing on foam surface with eyes close, here somatosensory system and visual system was compromised. Thus participants were solely dependent on vestibular system for postural control. Suggesting participants with motion sickness has impaired vestibular system resulting in impaired postural control.
Therefor suggesting individuals with motion sickness have impaired postural control compared to healthy counterparts.
Unilateral stance test with eyes open and closed condition was used to assess balance. The single-legged stance allows for the assessment of balance under conditions that introduce additional challenges to the postural-control system [35]. This test reduces the base of support and challenges postural-control system to make more adjustments in order to prevent a fall [35].
The centre of gravity sway velocity, which is the ratio of the distance travelled by the COG to the time of the trial (10s), was calculated for each trial. A touchdown by the non-stance leg was considered as fall. But an increased number of touchdowns might indicate a decrease in postural control [35].
As mentioned in figures 3 and 4 significant differences were found between the right leg unilateral stances in both eyes open and closed conditions. Similar significant differences were found for left leg unilateral stance in both eyes open and eyes closed conditions as shown in table and figures 5 and 6.
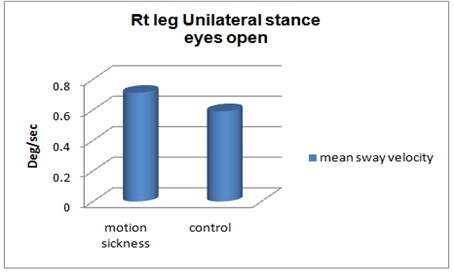 Figure 3: Unilateral stance right leg Scores Firm surface eyes open.
Figure 3: Unilateral stance right leg Scores Firm surface eyes open.
Inference: Figure 3 represent significant difference between mean sway velocity of healthy and motion sickness individuals on right leg unilateral stance on firm surface with eyes open condition.
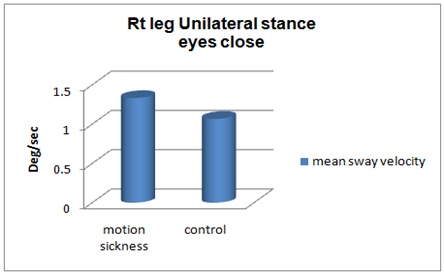 Figure 4: Unilateral stance right leg Scores Firm surface eyes close.
Figure 4: Unilateral stance right leg Scores Firm surface eyes close.
Inference: Figure 4 represent significant difference between mean sway velocity of healthy and motion sickness individuals on right leg unilateral stance on firm surface with eyes closed condition.(P=<0.001).
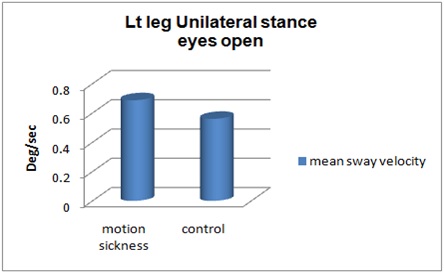 Figure 5: Unilateral stance left leg Scores Firm surface eyes open.
Figure 5: Unilateral stance left leg Scores Firm surface eyes open.
Inference: Figure 5 represent significant difference between mean sway velocity of healthy and motion sickness individuals on left leg unilateral stance on firm surface with eyes open condition.P=<0.001.
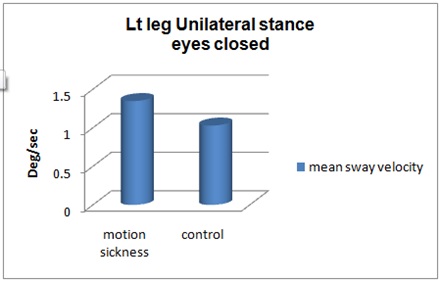 Figure 6: Unilateral stance left leg Scores Firm surface eyes closed.
Figure 6: Unilateral stance left leg Scores Firm surface eyes closed.
Inference: Figure 6 represent significant difference between mean sway velocity of healthy and motion sickness individuals on left leg unilateral stance on firm surface with eyes closed condition P=
Here due to unilateral support the somatosensory sensory input is reduced in both eyes open and closed testing position, thus challenging the visual and vestibular system.
