
Basic Reproductive Parameters in the Population of Przewalski’s Horse
*Corresponding Author(s):
Jana DolezalovaCzech University Of Life Sciences Prague, Department Of Animal Science, Faculty Of Agrobiology, Food & Natural Resources, Prague 165 00, Czech Republic
Tel:+420 22438 6501,
Email:dolezalovajana22@gmail.com
Abstract
The present study’s objectives were to determine basic reproductive parameters in the population of Przewalski’s horse and to identify and assess factors of potential relevance to these parameters. Input data were obtained from the worldwide online Przewalski’s Horse Studbook and comprised information from 1899 until 2016. Interbirth interval was used as a reference trait. The average interbirth interval was 380.2 days. The highest-frequency (42.2 %) interbirth interval was in the range from 361 to 390 days. A non-significant effect of foal’s sex was observed, with mean interbirth interval 1.5 day longer following the birth of a male foal. In contrast, interbirth interval was significantly influenced by age of mare (P < 0.01). A 1-year increase of maternal age extended the interbirth interval by 0.64 day. This study demonstrated that the main factor influencing length of interbirth interval in Przewalski’s horse was age of mare.
Keywords
Age of mare; Equus przewalskii; Interbirth interval; Foaling rate; Reproduction
INTRODUCTION
Przewalski’s horse (Equus przewalskiiPoljakov, 1881 [1] is considered the last living wild horse species [2]. It was discovered by the Russian general N. M. Przewalskij, who brought back the skull and skin of a wild horse from a campaign to Mongolia in 1879. Two years later, this discovery was described as Equus przewalskiiPoljakov [3]. A recent study has suggested that Przewalski’s horse was not a true wild horse but a wild descendant of formerly domesticated horses of the so-called Botai culture from the territory of today’s Kazakhstan [4]. Further investigations would be required, however, to conclude that decisively.
The last free-living herd of this species was seen in 1967. The last animals were spotted around that time in the Mongolian region presently called Gobi B at the spring of Chonin-us in 1968 [5]. Since 1996, Przewalski’s horse has been recorded on the International Union for Conservation of Nature (IUCN) Red List of Threatened Species as extinct in the wild. Due to a growing population of horses in captivity and reintroduction programmes currently in progress, the status of the species was re-evaluated as “threatened” in 2011 [6].
Since 1899, when the first transport from Mongolia took place, Przewalski's horses have been in more than 350 ZOOs and breeding stations around the world. Many of these breeds have disappeared for various reasons [7]. Over 7,000 individuals kept in 170 institutions are now registered in the Studbook [8].
Przewalski’s horse is the closest relative of domesticated horse (Equus caballus) [9,10], but it is distinguished by a larger number of 66 chromosomes versus the 64 in domesticated horse [10,11]. Such reproductive parameters as oestrus cycle length and gestation length are quite similar in Przewalski’s horse and domesticated horses Equus caballus. The two species may interbreed and produce fertile offspring.
Assessing basic reproductive parameters in the population of Przewalski’s horse and their development in time are essential for identifying factors that may in near future significantly influence the size of this population. Research in the population of Przewalski’s horse with the aim of long-term maintenance of the species’ gene pool has been recommended by [12].
The importance of the present study lies in the fact that it is the first attempt to evaluate comprehensively the selected reproductive parameters recorded in Przewalski’s Horse Studbook since 1899.
The investigation of reproductive parameters within the whole population of Przewalski’s horse is crucial for success of the reintroduction programmes currently ongoing.
MATERIALS AND METHODS
The study is based on data from the worldwide online Przewalski’s Horse Studbook, which has been maintained by Prague Zoo since 1959. The analysed data consisted of records for mares and their offspring. Input data were comprised information from 1899 until 2016.
With respect to the fact that it was impossible to observe the animals continuously and thus to determine accurately the parturition–conception interval, interbirth interval was chosen as a reference parameter. The records of interbirth intervals shorter than 300 days and age of mare at first foaling younger than 2.8 years were excluded from the analysis. These thresholds were determined on the basis of information from the literature regarding the physiological development and maturity of mares and survival rate prognosis for offspring. To determine the effect of age of mare on interbirth interval, each of the mares was assigned to one of five groups according to its age: (I) <5, (II) 5–10, (III) 10–15, (IV) 15–20, and (V) >20 years of age.
