Association of Ulcerative Colitis and Autoimmune Haemolytic Anemia
*Corresponding Author(s):
Berraida RDepartment Of Gastroenterology, Mohamed V Military Training Hospital, Rabat, Morocco
Email:rberraida@gmail.com
Abstract
Autoimmune diseases are group of diseases caused by the disruption of the immune system that attacks a part of the body of the subject as if it were a foreign substance to 2% of individuals suffer from autoimmune diseases. Autoimmunity is defined as an immune response against self-Ag (Autologous Ag) by self-reactive lymphocytes. The aim of our work is to report a case of a combination of Ulcerative Colitis (UC) and autoimmune haemolytic anemia, to highlight the diagnostic, therapeutic and evolutionary aspects.
INTRODUCTION
Autoimmune Hemolytic Anemia (AIHA) is a rare autoimmune disease. It is characterized by an increased destruction of red blood cells coated with autoantibodies by the reticuloendothelial system especially splenic. It is very rare in Morocco with a prevalence of 1.5 per 100,000. AIHA is manifested by haemolysis with more or less severe or sudden anemia, subacute evolution or unpredictable relapses. AIHA may be secondary or associated with drug intake, infection, primary immunodeficiency, other autoimmune disease (UC in our case).
Ulcerative colitis is part of Chronic Inflammatory Bowel Disease (IBD). It begins in young adults between 20 and 40 years of age and is characterized by continuous superficial lesions that start in the rectum and can spread throughout the colon without ever reaching other segments of the digestive tract.
It evolves in flares interspersed with periods of remissions (quiet periods without symptoms). It can be accompanied by extra-intestinal manifestations (articular, cutaneous and hepatic). The current treatments have for first objectives the control of the outbreaks, the prevention of relapses and the maintenance of an optimal quality of life for each patient.
OBSERVATION
A 26-years-old man, known for the UC since 2015 (rectal location and left colic), when the patient was 24 old years. The diagnostic is based on:
Clinical criteria: abdominal pain, dysentery syndrome
Endoscopic: congestive mucosa, ulcerated, fragile, hemorrhagic with transverse rectum lesions without healthy mucosal interval
Histological: Histological aspect compatible with an IBD type UC.
. The patient was treated with 5-ASA 500 mg: 4 cp/Day and recorded a good clinical and endoscopic evolution.
During his follow-up, biological tests was requested including a blood count which objectified a macrocytic anemia with an Hg: 8.8 g/dl, a VMA at 115fl, ferritinemia at 115g. Also the dosage of vitamin B12, folic acid were normal and fiberscopy was without abnormalities, assay reticulocyte rate which returned normal to 336000 mm3, total bilirubin was 16 mg/L with unconjugated bilirubin 10 mg/L, LDH at 347 UI/L, haptoglobin at 0.07g and the combs test was positive: AC hot IgG type + complement. The diagnosis of autoimmune haemolytic anemia associated with an UC was selected, the patient was put on steroid for 6 months (treatment of the RCH flare and treatment of autoimmune haemolytic anemia), and corticosteroids decrease as Hg exceeded 10 g/dl. And background treatment with 5-ASA. With a good clinical, biological and endoscopic evolution.
DISCUSSION
AIHA groups several clinic-biological entities. Their incidence is 1 to 2.5 per 100,000 and their prevalence is 5 to 20 per 100,000. Pediatric forms are generally acute and post-infectious and the adult forms are more often chronic. The etiological approach is directed; aside age, by the primitive or secondary clinical context [1].
The diagnosis of an autoimmune haemolytic anemia is based on the following elements in front of a patient typically presenting an anemic syndrome, subconjunctival and/or muco-cutaneous jaundice and often splenomegaly:
1. Anemia (
2. Hemolysis is confirmed by hyper-reticulocytosis (often?150000 mm3), elevation of unconjugated bilirubinemia, effusion of haptoglobinemia, increase of LDH level associated with the presence of hemoglobinuria.
