
Atypical Intraductal Papilloma of Breast Accompanied by Solid Lesions – Report of Two Cases
*Corresponding Author(s):
Jyh-Miin HuangDepartment Of General Surgery, St. Joseph’s Hospital, Yunlin, Taiwan
Tel:+886 963023378,
Email:sgmluca@gmail.com
Abstract
Intraductal papillary lesions are not so common among breast biopsy specimens. Possible presences of pseudoinvasion of small epithelial nests within the fibrotic tissue around an intraductal papilloma are well known, and the invasiveness could be ruled out by the presences of myoepithelial rimming about the tumor nests. Herein, we report two cases of atypical intraductal papilloma. A 42-year-old woman who had bloody discharge from left nipple. Excisional lumpectomy presents a central-located 3-cm intraductal papilloma accompanied by few satellite solid tumor nests in its fibrotic cystic wall. Loss of most myoepithelial rimming over an round solid papillary nest as well as a few invasion tongue-like structure on the margin of the other accessory tumor nest, which is proved to be pseudoinvasion lesion with strong CK5 immunostaining, unevenly patchy ER immunostaining, and rather low ki-67 labeling index. The other case of 69-year-old woman had extensive Ductal Carcinoma In Situ (DCIS) accompanied by an atypical intraductal papilloma with Atypical Ductal Hyperplasia (ADH) in the papillary lesion. The case one was treated with lumpectomy without adjuvant therapy, and gets the result of no tumor recurrence for two years. The case two accepted partial mastectomy for DCIS and the intraductal papillary lesion.
Keywords
Breast solid lesion; Ductal carcinoma in situ; Intraductal papilloma; Pseudo-invasion
INTRODUCTION
Intraductal papillary lesions are present in 1% to 10% of breast biopsy specimens [1,2]. Intraductal papilloma of breast is an event initiating within a subareolar dilated main mammary duct or from a cluster of peripheral dilated mammary ducts. Therefore, intraductal papilloms can be divided into two groups, a major group of solitary (central) intraductal papilloma, and a minor group of peripheral-located multiple intraductal papillomas [2-4]. The latter group seems to have higher ratio of malignant relationship [5]. Atypical Ductal Hyperplasia (ADH), Ductal Carcinoma In Situ (DCIS) and invasive ductal carcinoma are present in 29.4% of 68 solitary intraductal papilloma cases, reported by Gutman H., et al [2]. In the other article, itraductal papillomas of previous 153 core needle biopsies are found to have 9.2% of atypia, 2.6% of DCIS and 1.3% of invasive ductal carcinoma in latter excisional specimens [6]. However, pseudoinvasion pattern of small epithelial nests in the fibrotic tissue around an intraductal papilloma are well documented and should be kept in mind [7]. True invasion of the suspected small tumor nests can be proved only if the loss of peripheral myoepithelial rimming, and the invasion tongue around the intraductal papillary lesions may present out of the fibrous capsule [8-10].
Herein, we would like to present two cases of atypical intraductal papilloma accompanied with solid lesions, and discuss the significance of myoepithelial cell rimming, basal cell rimming or glandular reserve cells relating to the benignancy of proliferative lesion.
PRESENTATION OF CASE ONE
This is a case of 42-year-old female presenting bloody discharge from left nipple. Simultaneously, a tumor just locating at lateral aspect of areola is palpated. Lumpectomy was performed to remove the palpable mass. An intracystic tumor measuring 3.5x3.1x2.5 cm in size of the cystic cavity and 2.5x1.5x1.5 cm in size of the pedunculated intracystic tumor are identified. Microscopically, a pedunculated intraductal papilloma composed of benign papillary structure with two-cell layered lining epithelium, focal necrosis, and a focus of compact acinar arrangement demonstrating high Ki-67 labeling index (30%) (Figure 1A-B) and decreased myoepithelial rimming proved by partial loss of p63 Immunohistochemical (IHC) stain (Figure 1C) like atypical hyperplasia. More intracystic bleeding developed from the papilloma and its fibrous capsule nearby the hypercellular pedicle root. Ovoid tumor with central necrosis and fibrinous change (Figure 2A) in fibrotic capsule near the pedicle root is also present. The tumor cells are immunostained with CK5 (Figure 2B), unevenly patchy ER (Figure 2C) and PR immunostaining, and intermediate mitotic activity with Ki-67 labeling index = 10%. Noticeably, the surrounding margin of the tumor nest is not rimmed by myoepithelial cells, which can be proved by negative immunostaining for Smooth Muscle Actin (SMA) (Figure 2D). There are a few irregular pseudoinvasive fronts with low nuclear grade, low mitotic activity proved by low Ki-67 labeling index (Figure 2E), and complete loss of myoepithelial component proved by absence of p63 immunostaining (Figure 2F), protruding from the other mural solid tumor nest far from the cellular papilloma root.