To determine sex ratio changes and length of interbirth interval in period of time, were made with attention of data frequency 6 groups of time: until 1970, 1971-1980, 1981-1990, 1991-2000, 2001-2010, more than 2011. Data were analysed using the statistical analysis software package SAS 9.3 (SAS/STAT® 9.3, 2011). Basic statistical parameters were determined using MEANS and UNIVARIATE procedures. The REG procedure (STEPWISE method) was used to select an appropriate model. Various factors influencing interbirth interval (sex of foal, age of mare, and parity number) were examined.
The MIXED procedure followed by the Tukey–Kramer test was used to compare differences among groups. The statistical model for interbirth interval as dependent variable included the fixed effects of sex, parity number, age of mare at foaling, linear regression on year at foaling respectively.
The data were analysed also using the multifactorial analysis of variance according to the following equation
yijkl= μ + ai + bj+ ck + dl + b*(YEAR) + eijkl,
where:
yijkl is the dependent variable (interbirth interval),
μ is the mean value,
ai is the fixed effect of foal’s sex (i = females, n = 2971; i = males, n = 2796; i = abort, n = 96),
bj is the fixed effect of parity number (j = 1, n = 1331; j = 2, n = 1043; j = 3, n = 837; j = 4, n = 668; j = 5, n = 521; j = 6, n = 402; j = 7, n = 307; j = 8, n = 225; j = 9, n = 168; n = 10 and more, n = 360),
ck is fixed effect of age (in years) at foaling (k = ≤5, n = 1068; k = 5–10, n = 2438; k = 10–15, n = 1438; k = 15–20, n = 669; k = >20, n = 192),
dl is the fixed effect of mare as repeated factor,
b*(YEAR) is linear regression in year at foaling and eijkl is random error.
RESULTS
Over the observed period, the total number of mares was 3116. Total number of mares with at least one foal was 1330. The number of live-born foals was 5767, including 2971 females and 2796 males. The number of documented aborts and stillborns over the observed period was 96 (1.6%). The average number of foals per mare was 4.0. The highest number of foals, at 20, was seen in a mare that lived 27 years. Over the observed period, the total number of twins was 3 - in locations Askania Nova, Hustain Nuruu and Monarto). The average age of mares was 9.5 years, and the lowest and highest age at delivery was 2.8 and 27.9 years, respectively. The average mare age at first foaling was 5.4 years. The average interbirth interval was 380.7 days, while the shortest and longest values for live-born foals, respectively, were 300 and 753 days. The interbirth interval range seen most frequently (42.2 %) was that from 361 to 390 days (Figure 1). The average interbirth interval was 380.2 days following the birth of females, whereas it was about 1.5 day longer (381.7 days) in the case of male births.
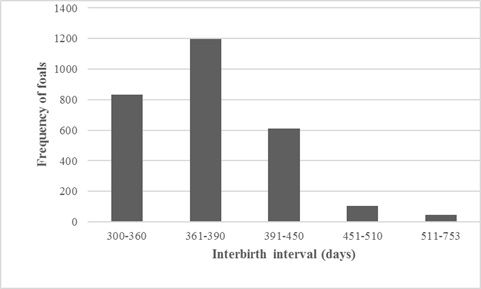 Figure 1: Interbirth interval length frequencies in the population of Przewalski’s horse (days).
Figure 1: Interbirth interval length frequencies in the population of Przewalski’s horse (days).
As shown in Table 1, age at foaling was significantly correlated with parity (r = 0.78; P < 0.001).
|
Parity |
Interbirth interval |
||
|
Mare age at foaling |
r |
0.781 |
0.071 |
|
P |
<0.001 |
0.001 |
|
|
n |
5803 |
2795 |
|
|
Parity |
r |
−0.026 |
|
|
P |
0.169 |
||
|
n |
2798 |
Table 1:Correlation between parity number and interbirth interval.
This correlation was confirmed by linear regression analysis (R2 = 0.005; P < 0.01) Figure 2). The value of the regression equation’s intercept was 374.15 ± 1.91 (P < 0.001). The slope value indicates an increase in interbirth interval with 1-year increase of age at foaling by 0.64 ± 0.17 days (P < 0.001).
