
Assessment of Patient Compliance to Compression Therapy
*Corresponding Author(s):
Jean-Francois UhlParis Cite University, France
Email:jeanfrancois.uhl@gmail.com
Introduction
Compression therapy is the main conservative therapy of Chronic Venous Disease (CVD). Its principle is to apply external pressure around the lower limb in order to reduce the transmural pressure of the venous system. The main devices to achieve this are Medical Compression Stockings (MCS), bandages, and wraps. Patient compliance is the key point for efficacy of compression therapy. Similarly to other areas of health care, compliance (or concordance) is underestimated and there is a high cost due to patient noncompliance [1,2].
Definition and Measurement of Patient Compliance
Adherence (or compliance [concordance]) to compression can be calculated as both a continuous and dichotomous measure [3,4]. The continuous measurement uses the Proportion of Days Covered (PDC), which means the number of days covered over a time interval. PDC is the preferred method for assessment of compliance in compression therapy. The dichotomous measure (compliant or noncompliant) considers whether a patient attains a specified PDC. This has been demonstrated in several studies because an improvement in wear time is achieved by an increase in the number of wear days and not the by average wear time per day. This is the reason why, for compression therapy, we propose measuring compliance by the number of wear days only. For example, a patient who wears a stocking five days out of seven would have a PDC of 71%, which is a fair adherence to compression.
Compliance in Phlebology
The most common issue in phlebology practice is that stockings are infrequently prescribed at the primary care level. When prescribed, they are not used by a high proportion of patients [5]. After several years of stocking use and even for severe stages of CVD, Raju, et al. reported a surprisingly low patient compliance rate of 37% [6]. This suggests that there are problems with use of Compression Stockings (CS), and mainly lack of patient education. Insufficient instructions from the practitioner about compression is likely to be a causative factor. However, intense patient education programs reported in Randomized Control Studies (RCTs) have still shown high levels of patient non-compliance [6-10]. It should be noted that there is an issue in all these studies: compliance is only assessed by patient self-reports, and thus we have no idea of the real compliance. This underlines the interest of special devices or methods to accurately assess the wear time of the compression device by the patient.
Adherence to Compression Therapy and Factors of Non-Compliance
To improve the clinical efficacy of compression, it is useful to determine the factors influencing compliance. Several studies have been published.
In 2004, Moffatt, et al. analyzed 10 studies on adherence to wearing MCS and bandages to treat venous ulcers [7]. The reasons found to explain the poor compliance were difficulties donning, ineffectiveness, and lack of comfort with a feeling of constriction.
Raju et al. studied 3144 patients with CVD for 8 years [6]. The compliance differed depending on the Clinical, Etiology, Anatomic, Pathophysiology (CEAP) classification: C0-C2 (67%), C3 (22%), C4 (4%), C5 (4%), and C6 (3%). Only 21% of patients reported using MCS daily, 16% less often, and 63% either did not use MCS at all or gave up on them after the trial. The reasons for non-use were mainly related to complaints regarding the physical properties of the MCS and poorly identified reasons (30%). The weaknesses of this study were: use of MCS knitting according to an American standard, with different pressures from French pressures, absence of data on compliance based on CEAP classification, and no prescription by practitioners that could have explained the importance of wearing MCS.
Zaija, et al. studied MCS wear adherence in 16,770 patients with CVD [5]. Compression was used by only 25.6% of patients. The more advanced the disease, the greater the adherence to MCS. Moreover, 5.3% of patients discontinued use due to its high cost, itchiness, and sweating. Their unsightly edema increased exuding lesions or difficulty in application.
In a series of 200 patients, Reich-Schupke, et al. showed that age over 60 years and Body Mass Index (BMI) over 25 kg/m2 made use more difficult [8].
Finlayson, et al. emphasized the importance of psychosocial factors: depressive syndromes are significantly associated with non-compliance [9].