The excisional margin is free of tumor involvement. The patient lives well after the lumpectomy without succeeding neither adjuvant therapy nor local recurrence for more than two years.
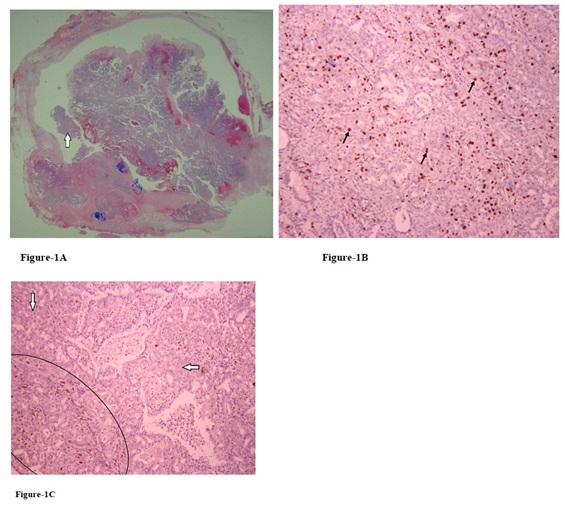
Figure 1 (A-C): Case one: A: A small area (arrow) of the large 2.5-cm papilloma with compact acinar arrangement (H&E stain, scanning view).
B: Higher Ki-67 labeling index up (arrows) to 30% is present in this unusually hyperplastic area (Ki-67, x200).
C: Lower rate of myoepithelial differentiation with less nuclear staining for p63 (arrows) comparing with the area of more myoepithelial cells (circle) is also identified (p63, x200).
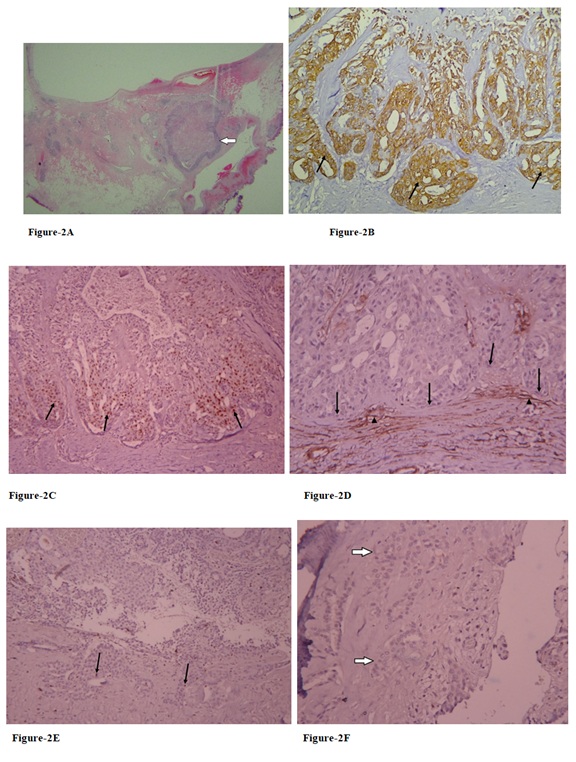
Figure 2(A-F): A: Ovoid tumor (arrow) with central degenerative change in fibrotic capsule near the pedicle root is seen (H&E stain, scanning view).
B: The tumor cells are mostly immunostained for high molecular weight cytokeratin (arrows) (CK5, x200).
