
Journal of Nanotechnology Nanomedicine & Nanobiotechnology Category: Medical
Type: Review Paper
Beauty of Fine Dots-Detection and Treatment of Alzheimer's Disease Using CdSe/ZnS Quantum Dots
*Corresponding Author(s):
Anida DevedzicDepartment Of Genetics, University Of Sarajevo Bosnia And Herzegovina, Balkans, Bosnia And Herzegovina
Tel:+003 8761455198,
Email:anidadevedzic@gmail.com
Received Date: Apr 08, 2017
Accepted Date: Jun 10, 2017
Published Date: Jun 29, 2017
Abstract
This paper presents a review on available researches, when it comes to the usage of quantum dots in detection and treatment of the most common form of dementia, and that is Alzheimer’s Disease (AD). Quantum Dots (QDs), as an essential material of nanotechnology give unique approaches and solutions for preventing and curing AD. In this paper CdSe/ZnS quantum dots and their application features are analyzed and proposed as the possible solution for inhibition of events in the organism, that occur before the patients show visible symptoms.
Scientists around the world truly believe, that nanotechnology in fact is the future of medicine and that this emerging technology, although still in a “new-born” state, would provide answers for the treatment of many to this date incurable disease, as AD itself. Discussed approaches are still in an experimental phase, because the possible toxicology of many nanomaterials isn’t fully inves-tigated.
Scientists around the world truly believe, that nanotechnology in fact is the future of medicine and that this emerging technology, although still in a “new-born” state, would provide answers for the treatment of many to this date incurable disease, as AD itself. Discussed approaches are still in an experimental phase, because the possible toxicology of many nanomaterials isn’t fully inves-tigated.
Keywords
Alzheimer’s Disease; Nanotechnology; Quantum Dots
INTRODUCTION
Alzheimer's Disease (AD) is a fatal neurodegenerative disease. It's the most common form of dementia and its occurrence is expected to double annually by the year 2050 [1]. In Europe, in 2015, 10.5 million people were registered with dementia. Besides Europe, figure 1. Shows the numbers of other world regions and their dementia patients. By 2018, dementia would be a trillion dollar disease worldwide [2]. What triggers the degenerative process of neural tissue is yet an unknown fact but it leads to specific neurodegenerative characteristics of malfunction and eventual death of neurons of the brain the most complex organ in the human body. Women are twice more affected with this disease than men are. Also Alzheimer is the third most expensive disease in the United States besides other causes of death, for example heart disease, which is statistically declining contrary to the specific state of the brain. The disease roughly affects people from the age 65 and above [1].
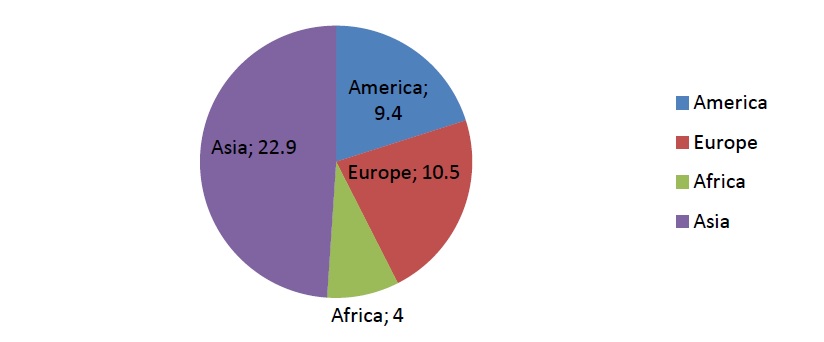
Figure 1: Estimated number of people living with dementia in each world region in 2015 (in millions) [1].
Possible biomarkers for AD are high concentrations of beta-amyloid and tau proteins. Amyloid beta-derived Diffusible Ligands (ADDLs) are small, toxic and highly amyloidogenic 42 amino acid amyloid beta peptides, that accumulate outside and tau proteins inside the neurons [3]. These accumulations form a proinflammatory and microglia-activating senile plaque that kills neurons and synapses as time progresses [3]. They are considered as precursors for amyloid fibrillation in Alzheimer's disease [4]. It is assumed that the action of secretases (ß and γ) lead to higher concen-trations of Aß peptides, that form plaques on glial and neuron receptors [5].
Another possible biomarker is the increasement of Reactive Oxygen Species (ROS) in the brain, which is a byproduct of oxygen metabolism and its appearance can't be avoided, because of the fact that the brain needs oxygen for its function [1].
At this moment there is no cure for AD and scientist are aiming to produce a technology for early treatment and diagnosis, before the actual clinical signs of AD occur [2]. When it comes to current treatments the most common are: Rivastigmine, Donepezil and Memantine. Rivastigmine treatment is an effective, but yet limited form of treatment, because of the strength of the Blood Brain Barrier (BBB) [2].
The blood brain barrier is a separation circulating blood, which acts as a protection against brain bacterial infections. Larger molecules and even antibodies can't cross this barrier, but viruses can. Because of this selective permeability feature, many drug treatments aren't successful [2].