During the 2013 American Venous Forum, Allaert presented a study on the MCS adherence of 2265 patients. Only 36.6% wore the stockings every day. The main reasons for poor compliance were: an insufficient number of pairs (25%), donning difficulties (24%), ablation issues (15%), and no improvement 9%. Benigni, et al. compared degressive pressure MCS and classic MCS with progressive pressure [10]. The latter have shown greater ease of installation and greater comfort. Coral reported a cross-sectional observational study in Brazil conducted from June 2017 to January 2019 [11], based on a patient questionnaire. The adherence rate in the study was 55.8%. The most common reason for not wearing stockings was financial. Secondary reasons were bad information and heat.
All these studies have the same limitation: real compliance was not assessed because the adherence to compression was reported only by patient self-reports.
Newly Available Methods to Assess Patient Compliance
Three new methods of assessment are available today: thermal sensor, movement sensor, and self-questionnaire. These techniques aim to assess the accurate wear time for each patient and to study the main factors of non-compliance.
Assessment With A Thermal Sensor [12-15]
The Thermotrack iBee22L thermal sensor is provided by Proges Company, France (http:// www.thermotrack.com). This device continuously records the skin temperature, making it possible to accurately record the wear time/days of compression over several weeks or months.
Description of the device
The Thermotrack thermal sensor, shown in Figure 1A, is a small disk that records the temperature at regular intervals during the entire duration of the study (1-12 weeks). Up to 4096 measurements recorded on the sensor memory can be displayed with a personal computer with a USB port (Figure 1B). The patient data are also available through a web server. The sensors are sewn into each stocking (Figure 1C).
 Figure 1: The THERMOTRACK device. A) The disk is 16 mm in diameter and 6 mm wide; B) the device records data, which can be transferred to a computer via USB; C) the sensor inserted inside the hem of a stocking.
Figure 1: The THERMOTRACK device. A) The disk is 16 mm in diameter and 6 mm wide; B) the device records data, which can be transferred to a computer via USB; C) the sensor inserted inside the hem of a stocking.
The accuracy of temperature measurement is 1 oC and ranges from 40 oC to 80 oC. This thermo button, commonly used to monitor the cold chain of the food industry, has a certification for a thermostatic environment. A thermal curve is obtained for each patient for the duration of the study (Figure 2).
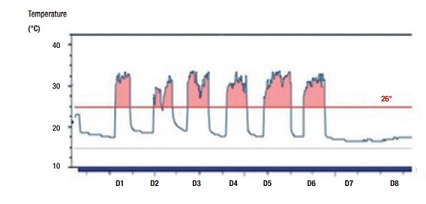 Figure 2: A representative thermal curve recorded in patient no. 4 over 8 days. The horizontal line (26 oC) shows all the moments when the stocking was worn (areas in pink).
Figure 2: A representative thermal curve recorded in patient no. 4 over 8 days. The horizontal line (26 oC) shows all the moments when the stocking was worn (areas in pink).
In a pilot study in France during the winter,15 the average skin temperature of the patients was found to be about 31 oC, which was significantly more than the indoor or outdoor temperatures Assessment of patient compliance to compression therapy 59 during the trial. Of note, 26 °C was considered the limit (Figure 2).
The thermal curves can be created by using the Microsoft Excel Sparklines function. The wear duration is then automatically computed by using an Excel formula from the daily temperature curve, considering the number of records above the limit of 26 oC. The wear days are easily computed manually from the curve.
The accuracy and reliability of this thermal sensor to compute the wear time of the stockings was previously validated by a pilot study15 presented at the AVF meeting in February 2015. Among 10 healthy subjects studied during 1 week, we found a perfect correlation of the recorded temperature curve with the daily events reported by the subjects on the questionnaire: 94% of the events (donning, removing, and washing) were identified by the iBee22L thermo button. So, this device could be used to accurately track and control the wear time of CS on a long-term basis.
