
Association of Adrenal Ganglioneuroma with Addison's Disease: A Case Study
*Corresponding Author(s):
Mnif FDepartment Of Endocrinology, Hedi Chaker Hospital, Sfax, Tunisia
Tel:+216 74242613,
Fax:+216 28656300
Email:fatmamnif05@yahoo.fr
Abstract
Adrenal ganglioneuroma is a rare tumor originating from the neural crest cells. The clinical presentation for most patients is asymptomatic, and most of those tumors are hormone silent. An association with autoimmune damage, particularly in Addison's disease has never been reported. We will describe a case of adrenal ganglioneuroma incidentally diagnosed in a 24-year-old female patient whose physical examination, routine laboratory studies and hormonal tests were within normal ranges. Abdominal computed tomography showed a solid oval tumor approximately 51x33 mm in the right adrenal gland without calcification or any remarkable signs of malignancy. Right adrenalectomy was performed for treatment purposes. Histological diagnosis of the tumor revealed the presence of a ganglioneuroma originating from the adrenal medulla. The immediate post-operative evolution was complicated by acute adrenal failure whose etiologic investigation was in favor of an Addison's disease.
Keywords
INTRODUCTION
Ganglioneuroma is a benign, differentiated neoplasm arising from neural crest tissue and is composed of mature ganglion cells and Schwann's cells in a fibrous stroma [1]. Its prevalence is 0.07 to 0.2% of differentiated tumors [2]. The pathogenesis of ganglioneuromas is unknown. They may arise anywhere along the paravertebral sympathetic plexus and only a small proportion of ganglioneuromas is of adrenal origin and occurs most commonly in children and young adults [3]. Characteristically, ganglioneuromas do not secrete excess catecholamines or steroid hormones. Because of this lack of hormone production, ganglioneuromas are usually clinically silent lesions detected in patients undergoing abdominal imaging studies for unrelated reasons. Despite CT imaging and MRI can we oriented to this pathology but it lacks sensitivity and specificity. Moreover, definitive diagnosis is done through histological examination. Assessment and management of the adrenal ganglioneuromas are similar to those of other adrenal tumors [4]. Our aim was to reported an inhabituel association of autoimmune damage, particularly Addison's disease and adrenal ganglioneuroma whose was never been reported.
CASE REPORT
A 24-year-old female, who came with an ascites of great abundance and transudative character in fine-needle aspiration cytology (protein=51g/l, white blood cells=60/mm 3, lymphocytes=65%) whose etiologic investigation was negative, namely the research of Mycobacterium tuberculosis in the urine and in the spittle, liver serology (hepatitis A, hepatitis B, hepatitis C and hepatitis E serology), serologic tests for autoimmune hepatitis (anti LKM1 - anti smooth muscle - anti mitochondria) and tumor markers. The evolution was marked by the persistence of an ascite blade of low abundance after drainage. During the investigation of this disease, a heterogeneous, hypoechogenic right adrenal solid mass, measuring 51x33 mm, was incidentally detected through abdominal CT scan. The patient was referred to our department for further examination.
Blood pressure was normal (110/70 mmHg). Physical examination demonstrated no significant finding. Routine laboratory tests were normal. Endocrine tests (Table 1), including Plasma Aldosteron Concentration (PAC), Plasma Rennin Activity (PRA), PAC/PRA ratio, diurnal cortisol rhythms, 24-h urinary catecholamines, and their metabolites were within normal ranges.
| Measured values | Normal values | |
| PAC (pg/ml) | 48,6 | ‹150 |
| PRA ( pg/ml) | 9,45 | ‹5 |
| PAC/PRA | 5 | ‹23 |
| Diurnal cortisol rhythms Cortisol (ng/ml) | C8H=117,9 C16H=75,8 C23H=22,7 | |
| Low dose dexamethasone Suppression Test | 7,6 ng/ml | ‹18 ng/ml |
| Urinary metanephrine | 3,25 µmol/24h | ‹5,5 µmol/24h |
Table 1: Laboratory test results of the patient.