C: Unevenly patchy ER immunoreactivity with brown nuclear staining (arrows) for these tumor cells (ER, x200).
D: Loss of myoepithelial differentiation over the rimming cells is proved by negative immunostaining (arrows) for SMA, but SMA Immunostaining over the surrounding myofibroblasts (arrow heads) (SMA, x400).
E: A few irregular pseudo invasive fronts with low mitotic activity (arrows) (Ki-67, x100).
F: complete loss of myoepithelial component proved by absence of p63 immunostaining (arrows) (p63, x400).
PRESENTATION OF CASE TWO
This is a case of 69-year-old female presenting bloody discharge from right nipple 7 years ago. Breast echo revealed a subareolar hypoechoic lesion measuring 0.37x0.27x0.49 cm. It is suspected as intraductal papilloma clinically without tissue proof. One year ago, a 0.43-cm circumscribed mass at 9 o’clock/5.8 cm from the nipple of right breast on sonography. Echo-guided core biopsy showed atypical ductal hyperplasia. The last outpatient department (OPD) visit follow-up echography of right breast revealed a 0.7x0.35x0.85 cm hypoechoic lesion at 9 o’clock/6 cm from nipple, a 0.57x0.53x0.66 cm “anechoic +hypoechoic cystic lesion” at 9.5 o’clock/6 cm from nipple, and a 0.8x0.4x0.6 cm hypoechoic lesion at 9 o’clock/3 cm from nipple. Finally, both resected hypoechoic lesions at 6 cm and 3 cm from nipple are proved to be extensive ductal carcinoma in situ with focal atypical ductal hyperplasia, strong ER and PR immunostaining, and preservation of myoepithelial rimming (Figure 3). The cystic lesion nearby the hypoechoic lesion (so-called solid lesion) of 6 cm from nipple reveals an atypical intraductal papilloma involved by atypical ductal hyperplasia (Figure 4).
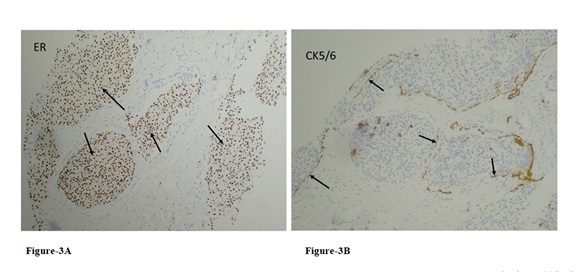
Figure 3 (A-B) Case two: A: Ductal carcinoma in situ with diffusely strong ER immunostaining (arrows) (ER, x100).
B: Myoepithelial rimming proved by linear or fragmented CK5/6 immunostaining (arrows) (CK5/6, x100) are found near the intraductal papillary lesion.
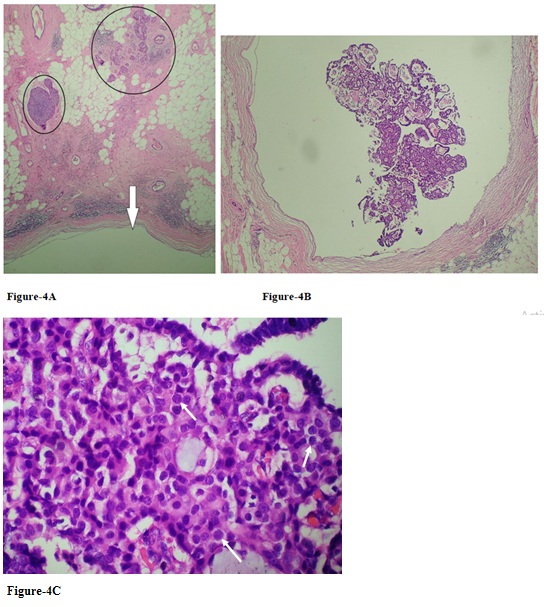
Figure 4 (A-C): The cystic lesion (arrow) measuring 0.57x0.53x0.66 cm, which is described on sonography, with associated solid lesion (circles)(H&E stain, x40) (A) reveals an intraductal papilloma (H&E stain, x100)(B) involved by atypical ductal hyperplasia with proliferation of monotonous cells (arrows) in the cores of focal papillary fronds (H&E stain, x400)(C)
DISCUSSION
Presence of myoepithelial cells in the situations of intraductal papilloma, ADH and DCIS can be proved by combination of part of the following IHC stains: p63, smooth muscle actin (SMA), and CD10 and high molecular weight cytokeratin, such as CK5/6 [11]. Atypical intraductal papilloma may have monocolonal proliferation of ductal epithelial cells with monotonous cell morphology and uniformly strong ER immunostaining on the ADH or DCIS lesions [7,11]. The parameters such as larger papilloma (> 1 cm) or older patient (> 54 years) may be the potential factors for coexistence of premalignant or malignant ductal lesion in the intraductal papilloma [6]. If the dimension of ADH is greater than 3 mm in a papilloma, then ductal carcinoma in situ in an intraductal carcinoma is admitted [7].