The Meditrack Study [12]
Forty-four active females, aged ≥18 years, seeking medical care for symptomatic primary CVD were enrolled in this study from November 1, 2015, to January 15, 2016. These patients were classified C2S according to the CEAP classification. All patients were asked to carry a cell phone and were enrolled and followed by the same practitioner for 4 weeks. Exclusion criteria were pregnancy, ongoing treatment for CVD, history of deep vein thrombosis, foot static disorders or ankle stiffness, or any orthopedic history of the lower limbs. Patients with a low probability of having symptoms of venous origin were also excluded. For this purpose, we used a specific score (0-4) for symptom imputability [16]. Patients with a score < 3 were excluded.
Study Design
This study was organized as a single-blinded randomized controlled trial. Informed consent was first obtained from all selected patients after an explanation of the study. The patients were then randomized into two groups, each containing 20 subjects:
- Group 1: received minimal recommendations about CS use by the physician at the office;
- Group 2: received in-depth recommendations by the physician and additional recommendations by SMS twice a week;
- All patients received a CS providing a pressure of 15-20 mmHg at the ankle, namely point B (Mediven® Elegance French class 2).
Parameters Assessed During this Study
A customized computer program was used, the Computer Venous Registry (CVR) [17], to provide an automated CEAP classification and symptom scoring for each limb [18]. The data entered for each patient included the examination date, birth date, sex, BMI, and personal history.
Venous mapping of the superficial and deep systems was performed by using color Duplex ultrasound in all patients. The basic CEAP classification was documented by using the definitions proposed by the expert panel at the 2001 world meeting of the International Union of Phlebology in Rome. Venous symptoms were documented by using a 10-point Visual Analog Scale (VAS).
The patient-reported Quality of Life (QoL) was assessed by using the Chronic Venous Disease Quality-of-Life Questionnaire (CIVIQ) [19], a disease-specific QoL instrument dedicated to CVD and validated in this condition. In addition to the global CIVIQ scale, its four dimensions (pain, physical, psychological, and social components) were analyzed separately. Venous symptoms assessed by a VAS and CIVIQ were repeated at the conclusion of the 4-week study.
Statistical Methods
Statistical analysis was performed blinded with JMP software (version 12 pro for Mac).
The mean comparison of the wear duration/ days was tested with the Student’s t-test, with P < 0 .05 considered as significant.
Results
There were no differences between the two groups regarding age, BMI, symptom imputability scoring, pain score, heaviness score, and type of CS. The study was performed in France during the winter. The average skin temperature of the patients was 31 °C, which was significantly greater than the indoor or outdoor temperatures during the trial.
The authors compared the temperature curves of the two groups regarding the daily, weekly, and monthly wear time in hours, and the number of days worn (wear days) per week and per month (Table 1). Student’s t-test showed a very significant difference between the two groups. In Group 2, the average number of wear days for the 4 weeks of the trial increased by 33%: 18.9 versus 13.4 in Group 1 (P < 0.01).
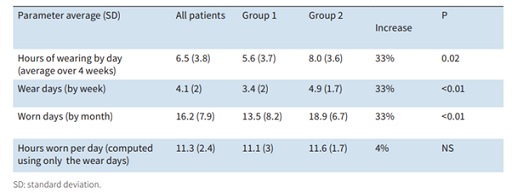 Table 1: Comparison of the real wear duration/days in the two groups (based on the 4-week study).
Table 1: Comparison of the real wear duration/days in the two groups (based on the 4-week study).
Similarly, the average number of wear days each week increased by 33%: 4.9 in Group 2 versus 3.4 in Group 1 (P < 0.001) (Figure 3). However, there was no difference in the average number of hours on days when the CS were worn by patients in each group (11.1 in Group 1 and 11.6 in Group 2). This is the reason the average number of wear days per week (PDC) was considered the best criterion to assess compression adherence.