3. The autoimmune nature of this hemolytic anemia is based on the Coombs Direst Test (TCD), which is positive in 95% of cases [2,3]. According to the immunochemical characteristics of the autoantibody (specificity and “thermal optimum” of the antibody), there are mainly 2 types of AIHA:
a) AIHA with so-called “hot” auto antibodies, linked to the presence of an autoantibody whose maximal hemolytic activity (“thermal optimum”) is working at temperatures close to the physiological body temperature of 37°C. They represent about 70% of all AIHA and are in half of times associated with an underlying disease: immunodeficiency, systemic lupus most often among young adults (mainly women), lymphoid blood disease often low-grade among older subjects (non-Hodgkin’s lymphoma, chronic lymphocytic leukemia) [4-7].
b) “Cold” antibody AIHAs are found due to the presence of antibodies called “cold agglutinins” that are active at low temperatures. These can be either acute or transient evolution in particular when they are of post-infectious origin (mycoplasma infection, infectious mononucleosis) among children or young adults, or of chronic evolution among the 50 year old (and more subjects) corresponding to what is called “chronic Cold Agglutinin Disease" (CAD). The latter is associated in most cases with monoclonal gammopathy of kappa IgM class with anti-red cell antibody activity of the “cold agglutinin” type and is similar to a low grade lymphoid hemopathy B.
The initial management of an AIHA is most often, with the exception of cold agglutinin disease in adults, based on corticosteroid therapy, which must be started urgently and prolonged. Corticosteroid dependence is frequently observed which indicates in about 50 to 60% of cases, a second-line treatment (ritxuximab, splenectomy or immunosuppressor). At diagnosis, as at any time in the natural history of the disease, AIHA can lead to potentially severe acute anemia, with transfusion urgency, justifying specialized treatment in emergency.
AIHA is considered idiopathic by elimination, when it is the sole manifestation of the disease. Otherwise, it is considered either a complication, or one of the components of a more complex immunological constellation, or as an associated disease without a well-established link. Half of the cases were previously considered as idiopathic forms, but currently the secondary or associated forms have become predominant between 60 to 80% depending on the series. These considerations lead to a systematic search for an underlying pathology of which AIHA is, so to speak, a secondary disease.
Ulcerative colitis is part of Chronic Inflammatory Bowel Disease (IBD). It is characterized by continuous superficial lesions that start in the rectum and can spread over the entire colon without ever reaching other segments of the digestive tract. It evolves in flares interspersed with periods of remissions (quiet periods without symptoms). It can be accompanied by extra-intestinal manifestations (articular, cutaneous, liver, etc). It is very common in industrialized countries: North-West Europe and the United States with nearly 3,000 new cases a year, also France is less affected. Most of the disease is between 30 and 40 years old. The most common symptom encountered during the course of UC is the presence of blood in the stools (rectorrhagia). This can be accompanied by mucus, diarrhea and abdominal pain. Severe outbreaks of the disease can lead to weight loss, significant fatigue and fever.
In about 1/3 of the cases the disease begins with lesions limited only on the rectum, 1/3 of the patients will have an attack from the outset maximum on the entire colon (pancolitis). After 20 years of evolution, this pancreatic involvement is found in 50% of patients. Extra-intestinal manifestations may appear: joints (arthralgia, lumbago), skin (erythema nodosum) or in the eyes and liver. In children, ulcerative colitis induces malnutrition phenomena, which can cause significant stunting and weight loss. A number of non-rare and sometimes inaugural complications increase the burden of the medical and surgical management of these patients: severe acute colitis, cancerous degeneration whose risk is 18 times higher than that of the general population after 20 years of evolution of a pancolitis form.
In principle, it is necessary to discuss the diagnosis of UC which may be asymptomatic. (Without a clear gastrointestinal clinical sign) when there is a moderate and recent change in intestinal transit associated with an impairment of the general condition (loss of weight, loss of appetite) [8,9]. Biologically it can be highlighted, an inflammatory biological syndrome associating a protein C of the high inflammation (CRP), anemia associating a lowered Hemoglobin level (Hb). PANCA antibodies (Perinuclear Antineutrophilic Cytoplasmic Antibodies) are positive in ulcerative colitis and may guide the diagnosis [10,11]. The endoscopic aspects range from the simply odemic or granitic mucosa masking the submucosal vessels and may or may not bleed on contact with a mucosa covered with pus, ulcerated in places, bleeding spontaneously in a sheet. Lesions are diffuse, without healthy mucosal interval. Pseudo-polyps have often formed and are seen in old CHNs. The rectal bulb often appears of reduced volume (Figure 1).