Entrapping of small epithelial nests in the fibrotic tissue around the capsule of papilloma should not be interpreted as malignancy directly, except for loss of myoepithelial cell rimming over the tumor nests of putative stromal invasion [8]. The myoepithelial cells surround benign mammary duct and preinvasive in situ carcinoma. However, the myoepithelial cells will disappear when they are outnumbered by invasive cancer cells.
The rimming myoepithelial cells are derived from ductal luminal epithelial cells, and the functions of myoepithelial cells are basement membrane production, involvement in tissue polarity and morphogenesis of mammary glands, and natural tumor suppressor [12].
These two cases have solid lesion around the intraductal papilloma. Case one has an unusual solid papillary lesion with loss of myoepithelial differentiation nearby the large atypical intraductal papilloma, but extensive production of CK5 antigen could be related to benign papilloma/Usual Ductal Hyperplasia (UDH) [13]. We noted that the loss of myoepithelial rimming of this solid lesion might mimic the loss of basal cell rimming over atrophic prostatic glands, like a degenerative process. Cautiously, old solid papillary lesions with focal central necrosis and fibrinous degeneration, and loss of myoepithelial rimming in case one may be hard to be distinguished from solid papillary carcinoma, encapsulated papillary carcinoma or intracystic papillary carcinoma [14]. Although benign ductal epithelial cells, such as UDH, may gain high molecular weight cytokeratin CK5 antigenicity, and mentioned as stem cell differentiation, the possibility of aging process of the ductal cells still exist on account of their low proliferative activity and production of high molecular weight cytokeratin over the ductal luminal cells, like our experience about atrophic change of uterine endocervical glands displaying CK5 (+) glandular cells [13] (Figure 5).
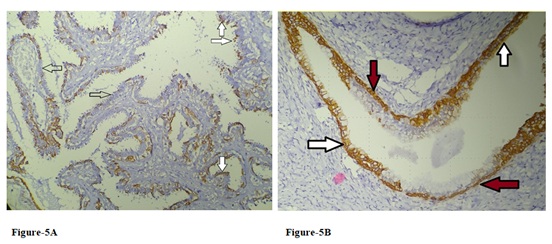 Figure 5 (A-B): A: In addition to myoepithelial cells lying at the base of papillary epithelial lining, loss of myoepithelial differentiation (empty arrows), and CK5 immunoreactivity at superficial cells (solid arrows) without underlying myoepithelial layer are another two fashions in the large papilloma of case one (CK5, x100).
Figure 5 (A-B): A: In addition to myoepithelial cells lying at the base of papillary epithelial lining, loss of myoepithelial differentiation (empty arrows), and CK5 immunoreactivity at superficial cells (solid arrows) without underlying myoepithelial layer are another two fashions in the large papilloma of case one (CK5, x100).
B: Atrophic change of postmenopausal uterine endocervical glands may reveal CK5 (+) atrophic glandular cells (white arrows) contrast with the CK5 (+) reserve cell over basal layer (red arrows) (CK5, x200).