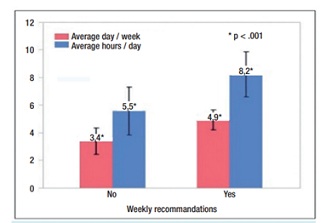 Figure 3: Comparison of the wear time between the two groups. The average number of days per week is shown in pink, and the average hours per day is shown in blue. In the group with recommendations (on the right), there was a significant increase in the patient compliance for both criteria (P < 0.001).
Figure 3: Comparison of the wear time between the two groups. The average number of days per week is shown in pink, and the average hours per day is shown in blue. In the group with recommendations (on the right), there was a significant increase in the patient compliance for both criteria (P < 0.001).
Relationship Between Wear Time and Quality of Life
There was a significant correlation between the wear time and two parameters of the QoL: psychic and social (both P < 0.001). Paradoxically, there was no correlation with the two other parameters of the CIVIQ, physical and pain.
Discussion
The accuracy and reliability of the Thermotrack device were demonstrated in a pilot study [15], and also strongly supported by the Industrial reports. However, our study does have several limitations. The first is the interpretation of the thermal curve: In the present study, the measuring device was around 97% accurate with error range of around 3%. In fact, the maximum error per recording cycle was 19 minutes for an average of 11 hours of wear time (which is about 3%). The second limitation regards the Thermotrack device itself: The external temperature should not exceed 23 oC to have a clear cut-off value much lower than the skin temperature (around 30 oC). So, this device is not reliable during hot weather. The third issue is the choice of below-knee or thigh-high stockings according to patient preference, but this variation was not different in the two groups. The fourth limitation is that only one stocking of each pair contained the temperature device, so the other stocking may have been worn and the time not recorded, but this is probably similar in the two groups. The fifth limitation is the short duration of this study (4 weeks). However, it seems that habits developed during the initial period of wearing CS play an important role in long-term compliance (expert advice, as in the French high authority for health report) [20].
A medium-term compliance study of 3-6 months should be done to confirm our results. Another limitation is that our study was at only one center. Lastly, the lack of improvement in the pain score when using the compression, as measured by the CIVIQ, is surprising in our study, but it could be explained by the small number of patients. In addition, the final pain score was not recorded.
According to the data of the Bonn vein studies [21], 28.7% of C2 patients wore CS and 38% wore a compression device including bandages. The Bonn Vein Study 2 also showed that 67.4% of patients who stopped wearing MCS had only temporary reasons for their use (pregnancy, treatments). Therefore, presumably 74% of patients who have a long-term indication regularly wear CS. This is a high level of compliance compared with the other studies. The issue with these results, however, is the lack of compliance definition.
Summary of the Meditrack Study
To our knowledge, this is the very first study demonstrating actual compliance rates in wearing CS. Adherence was measured accurately, thanks to the thermal curves by a small sensor (Thermotrack) inserted into the MCS. In this small homogenous group of 40 women classified as C2S, the extensive and repeated recommendations by SMS given by the practitioner during the 4-week period significantly increased patient compliance by 33%, from 48% in the control group to 71% in the recommendation group (P < 0.001) (Figure 3).
Buenos also used the thermo button to assess in one patient how often her wore his full body compression system [13], called “second skin ROSA,” to manage obesity. Park published a research paper reporting recent advances in materials science and biomedical engineering: a new skin-interface sensor that records both temperature and pressure to be used as a smart monitor for compression therapy [14], controlled by a smartphone. This flexible, lightweight, wireless device supports simultaneous moni-toring of temperatures and the interface pres-sures between compression garments and the skin. Experimental studies on healthy subjects demonstrated accurate and stable monitoring performance. Clinical studies with patients that exhibit different pathologies illustrate capabilities for measuring pressures for different body types and skin conditions. Specific demonstrations highlight continuous tracking while sleeping, walking, and cycling. This technology has clear potential for use not only at the clinic but also at home, for improved health outcomes by enabling the precise adjustment of compression therapy. Additional options include direct integration with existing MCS products as “smart” stockings.