Abdominal CT scan showed a round right adrenal mass of irregular outline, having a diameter of 51x33 mm and its spontaneous density was 36UH with heterogeneous contrast enhancement and without calcification. Figure 1a and b, the left adrenal gland was normal. Magnetic resonance imaging was not performed.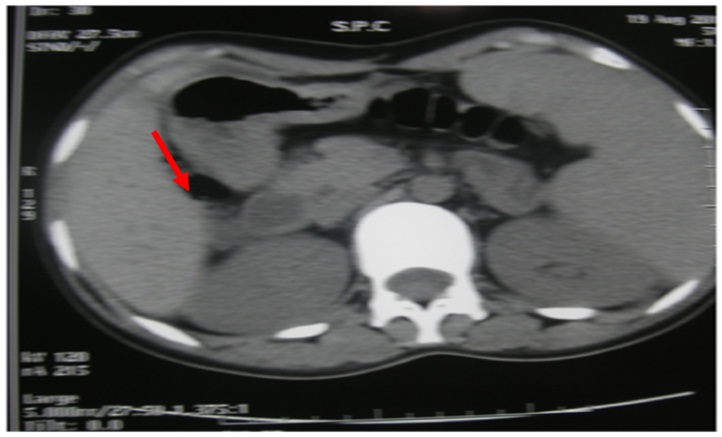
Figure 1a: Abdominal CT scan without contrast demonstrated a round right adrenal mass (51 x 33 mm).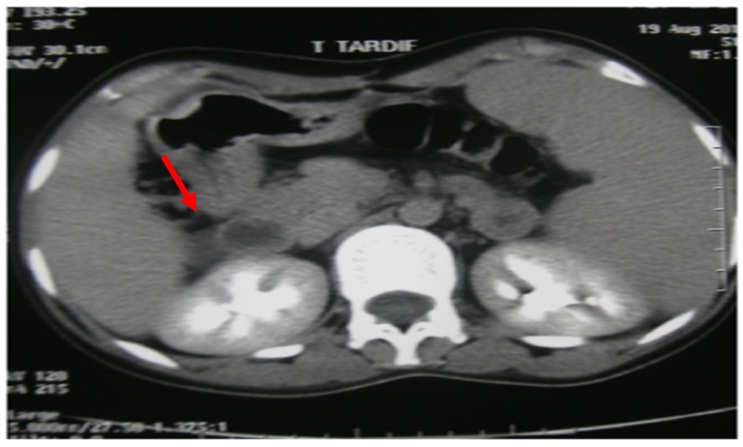
Figure 1b: Abdominal CT scan with contrast demonstrated a large, right heterogeneous adrenal lesion.
Right Laparoscopic adrenalectomy was performed through the transabdominal route under general anesthesia. The removed tumor was well circumscribed, measuring 60 x50 x15 mm. The histological diagnosis was that of adrenal ganglioneuroma by showing mature ganglion cells spindle shaped, called Schwann cells (Figure 2a and b). The immunohistochemical analysis was not performed.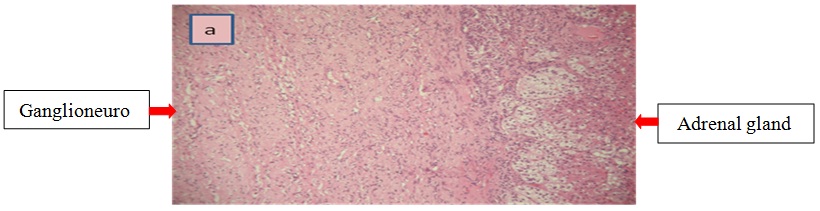
Figure 2a: Histological examination of the adrenal tumor.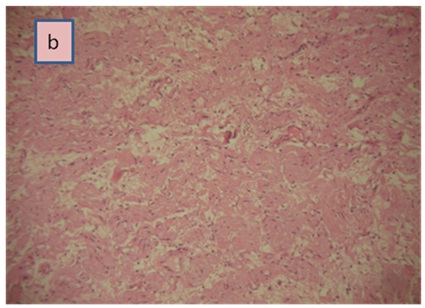
Figure 2b: Ganglion cells surrounded by Schwann cells (H&E, × 100).
Four days after the operation, the patient experienced nausea, vomiting and abdominal pain with a hypotension of 90/60 mmHg. At first, we had thought of a possible postoperative complication such as deep bruising or abscess of the abdominal wall of the operation site. These two possibilities were eliminated with a normal abdominal ultrasound scan. Clinical examination, however, was strongly in favor of an adrenal failure which could not be explained by the unilateral adrenalectomy of a non-secreting adrenal adenoma. Laboratory tests were normal except for the level of plasma cortisol which was reduced to 57 ng/ml. Adrenal failure was confirmed by two synacthen 250 µg tests which were performed first 15 days and then two and a half months after surgery (Table 2). Besides that, the other hormonal tests were not controlled.
| Time (min) | 0 | 60 | |
| 15 days after surgery | Cortisol (ng/ml) | 57,2 | 113,2 |
| Two and a half months after surgery | Cortisol (ng/ml) | 39,2 | 77,5 |
Table 2: Results of synacthen tests of the patient after surgery.