In case one, three ominous signs about this atypical intraductal papilloma include A) atypical change in focal intraductal papilloma, B) atypical solid lesion around it, and C) invasion-like fronts with lower nuclear grade from the mural tumor far from the pedicle root of papilloma may misleading our interpretation. However, after adequate immunohistochemical studies, it turns out to be an atypical intraductal papilloma with surrounding benign solid lesion and loss of myoepithelial rimming, that might be an unusual degenerative process.
The other case is an extensive ductal carcinoma in situ probably causing the formation of atypical intraductal papilloma, which is involved by ADH. Previous neoplastic lesion might induce secondary intraductal papilloma with or without atypia should be kept in mind as well.
CONCLUSION
The solid lesion or invasion-like lesion around the intraductal papilloma may be the consequence or the cause of intraductal papilloma formation. The atypical or premalignant lesion in the intraductal papilloma may be a relevant or independent event related to the peri-papilloma solid lesions with pseudoinvasion.
ACKNOWLEDGMENTS
The authors would like to thank the colleagues of Department of Anatomic Pathology, St. Joseph’s Hospitalsien, Yunlin, Taiwan -- Dr. Jong-Shiun Chen for his thoughtful opinions, and medical technician Pei-Chi Hsien for her fine technique and aid.
REFERENCES
- Karadeniz E, Arslan S, Akcay MN, Subai ID, Demirci E (2016) Papillary Lesions of Breast. Chirurgia (Bucur) 111: 225-229.
- Gutman H, Schachter J, Wasserberg N, Shechtman I, Greiff F (2003) Are Solitary Breast Papillomas Entirely Benign ? Arch Surg 138: 1330-1333.
- Li A, Kirk L (2020) Intraductal Papilloma. StatPearls, USA.
- Troxell ML, Boulos F, Denkert C, Horii R, Yamaguchi R (2019) Intraductal papilloma. WHO Classification of Tumors – Breast Tumors, 5th Ed 52-56.
- Weidner N (2009) Intraductal papilloma of breast. Modern Surgical Pathology (Second Edition) Page No: 569-571.
- Kiran S, Jeong YJ, Nelson ME, Ring A, Johnson MB, et al. (2018) Are we overtreating intraductal papillomas? J Surg Res 231: 387-394.
- Jorns JM (2016) Papillary Lesions of the Breast: A Practical Approach to Diagnosis. Arch Pathol Lab Med 140: 1052-1059.
- Wei S (2016) Papillary Lesions of the Breast - An Update. Arch Pathol Lab Med 140: 628-643.
- Deugnier MA, Teulière J, Faraldo MM, Thiery JP, Glukhova MA (2002) The importance of being a myoepithelial cell. Breast Canc Res 4: 225-230.
- Sinn HP and Kreipe H (2013) “A Brief Overview of the WHO Classification of Breast Tumors, 4th Edition, Focusing on Issues and Updates from the 3rd Edition.” Breast Care (Basel) 8: 149-154.
- Pal SK, Lau SK, Kruper L, Nwoye U, Garberoglio C, et al. (2010) Papillary Carcinoma of the Breast: An Overview. Breast Cancer Res Treat 122: 637-645.
- Gudjonsson T, Adriance MC, Sternlicht MD, Petersen OW, Bissell MJ (2005) Myoepithelial Cells: Their Origin and Function in Breast Morphogenesis and Neoplasia. J Mammary Gland Biol Neoplasia 10: 261-272.
- Martinez AP, Cohen C, Hanley KZ, Li X (2016) Estrogen Receptor and Cytokeratin 5 Are Reliable Markers to Separate Usual Ductal Hyperplasia From Atypical Ductal Hyperplasia and Low-Grade Ductal Carcinoma In Situ. Arch Pathol Lab Med 140: 686-689.
- Hameed O, Humphrey PA (2010) Pseudoneoplastic Mimics of Prostate and Bladder Carcinomas. Arch Pathol Lab Med 134: 427-443.
Citation: Shih CM, Chen RH, Huang JM (2020) Atypical Intraductal Papilloma of Breast Accompanied by Solid Lesions – Report of Two Cases. J Cytol Tissue Biol 7: 027.
Copyright: © 2020 Chi-Min Shih, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