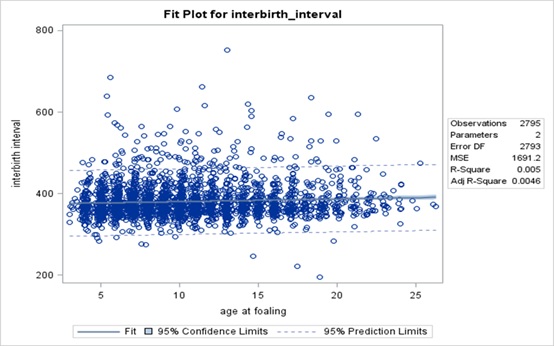 Figure 2: Relationship between age of mare at foaling and interbirth interval.
Figure 2: Relationship between age of mare at foaling and interbirth interval.
The analysis of variance results for the effects of sex, parity number, and age at foaling on interbirth interval in the population of Przewalski’s horse are given in Table 2. The equation used explained 3.3 % (P < 0.001) of interbirth interval variance. It is evident that the interbirth interval was significantly affected especially by parity number and age at foaling, whereas the sex of foal had no significant effect on this trait.
|
|
MODEL |
Sex |
Parity |
Age at foaling |
Year of foaling |
||||||
|
r2 |
P |
F-test |
P |
F-test |
P |
F-test |
P |
F-test |
P |
||
|
Interbirth interval |
0.033 |
<0.01 |
1.8 |
0.165 |
17.54 |
<0.01 |
6.03 |
<0.01 |
10.15 |
|
<0.01 |
Table 2: Analysis of variance.
Figure 3 shows the effect of parity number on interbirth interval. Increased parity number was associated with decreasing interbirth interval.
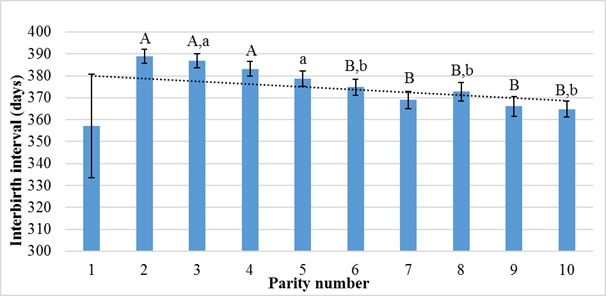 Figure 3: Effect of parity number on interbirth interval.
Figure 3: Effect of parity number on interbirth interval.
Statistical significance is indicated by the different letter on the columns A, B (P < 0.01); a,b (P < 0.05).
Figure 4 shows the effect age of mare at foaling on interbirth interval (P < 0.05). Increased age of mare was associated with increasing interbirth interval.
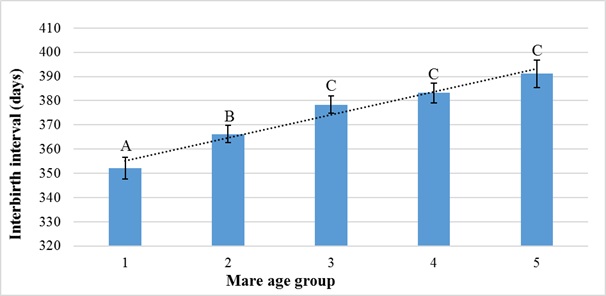 Figure 4: Effect of mare age at foaling on interbirth interval.
Figure 4: Effect of mare age at foaling on interbirth interval.
Interbirth intervals observed in mare age groups 1: 20 years were 352, 366, 378, 383, and 391 days, respectively. Statistical significance is indicated by the different letter on the columns A, B, C(P < 0.01).
Sex ratio and interbirth interval length in period of time is shown in Figure 5. The sex ratio remained almost unchanged during the observation period. More fillies were born than colts, statistically no significant. Over the years, the interbirth interval length has been shortening. Between years 1899 -1970, the interbirth interval was shortened by 9 days from 1971, until the end of the observation period by 3 days.
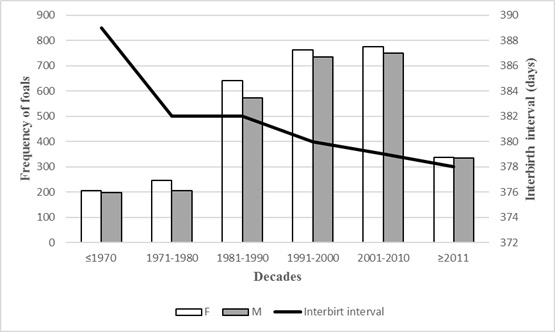 Figure 5: Sex ratio and interbirth interval of przewalski foals born in period of time.