As part of etiological investigation for adrenal failure, adrenal antibodies and 21-hydroxylase antibodies, which are specific markers of the adrenal gland, were strongly positive to 2866.9 U/ml [1-100 UI/ml], confirming the diagnosis of Addison's disease associated with adrenal ganglioneuroma. However, our patient haven't any other signs of autoimmunity particularly she have not goiters, she has a regular cycle and the rest of the immunological tests including anti TPO tests and anti thyroglobulin were negatives.
DISCUSSION
Ganglioneuroma is a benign tumor of the sympathetic nervous system that usually occurs in older children and young adults with an average onset age of 25.3 years [5,6]. There is a female predominance with a sex ratio of 0,75 [7]. Most GNs are sporadic but they can also associate with neurofibromatosis type II and multiple endocrinologic neoplasma type II. GNs may arise anywhere along the paravertebral sympathetic plexus [8], more common in the retroperitoneum (40%) and rarely found in adrenal glands (1-6%) [9]. Adrenal GNs do not typically secrete exogenous hormones; thus the clinical presentation in most cases is asymptomatic with an incidental diagnosis [10]. However, sometimes, these tumors are manifested by non specific pain, or by signs of digestive, urinary, neurological or vascular compression [7,11]. In our case where the discovery of the ganglioneuroma came as a result of the investigation of an ascite of great abundance that can be reactive to the large size of adrenal GN. In fact, any retroperitoneal tumors of a large size are capable of causing a variety of digestive disturbances which may include reactive ascites [12].
Concerning radiological examination, upon CT, AGNs usually appear as oval or round, homogenous and well-circumscribed masses [13,14], which often partially or completely surround major blood vessels. AGNs develop as slightly hypodense masses, with multiple punctate calcifications visible internally. In our case the mass was well circumscribed, measuring 6 cm in long axis, hypodense without calcification or invasion of adjacent structures. However, both CT and MRI do not allow distinguishing GN from others tumors such neuroblastomas or pheochromocytomas, and to obtain a definitive diagnosis histological exams is necessary.
Microscopically ganglioneuromas have consists of a spindle cell tumor composed of neuritic processes, Schwann cells and perineural cells and show numerous ganglion cells [15,16]. Immunohistochemical results, including S100, CD56, synaptophysin and NSE positivity, are also important for forming a diagnosis [17].
The recommendation of surgery for an adrenal mass is somewhat controversial for non functional adrenal mass between 4-6 cm, because the likelihood of adrenal cancer has been known to increase in adrenal lesions with a diameter greater than 40 mm. However non-secreting adrenal incidentalomas larger than 40 mm with suspicious features of malignancy on imaging procedures should be treated using adrenalectomy because of the increased prevalence of malignancy [18]. In case or size tumor than 6 cm we generally use surgery as the case of our patient, right laparoscopic adrenalectomy was performed through the trans-abdominal route and diagnosis of adrenal GN was retained after histological study.
Addison's disease or Primary Adrenal Insufficiency (PAI) is caused by autoimmune destruction of the adrenal cortex. The diagnosis of PAI is often delayed by many months, and most patients present with symptoms of acute adrenal insufficiency as is the case of our patient who prior the surgery the 8 AM cortisol levels 117,9 ng/ml (322 nmol/l) such levels may well be found in preclinical states of PAI. Moreover the acute adrenal failure was likely precipitated by surgery, adrenalectomy and perhaps also anesthesics. The common autoimmune form of PAI is characterized by the presence of steroid 21-hydroxylase autoantibodies with high specificity (90%) and sensibility (85%) [18,19] and if the test is positive, further etiologic evaluation is not generally necessary, however, other causes should be sought if no autoantibodies are detected. Whereas the association of ganglioneuroma with (PAI), has never been reported. Finally new investigations should be advanced in the fields of molecular and genetic pathology of ganglioneuroma and may be some connection with adrenal failure especially (PAI).
CONCLUSION
Adrenal ganglioneuroma occurs rarely in adults and preoperative diagnosis is difficult. It needs careful evaluation and surgical treatment. The surgical removal of this ganglioneuroma in our patient revealed adrenal failure of autoimmune origin (Addison's disease). The combination of these two diseases has not yet been reported and still requires further research.