Assessment With A Movement Sensor Device
Evaluation of an accelerometer-based device to monitor compliance on patients wearing MCS has been made by Grenier et al. [22]. The electronic monitoring device uses a 3D accelerometer. Movements are recorded with an electronic monitoring chip, the CR1616 button cell, embedded in the MCS (Figure 4).
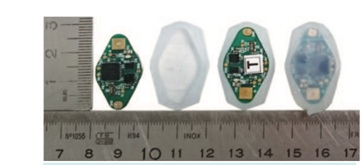 Figure 4: From left to right: the monitoring chip, silicone base, the device on the base, and the final device sealed by potting silicone.
Figure 4: From left to right: the monitoring chip, silicone base, the device on the base, and the final device sealed by potting silicone.
The duration of recording is up to 340 days with sampling every 15 minutes. Raw data are analyzed, filtered, and then converted to wear time by using a dedicated program providing a daily wear time summary file and a graphic display of the event vectors.
The first trial was conducted on five patients. The difference between the weekly wear times reported by the patients and the durations measured by the monitoring device was < 60 minutes for 79% of the wear days. The results of the monitoring devices also demonstrated 85% accuracy on the days when the elastic CS were not worn. The limitation of this study was the small number of patients.
In summary, this device seems interesting to measure the wear time of MCS, in particular in the summer or in hot countries where Thermotrack is inappropriate. In addition, several new skin-integrated biosensors have been developed to increase their use in medicine, as shown by Rodrigues, et al. and GE et al. [23,24].
Assessment with a Dedicated Self-Report [25]
The aim of this study was to validate a short self-questionnaire measuring patient adherence to wearing elastic CS and to validate its psychometric properties: acceptability, test-retest reliability, internal consistency and external validity. The gold standard of compliance was established by a Thermotrack thermal sensor [12]. The PDC was computed for each patient (79 patients during 4 weeks).
The questionnaire was first optimized, a process that involved reducing the number of items (analysis of internal and external consistencies). Then, the questionnaire was correlated with the PDC. Next, a multicomponent analysis with varimax rotation was performed, selecting the most discriminant items. Finally, the correlation of each item was correlated again to the PDC. Fifteen items were selected to build the final questionnaire. The result was the computation of a score for adhesion called “Adhesig” to predict the real patient adherence to compression. Its specificity is 63%, which is perfectly usable in practice. In phlebological practice, it could be an interesting tool to detect the non-compliant patients.
Conclusion
Patient adherence (or compliance [concordance]) to compression therapy is the key to therapy efficacy, together with the good choice of the interface pressure. Several new tools are available today to assess it more accurately, and thus to analyze the factors of non-compliance. Additional studies are needed to better investigate these factors. The first step in daily practice is to detect non-adherence to compression by using a short questionnaire.
References
- Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS (2005) Impact of medication adherence on hospitalization risk and healthcare cost. Med Care 43: 521-530.
- Iskedjian M (2002) Estimating the economic burden of hospitalization due to patient nonadherence in Canada. Value Health 5: 470.
- Hess LM, Raebel MA, Conner DA, Malone DC (2006) Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother 40: 1280-1288.
- Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, et al. (2007) Medication compliance and persistence: terminology and definitions. Value Health 11: 44-7
- Ziaja D, Kocelak P, Chudek J, Ziaja K (2011) Compliance with compression stockings in patients with chronic venous disorders. Phlebology 26: 353-360.
- Raju S, Hollis K, Neglen P (2007) Use of compression stockings in chronic venous disease: patient compliance and efficacy. Ann Vasc Surg 21: 790-795.
- Moffatt CJ (2004) Factors that affect concordance with compression therapy. J Wound Care 13: 291-294.
- Reich-Schupke S, Murmann F, Altmeyer P, Stücker M (2012) Compression therapy in elderly and overweight patients. VASA 41: 125-131.