Anne Shumway-cook and Fay Bahling Horak explained that even multiple sensory inputs are available central nervous system generally relies on one system at a time and in case of healthy individuals its mainly the somatosensory system. But when somatosensory system inputs are compromised central nervous system uses vestibular inputs to resolved conflict and maintain balance [36].
The theory proposed by Bos and Bles and Bles et al, explained all situations provoking motion sickness are characterized by a condition in which the sensed vertical is at variance with the subjective vertical that is expected from previous motion experience. Suggesting, motion sickness might be occurring due to canal-otolith conflict or otolith asymmetry [1].
In case of eyes closed condition of unilateral stance both visual and somatosensory systems inputs are reduced due to reduced base of support and eyes closed condition. Thus the task of postural control relies only on the vestibular system which may be affected in individuals with motion sickness. Thus individuals with motion sickness have impaired postural control compared to healthy counterparts.
CONCLUSION
This study shows that sway velocity was more for the individuals with motion sickness in unilateral stance as well as CTSIB.Increased sway velocity indicates impaired balance. Hence this study concluded that individuals with motion sickness have impaired balance compared to healthy counterparts.
CLINICAL IMPLICATION
In this study it was found that individuals with motion sickness have impaired balance mainly due to vestibular system compromise.
Therefore vestibular rehabilitation can be given to individuals with motion sickness to improve postural control.
FUTURE SCOPE OF STUDY
- A study can be conducted to assess to whether balance intervention improves balance in motion sickness affected population.
- To evaluate efficacy of vestibular rehabilitation in motion sickness population.
LIMITATIONS
Dynamic balance was not assessed in this study
REFERENCES
- Buyuklu F, Tarhan E, Ozluoglu L (2009) Vestibular functions in motion sickness susceptible individuals. European Archives of Oto-Rhino-Laryngology 266: 1365-1371.
- Sharma K (1997) Prevalence and Correlates of Susceptibility to Motion Sickness. Acta geneticae medicae et gemellologiae: twin research 46: 105-121.
- Shupak A, Gordon CR (2006) Motion sickness: advances in pathogenesis, prediction, prevention, and treatment. Aviation, space, and environmental medicine 77: 1213-1223.
- Golding JF, Mueller AG, Gresty MA (2001) A motion sickness maximum around the 0.2 Hz frequency range of horizontal translational oscillation. Aviation, space, and environmental medicine 72: 188-192.
- Lackner JR (2014) Motion sickness: more than nausea and vomiting. Experimental brain research 232: 2493-2510.
- Golding JF (2006) Motion sickness susceptibility. Autonomic Neuroscience: Basic and Clinical 129: 67-76.
- Bertolini G, Straumann D (2016) Moving in a moving world: a review on vestibular motion sickness. Frontiers in neurology 7: 14.
- Reason JT (1978) Motion sickness adaptation: a neural mismatch model. Journal of the Royal Society of Medicine 71: 819-829.
- Riccio GE, Stoffregen TA (1991) An ecological theory of motion sickness and postural instability. Ecological psychology 3: 195-240.
- Stoffregen TA, Smart Jr LJ (1998) Postural instability precedes motion sickness. Brain research bulletin 47: 437-448.
- Takeda N, Morita M, Horii A, Nishiike S, Kitahara T, et al. (2001) Neural mechanisms of motion sickness. Journal of Medical Investigation 48: 44-59.
- Krueger WW (2011) Controlling motion sickness and spatial disorientation and enhancing vestibular rehabilitation with a user-worn see-through display. The Laryngoscope 2: 17-35.
- Schmäl F (2013) Neuronal mechanisms and the treatment of motion sickness. Pharmacology 91: 229-241.
- Prieto TE, Myklebust JB, Hoffmann RG, Lovett EG, Myklebust BM (1996) Measures of postural steadiness: differences between healthy young and elderly adults. IEEE Transactions on biomedical engineering 43: 956-966.
- Portela FM, Rodrigues EC, de Sá Ferreira A (2014) A critical review of position-and velocity-based concepts of postural control during upright stance. Human Movement 15: 227-233.