Figure 5: Sex ratio and interbirth interval of przewalski foals born in period of time.
DISCUSSION
As reported by Dorj, et al. [13], Przewalski’s horse mares are capable of reproduction from 2 years of age. Depending on food and water availability, a similar age at first parturition has also been reported in wild Equidae species [14]. A progressive domestication process is reflected in reaching sexual maturity earlier, sometimes even well before reaching physical maturity. In extreme cases, this may negatively affect foetus development, mare health, and birthing difficulty [15]. First parturition in wild horses typically occurs at 4–11 years of age [5]. Similarly, Slotta-Bachmayr, et al. [16] reports that wild horse mares foal no sooner than at 4 years of age. Average mare age at first foaling of 5.4 years was observed in the present study. On average, 27.6% of first parturitions occurring between 3 - 4 years of age and 66% of mares until age of 6. Dorj, et al. reported in the population of Przewalski’s horse living in Hustain National Park on average, 24.7% of first parturitions occurring at 3 years of age and 55.6% at 4 years of age [13].
The ability to give birth at an early age depends especially on individual characteristics of mares and on an abundance of food and water [14].
It has previously been reported that average gestation length in the domestic horse is 333 days, with variation ranging from 310 to 360 days [17]. Mean gestation length reported by Volf J [2] was 335 days in both species, with variation from 328 to 348 days for the domestic horse and 328 to 343 daysfor Przewalski’s horse. Boyd LE, et al. reported gestation length and variation in Przewalski’s horse of 11 months and 45 to 50 weeks, respectively, and gestation length variation in domestic horse of 327 to 357 days [9]. Similar variation has been reported also by Ransom JI [14]. The gestation length of Przewalski’s mares in captivity has ranged from 320 to 343 days.
Instead of gestation length, interbirth interval was chosen for this study as a reference parameter, because it was impossible to continuously observe mating in the entire population of Przewalski’s horse. The average interbirth interval in this study was 382 days. If mares can potentially conceive 7 to 9 days after parturition, which would be similar to that observed in domestic horse, then the gestation length would be 373 to 375 days. This, however, would be inconsistent with the gestation length reported for both Przewalski’s horse and domestic horse in other studies. With respect to similar reproduction physiology in the two species [18], we conclude that the first oestrus after parturition in Przewalski’s horse is not always fully developed and that mares conceive at the second or third oestrus. Such a hypothesis may explain the shorter gestation length reported in other studies.
The interval between two consecutive parturitions depends upon the individual qualities of a given mare and the availability of resources. Young wild Przewalski’s mares seldom foal in the year following after birth of the first offspring [14]. After second and third parturitions, mares become more experienced and foal usually every year. Therefore, we had hypothesized that the first interbirth interval in the population of Przewalski’s horse would be the longest. This, however, was not confirmed by the results, and the first interbirth interval was even shorter than those following, probably due to large variation in length of the first interbirth interval as evidenced by high statistical error.
A tendency towards decreasing interbirth intervals with increasing parity has been reported also in Jeju pony [19] and indicates that Przewalski’s mares conceive (and foal) for the first time prior to completing their physical development. Thus, the mares are not able to secure sufficient resources to invest in a foal, to complete their development, and to conceive again. The longest interbirth intervals observed in young Przewalski’s mares corresponded with the second and third parities (Figure 3), and these were probably caused by longer parturition–conception intervals. During later pregnancies the requirement for completing physical development becomes less important and ultimately disappears completely, and thus the mares have more energy available for the following conception. Every subsequent pregnancy becomes more routine and, providing that sufficient resources are available, interbirth intervals is reduced due to earlier conceptions. In addition, the uterus surface area used for nutrient exchange grows with each pregnancy. As reported by [20] in Anglo-arabian mares, primiparous dams produced lighter and smaller foals and placentas at term and have a reduced placental efficiency compared to multiparous dams.
Uterus surface growth connected with good condition of placental efficiency results in faster foetus development and its earlier delivery, and thus in a reduction of gestation and interbirth interval lengths.