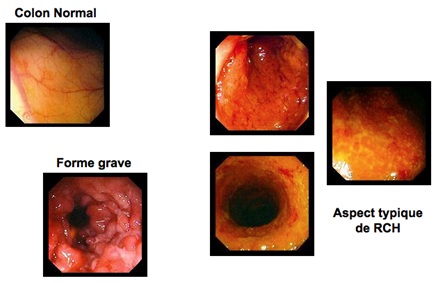 Figure 1: Endoscopic appearance of a normal and inflamed colon during UC.
Figure 1: Endoscopic appearance of a normal and inflamed colon during UC.
The treatment of ulcerative colitis is sometimes surgical, after failure of medical treatments. Dietary measures are paramount. Depending on the intensity and severity of the disease outbreak, the intestinal exclusion diet will be used:
Diet without residues in minor forms allowing resting of the colon;
Enteral feeding method with low continuous flow: digestive probe placed in the duodenum with electric pump in medium forms;
Parenteral nutrition (intravenous infusions or central venous catheter) with absolute digestive rest in severe forms. The so - called immunosuppressive drugs such as cyclosporine or azathioprine inhibit certain cells of the immune system. They are prescribed in moderate forms of the disease or as a preventive treatment for relapses. Most often, specialists will prescribe anti-TNF agents (adalimumab, golimumab, infliximab) which are monoclonal antibodies acting as immunomadulators. These are basic treatments. However, these treatments are not free of side effects that can be severe.
CONCLUSION
The combination of UC and autoimmune haemolytic anemia is very rare and is considered to be one of the components of a more complex immunological constellation or an associated disease with no well-established link.
REFERENCES
- Gautier E, Homberg JC (1994) Anémies hémolytiques autoimmunes. In: Najman A, Verdy E, Potron G, Isnard F (eds.). Hématologie, Ellipses, Paris. 16: 391-406.
- Engelfriet CP, Overbeeke MA, von dem Borne AE (1992) Autoimmune hemolytic anemia. Semin Hematol 29: 3-12.
- Gehrs BC, Friedberg RC (2002) Autoimmune hemolytic anemia. Am J Hematol 69: 258-271.
- Aladjidi N, Leverger G, Leblanc T, Picat MQ, Michel G, et al. (2011) New insights into childhood autoimmune hemolytic anemia: a French national observational study of 265 children. Haematologica 96: 655-663.
- Barcellini W, Fattizzo B, Zaninoni A, Radice T, Nichele I, et al. (2014) Clinical heterogeneity and predictors of outcome in primary autoimmune hemolytic anemia: a GIMEMA study of 308 patients. Blood 124: 2930-2936.
- Roumier M, Loustau V, Guillaud C, Languille L, Mahevas M, et al. (2014) Characteristics and outcome of warm autoimmune hemolytic anemia in adults: New insights based on a single-center experience with 60 patients. Am J Hematol 89: 150-155.
- Vaglio S, Arista MC, Perrone MP, Tomei G, Testi AM, et al. (2007) Autoimmune hemolytic anemia in childhood: serologic features in 100 cases. Transfusion 47: 50-54.
- Bouhnik Y (2005) [Diagnostic tools in inflammatory bowel diseases]. Rev Prat 55: 977-983.
- Hommes DW, van Deventer SJ (2004) Endoscopy in inflammatory bowel diseases. Gastroenterology 126: 1561-1573.
- Beaugerie L (2000) La démarche diagnostique. In: Rampal P, Beaugerie L, Marteau P, Corthier G (eds.). Colites infectieuses de l'adulte. John Libbey Eurotext, Paris.
- Moum B, Ekbom A, Vatn MH, Aadland E, Sauar J, et al. (1997) Inflammatory bowel disease: re-evaluation of the diagnosis in a prospective population based study in south eastern Norway. Gut 40: 328-332.
Citation: Berraida R, Alaomari A, Hamdouchi HEI, Jamal S, Touibi Y, et al. (2018) Association of Ulcerative Colitis and Autoimmune Haemolytic Anemia. J Gastroenterol Hepatology Res 3: 018.
Copyright: © 2018 Berraida R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.