- Proske U, Gandevia SC (2012) The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiological reviews 92: 1651-1697.
- Vennila K, Aruin AS (2011) Postural control in response to a perturbation: role of vision and additional support. Exp Brain Res 212: 385-397.
- Magnusson M, Enbom H, Johansson R, Pyykkö I (1990) Significance of pressor input from the human feet in anterior-posterior postural control. The effect of hypothermia on vibration-induced body-sway. Actaoto-laryngologica 110: 182-188.
- Bispo AS, dos Santos Silva AL, de Mello Pinto MV, Baraúna MA, Caratinga MG, et al. (2010) Vestibular system and postural control. AO SPINE, Davos, Switzerland.
- Lord SR (2006) Visual risk factors for falls in older people. Age Ageing 35: 42-45.
- Bardy BG, Warren WH, Kay BA (1999) The role of central and peripheral vision in postural control duringwalking. Perception & psychophysics 61: 1356-1368.
- Fransson PA, Magnusson M, Johansson R (1998) Analysis of adaptation in anteroposterior dynamics of human postural control. Gait & posture 7: 64-74.
- Browne JE, O'Hare NJ (2001) Review of the different methods for assessing standing balance. Physiotherapy 87: 489-495.
- Clark S, Rose DJ, Fujimoto K (1997) Generalizability of the limits of stability test in the evaluation of dynamic balance among older adults. Archives of physical medicine and rehabilitation 78: 1078-1084.
- Boughen J, Dunn K, Nitz J, Johnston V, Khan A (2013) A new method of interpreting the centre of gravity location using the modified Clinical Test of Sensory Interaction on Balance: A reliability study. Hong Kong Physiotherapy Journal 31: 64-68.
- Sienko KH, Whitney SL, Carender WJ, Wall C (2017) The role of sensory augmentation for people with vestibular deficits: Real-time balance aid and/or rehabilitation device?. Journal of Vestibular Research 27: 63-76.
- Villard SJ, Flanagan MB, Albanese GM, Stoffregen TA (2014) Postural Instability and Motion Sickness in a Virtual Moving Room. Hum Factors 50: 332-345.
- Sturnieks DL, St George R, Lord SR (2008) Balance disorders in the elderly. Neurophysiologie Clinique/Clinical Neurophysiology 38: 467-478.
- Golding JF (1988) Motion sickness susceptibility questionnaire revised and its relationship to other forms of sickness. Brain Res Bull 47: 507-516.
- Bouche K, Stevens V, Cambier D, Caemaert J, Danneels L (2006) Comparison of postural control in unilateral stance between healthy controls and lumbar discectomy patients with and without pain. European Spine Journal 15: 423-432.
- Rine RM, Schubert MC, Balkany TJ (1999) Visual-vestibular habituation and balance training for motion sickness. Physical therapy 79: 949-957.
- Hromatka BS, Tung JY, Kiefer AK, Do CB, Hinds DA, et al. (2015) Genetic variants associated with motion sickness point to roles for inner ear development, neurological processes and glucose homeostasis. Hum Mol Genet 24: 2700-2708.
- Masani K, Popovic MR, Nakazawa K, Kouzaki M, Nozaki D (2003) Importance of body sway velocity information in controlling ankle extensor activities during quiet stance. Journal of Neurophysiology 90: 3774-3782.
- Paillard AC, Quarck G, Paolino F, Denise P, Paolino M, et al. (2013) Motion sickness susceptibility in healthy subjects and vestibular patients: effects of gender, age and trait-anxiety. Journal of Vestibular Research 23: 203-209.
- Palmieri RM, Ingersoll CD, Stone MB, Krause BA (2002) Center-of-pressure parameters used in the assessment of postural control. Journal of Sport Rehabilitation 11: 51-66.
- Humway-Cook A, Horak FB (1986) Assessing the influence of sensory interaction of balance. Suggestion from the field. Physical therapy 66: 1548-1550.
Citation: Joshi S, Ambolkar A (2020) Assessment of Balance in Individuals with and without Motion Sickness: A Comparative Study. J Phys Med Rehabil Disabil 6: 056.
Copyright: © 2020 Snehal Joshi, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