An effect of age of mare on interbirth interval (P < 0.05) in the present study is in contrast to findings reported in Equus quagga zebra [21] and in Equus burchelli zebra [22]. Increased age of mares was associated with increasing interbirth intervals by 0.59 day per year of mare age (Figure 4). Extended interbirth intervals in relation to onset of the aging process probably are due to deteriorated utilization of resources by older mares, which need more time to acquire sufficient energy to conceive again. Another caused was published by [23]. Mares older than 13-14 years usually require more mating per conception, which is explained by progressive degenerative changes in the endometrium and increased susceptibility to infections Gluckman,et al. [23]. The degenerative changes in the endometrium might potentially reduce its nutritive capacity for the development of the fetus [24]. Age-related decrease in fertility linked primarily to reduced oocyte quality rather than to impaired uterine function have been reported in warmblood, Friesian, Standardbred, Arabian, Cobs and Quarter-horse breeds [25]. A similar research has not been done in a Przewalski horse population.
Our results show the most fertile age for Przewalski’s mares to be between 5 and 10 years. This is in agreement with the findings of Dorj, et al. [13], who reported the optimum reproductive age in Przewalski’s horse to be from 5 to 15 years, and of Ransom, et al.[14], who determined the most fertile period to be from 6 to 16 years of age.
Barnier, et al. reported significantly longer interbirth intervals following the birth of male foals than after female foals in Equus quagga zebra [21]. Additional costs to a mare associated with a male foal resulting in a 1.5 day longer interbirth interval were also suggested in this study with Przewalski’s horse, but the differences lacked statistical significance. Greater demands of male foetuses resulting in longer gestations have been reported in Hannoverian warmblood [26], Warmblood [27,28]. English Thoroughbred, [29] mares [30] reported in their study that the reason for the variation of gestation length associated with the gender of the foal has not yet been completely elucidated. However, it is agreed that male body development is greater than female’s and therefore, if the birth occurs when the fetal development is complete, the gestation length of a colt would be longer [31].
In the przewalski horse population interbirth interval length decrease has founded during observation period. The greatest decline was recorded until 1970 and was probably associated with a lower frequency rate and inaccurate data recording. From 1971 until the end of the observation period, the length of the interbirth interval decreased by 3 days. Breeding management is almost similar during observation period. So, they caused might be connected with the attitude of breeders to this species. Since the 1970s, when the last wild individual of this species was seen in nature [5], the interest in saving this species and its return to the wild began to increase. Increased awareness and education of breeders might be one of the main factors in reducing the length of the interbirth interval in the worldwide population of Przewalski horses.
CONCLUSION
Interbirth intervals observed in the population of Przewalski’s horse significantly increased with the increasing age of mares. Mean interbirth interval following birth of a male foal was 1, 5 day longer, but the effect of foal’s sex lacked statistical significance.
CONFLICTS OF INTEREST
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
REFERENCES
- Groves C, Grubb P (2011) Ungulate Taxonomy. The Johns Hopkins Univ Press, Baltimore, USA.
- Volf J (2002) Odyseadivokýchkoní. Academia. Praha.
- Kus E (1994) Aklimatizace a perspektivyreintrodukcekoníPrevalského v Zaaltajské Gobi. Zoo Praha, Gazela.
- Gaunitz C, Fages, A, Hanghøj K, Albrechtsen A, Khan N, et al. (2018) Ancient genomes revisit the ancestry of domestic and Przevalski’s horses. Science 360: 111-114.
- Volf J (2000) Significant ungulates of the Mongolian Gobi. Proc. from the meeting, Zoo Praha, Gazella.
- Walzer C, Kaczensky P, Zimmermann W, Stauffer C (2007) Przewalski´s horse reintroduction to Mongolia: Status and Outlook. WAZA magazine. WAZA 77: 3-6.
- Ibler B, Kolter L, Šimek J (2019) EEP for the Przewalski horse (Equus ferus przewalskii) Recommendations and Analyses. Zoo Köln.
- Šimek J (2018) International Przewalski's Horse Studbook Equus ferusprzewalskii. Praha (SPARKS version).
- Boyd LE, Houpt KA (1994) Przewalski’s horse. The history and Biology of an endangered species. State University of N. Y. Press, Albany.