REFERENCES
- Erem C, Kocak M, Cinel A, Erso HO, Reis A (2008) Dopamine-secreting adrenal ganglioneuroma presenting with paroxysmal hypertension attacks. Saudi Med J 29: 122-125.
- Moriwaki Y, Miyake M, Yamamoto T, Tsuchida T, Takahashi S, et al. (1992) Retroperitoneal ganglioneuroma : a case report and review of the Japanese literature. International Medecine 31: 81-85.
- Arredondo Martínez F, Soto Delgado M, Benavente Fernández A, Basquero González B, Zurera Cosano A, et al. (2003) [Adrenal ganglioneuroma. Report of a new case]. Actas Urol Esp 27: 221-225.
- Gupta R, Dinda AK (2007) Ganglioneuroma of the adrenal gland: a rare case. Indian J Pathol Microbiol 50: 782-784.
- Leavitt JR, Harold DL, Robinson RB (2000) Adrenal ganglioneuroma: a familial case. Urology 56: 508.
- Shawa H, Elsayes KM, Javadi S, Morani A, Williams MD, et al. (2014) Adrenal ganglioneuroma: features and outcomes of 27 cases at a referral cancer centre. Clin Endocrinol (Oxf) 80: 342-347.
- Hafiani M, Rabii R, Debbagh A, Rais H, Bennani S, et al. (1998) Retroperitoneal ganglioneuroma : report of a case. Ann Urol 32: 20-22.
- Ichikawa T, Koyama A, Fujimoto H, Honma M, Saiga T, et al. (1993) Retroperitoneal ganglioneuroma extending across the midline: MR features. Clin Imaging 17: 19-21.
- Spinelli C, Rossi L, Barbetta A, Ugolini C, Strambi S (2015) Incidental ganglioneuromas: a resentation of 14 surgical cases and literature review. Journal of Endocrinological Investigation 38: 547-554.
- Chen CL, Huang ST, Chang PL, Ng KF (2000) Adrenal ganglioneuroma: report of five cases. Chang Gung Med J 23: 550-554.
- Garcia G, Anfossi E, Prost J, Ragni E, Richaud C, et al. (2002) [Benign retroperitoneal schwannoma: report of three cases]. Prog Urol 12: 450-453.
- McCarthy S, Duray PH (1983) Giant retroperitoneal neurilemoma: a rare cause of digestive tract symptoms. J Clin Gastroenterol 5: 343-347.
- Radin R, David CL, Goldfarb H, Francis IR (1997) Adrenal and extra-adrenal retroperitoneal ganglioneuroma: imaging findings in 13 adults. Radiology 202: 703-707.
- Legmann P (2009) [Adrenal incidentaloma: management approaches: CT - MRI]. J Radiol 90: 426-443.
- Chang CY, Hsieh YL, Hung GY, Pan CC, Hwang B (2003) Ganglioneuroma presenting as an asymptomatic huge posterior mediastinal and retroperitoneal tumor. J Chin Med Assoc 66: 370-374.
- Hafiani M, Rabii R, Debbagh A, Rais H, Bennani S, et al. (1998) [Retroperitoneal ganglioneuroma. Apropos of a case]. Ann Urol (Paris) 32: 20-22.
- Zhang Y, Nishimura H, Kato S, Fujimoto K, Ohkuma K, et al. (2001) MRI of ganglioneuroma: histologic correlation study. J Comput Assist Tomogr 25: 617-623.
- Tanaka H, Perez MS, Powell M, Sanders JF, Sawicka J, et al. (1997) Steroid 21-hydroxylase autoantibodies: measurements with a new immunoprecipitation assay. J Clin Endocrinol Metab 82: 1440-1446.
- Laureti S, De Bellis A, Muccitelli VI, Calcinaro F, Bizzarro A, et al. (1998) Levels of adrenocortical autoantibodies correlate with the degree of adrenal dysfunction in subjects with preclinical Addison's disease. J Clin Endocrinol Metab 83: 3507-3511.
Citation: Mnif F, Graja S, Mnif MF, Bouaziz Z, Kammoun M, et al. (2015) Association of Adrenal Ganglioneuroma with Addison's Disease: A Case Study. J Clin Stud Med Case Rep 2: 022.
Copyright: © 2015 Mnif F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