- Finlayson K, Edwards H, Courtney M (2010) The impact of psychosocial factors on adherence to compression therapy to prevent recurrence of venous leg ulcers. J Clin Nurs 19: 1289-1297.
- Benigni JP, Branchoux S, Bacle I, Taieb C (2013) Difficulty associated with donning medical compression stockings: results from a survey comparing two different compression stockings. Women’s health (London Engl) 9: 291-300.
- Coral FE, Guarinello GG, Cavassola AP, Rocha ALM, Guidi MM, et al. (2021) Chronic venous insufficiency and graduated compression stockings: analysis of public health system patients’ adherence to treatment. J Vasc Bras 20: 20200034.
- Uhl JF, Benigni JP, Chahim M, Fréderic D (2018) Prospective randomized controlled study of patient compliance in using a compression stocking: Importance of recommendations of the practitioner as a factor for better compliance. Phlebology 33: 36-43.
- Buenos P, Buenos L (2016) Mesure de l’observance du port d’un vêtement compressif grâce à un thermo bouton. Phlébologie 69: 53-57.
- Park Y, Kwon K, Kwak SS, Yang DaS, Kwak JW, et al. (2020) Wireless, skin-interfaced sensors for compression therapy. Sci Adv 6: 1655.
- Uhl J, Benigni J, Chahim M, Cornu-Thenard A (2015) Use of Compression Stockings in Chronic Venous Disease: Validation of a New Device to Assess Patient Compliance. J Vasc Surg Venous Lymphat Disord 3: 131.
- Carpentier PH, Poulain C, Fabry R, Chleir F, Guias B, et al. (2007) Ascribing leg symptoms to chronic venous disorders: the construction of a diagnostic score. J Vasc Surg 46: 991-996.
- Uhl JF, Cornu Thenard A, Antignani PL (2005) The computerized venous registry (CVR) or the CEAP in daily practice. Int Angio 21: 7
- Eklof B, Rutherford RB, Bergan JJ, Carpentier PH, Gloviczki P, et al. (2004) Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg 40: 1248-1252.
- Launois R, Reboul-Marty J, Henry B (1996) Construction and validation of a quality of life questionnaire in chronic lower limb venous insufficiency (CIVIQ). Qual Life Res 5: 539-554.
- French High Authority for Health (HAS) (2010) Dispositifs de compression medicale a usage individuel. Utilisation en pathologie vasculaire. Revision de la liste des produits et prestations remboursables.
- Rabe E, Hertel S, Bock E, Hoffmann B, Jöckel K-H, et al. (2013) Therapy with compression stockings in Germany-results from the Bonn Vein Studies. J Dtsch Dermatol Ges 11: 257-261.
- Grenier E, Rastel D, Chaigneau C (2018) Evaluation of an accelerometer-based device to monitor compliance on patients wearing medical compression stockings. JTAVR 3: 25-32.
- Rodrigues D, Barbosa AI, Rebelo R, Kwon IK, Reis RL, et al. (2020) SkinIntegrated Wearable Systems and Implantable Biosensors: A Comprehensive Review. Biosensors (Basel) 10: 79.
- Dong XC, Ge G, Cai Y, Dong Q (2018) A flexible pressure sensor based on rGO/polyaniline wrapped sponge with tunable sensitivity for human motion detection. Nanoscale 10: 10033-10040.
- Allaert FA, Rastel D, Graissaguel A, Sion D, Hamel-Desnos C, et al. (2019) Design and evaluation of the psychometric properties of a self-questionnaire on patient adherence to wearing elastic compression stockings. Phlebology 34: 25-31
Citation: Uhl J-F, Benigni J-P (2022) Assessment of Patient Compliance to Compression Therapy. J Angiol Vasc Surg 7: 089.
Copyright: © 2022 Jean-Francois Uhl, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