- Kefena E, Mekasha Y, Han JL, Rosenbom S, Haile A, et al. (2011) Discordances between morphological systematics and molecular taxonomy in the stem line of equids: A review of the case of taxonomy of genus Equus. Livest Sci 143: 105-115.
- Steiner CC, Ryder OA (2011) Molecular phylogeny and evolution of the Perissodactyla. Zool J Linn Soc 163: 1289-1303.
- Kus E (2000) International symposium for preservation of the Przewalski Horse. ZOO Praha. Gazella.
- Dorj U, Namkhai B (2013) Reproduction and Mortality of Re-introduced Przewalski’s Horse Equus przewalskii in Hustai National Park, Mongolia. J Live Sci 7: 623-629.
- Ransom JI, Kaczensky P (2016) Wild Equids: Ecology, Management and Conservation. Johns Hopkins Univ Press, Baltimore, USA.
- Volf J (1989) Die „wilde“ odergezielteAufzucht von Przewalskipferden (Equus przewalskiiPoljakov 1881). Zool Garten 59: 402-410.
- Slotta-Bachmayr L, Boegel R, Kaczensky P, Stauffer C, Walzer C (2004) Use of population viability analysis to identify management priorities and success in reintroducing Przewalski´s horses to southwetstern Mongolia. J Wildlife Manage 68: 790-798.
- Dušek J, Misar D, Müller Z, Navrátil J, Rajman J, et al. (2007) Chovkoní. Brázda. Praha.
- Collins CW, Monfort SL, Vick MM, Weis RB, Wildt DE, et al. (2008) Reproductive seasonality and follicular dynamics in the Przewalski´s horse. Reprod Fertil Dev 21: 176-176.
- Rho JR, Srygley RB, Choe JC (2004) Age-specific fertility rates of the Jeju pony (Equus caballus). J Ethol. 22: 113-117.
- Robles M, Dubois C, Gautier C, Dahirel M, Guenon I, et al. (2018) Maternal parity affects placental development, growth and metabolism of foals until 1 year and a half. Theriogenology 108: 321-330.
- Barnier F, Grange S, Ganswindt A, Ncube H, Duncan P (2012) Inter-birth interval in zebras is longer following the birth of male foals than after female foals. Acta Oecol 42: 11-15.
- Pluhácek J, Bartoš L, Culík L (2006) High-ranking mares of captive plains zebras Equus burchelli have greater reproductive success than low-ranking mares. Appl Anim Behav Sci 99: 315-329.
- Gluckman PD, Hanson MA (2004) Maternal constraint of fetal growth and its consequences. Sem Fetal Neon Med 9: 419-425.
- Satué K, Felipe M, Mota J, Munoz A (2011) Gestational length in Carthusian broodmares: effects of breeding season, foal gender, age of mare, year of parturition, parity and sire. Pol J Vet Sci 14: 173-180.
- Cuervo-Arango J, Claes AN, Stout TA (2019) A retrospective comparison of the efficiency of different assisted reproductive techniques in the horse, emphasizing the impact of maternal age. Theriogenology 132: 36-44.
- Christmann A, Sieme H, Martinsson G, Distl O (2017) Genetic and environmental factors influencing gestation length and parturition conception interval in Hanoverian warmblood. Livest Sci 199: 63-68.
- Kuhl J, Stock KF, Wulf M, Aurich C (2015) Maternal Lineage of Warmblood Mares Contributes to Variation of Gestation Length and Bias of Foal Sex Ratio. PLOS ONE.10: 1- e0139358.
- Wulf M, Erber R, Ille N, Beythien E, Aurich J, et al. (2017) Effects of foal sex on some perinatal characteristics in the immediate neonatal period in the horse. J Vet Behav 18: 37-42.
- Davies-Morel DMC, Newcombe JR, Holland SJ (2002) Factors affecting gestation length in the thoroughbred mare. Anim Reprod Sci 74: 175-185.
- Wilsher S, Allen WR (2003) The effects of maternal age and parity on placental and fetal development in the mare. Equine Vet J 35: 476-483.
- https://przwhorse.zoopraha.cz
Citation: Dolezalova J, Eichler V, Duchacek J,Kus E, Neumann C, et al. (2020) Basic Reproductive Parameters in the Population of Przewalski’s Horse. Archiv Zool Stud 3: 015.
Copyright: © 2020 Jana Dolezalova, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