Paul Erlich an American biologist gave a visual description of how the BBB partitions the brain from the rest of the blood stream, by injecting a dye stain into the bloodstream of an animal, after which all the organs except the brain were colored. He repeated the same by injecting the stain in the cerebral spinal fluid, which resulted in dyeing of the brain only [2].
To overcome this drug deliver difficulty, scientists came up with a “Trojan Horse”tactic. And here comes to the light the use of nanoparticles, because of their characteristic they are the ideal solution for crossing the BBB, instead of viral vectors, which could activate the production of antibodies and other peptides. Also a study showed that Rivastigmine is three times more effective when attached to the nanoparticle and injected intravenously, than as a free form of drug [2].
Nanoparticles are ideal for this kind of treatment; because they increase the duration of the drug circulates across the BBB, making them easily interact with specific molecules on the luminal side of the barrier [6].
Nanotechnology alongside with the usage of nanoparticles represents a great solution in order to prevent the damage of the subject's memory and its functional and cognitive skills [1]. Richard Feynman is considered the father of nanotechnology because he was the first who introduced the idea about controlling and manipulating atoms or any other subjects in the range of nanometer scale. He proposed this idea during his lecture titled “There is plenty of room at the bottom“ in December 1959 at the CalTech Institute [3]. It is a multidisciplinary field that involves design, synthesis, characterization and application of materials and devices, which size is in the nanometer range [4].
Quantum Dots (QDs) are colloidal semi-conductive fluorescent nanocrystals or a form of semiconductor nanoparticles whose low concentrations in the circulation are sufficient to inhibit the fibrillation process [6,7]. They can withstand significantly more cycles of excitations and light emissions than any other typical organic molecule [8].

Figure 1: Estimated number of people living with dementia in each world region in 2015 (in millions) [1].
Possible biomarkers for AD are high concentrations of beta-amyloid and tau proteins. Amyloid beta-derived Diffusible Ligands (ADDLs) are small, toxic and highly amyloidogenic 42 amino acid amyloid beta peptides, that accumulate outside and tau proteins inside the neurons [3]. These accumulations form a proinflammatory and microglia-activating senile plaque that kills neurons and synapses as time progresses [3]. They are considered as precursors for amyloid fibrillation in Alzheimer's disease [4]. It is assumed that the action of secretases (ß and γ) lead to higher concen-trations of Aß peptides, that form plaques on glial and neuron receptors [5].
Another possible biomarker is the increasement of Reactive Oxygen Species (ROS) in the brain, which is a byproduct of oxygen metabolism and its appearance can't be avoided, because of the fact that the brain needs oxygen for its function [1].
At this moment there is no cure for AD and scientist are aiming to produce a technology for early treatment and diagnosis, before the actual clinical signs of AD occur [2]. When it comes to current treatments the most common are: Rivastigmine, Donepezil and Memantine. Rivastigmine treatment is an effective, but yet limited form of treatment, because of the strength of the Blood Brain Barrier (BBB) [2].
The blood brain barrier is a separation circulating blood, which acts as a protection against brain bacterial infections. Larger molecules and even antibodies can't cross this barrier, but viruses can. Because of this selective permeability feature, many drug treatments aren't successful [2].
Paul Erlich an American biologist gave a visual description of how the BBB partitions the brain from the rest of the blood stream, by injecting a dye stain into the bloodstream of an animal, after which all the organs except the brain were colored. He repeated the same by injecting the stain in the cerebral spinal fluid, which resulted in dyeing of the brain only [2].
To overcome this drug deliver difficulty, scientists came up with a “Trojan Horse”tactic. And here comes to the light the use of nanoparticles, because of their characteristic they are the ideal solution for crossing the BBB, instead of viral vectors, which could activate the production of antibodies and other peptides. Also a study showed that Rivastigmine is three times more effective when attached to the nanoparticle and injected intravenously, than as a free form of drug [2].
Nanoparticles are ideal for this kind of treatment; because they increase the duration of the drug circulates across the BBB, making them easily interact with specific molecules on the luminal side of the barrier [6].
Nanotechnology alongside with the usage of nanoparticles represents a great solution in order to prevent the damage of the subject's memory and its functional and cognitive skills [1]. Richard Feynman is considered the father of nanotechnology because he was the first who introduced the idea about controlling and manipulating atoms or any other subjects in the range of nanometer scale. He proposed this idea during his lecture titled “There is plenty of room at the bottom“ in December 1959 at the CalTech Institute [3]. It is a multidisciplinary field that involves design, synthesis, characterization and application of materials and devices, which size is in the nanometer range [4].
Quantum Dots (QDs) are colloidal semi-conductive fluorescent nanocrystals or a form of semiconductor nanoparticles whose low concentrations in the circulation are sufficient to inhibit the fibrillation process [6,7]. They can withstand significantly more cycles of excitations and light emissions than any other typical organic molecule [8].
WHY ARE QUANTUM DOTS SO UNIQUE?
The QDs unique structure consists of a semiconductor material core (cadmium mixed with selenium or tellurium), and coated with an additional semiconductor shell (usually zinc sulfide) with a 3D confinement [9,10]. They have special fluoresce properties that include minimal photo bleaching, optimal stability, high signal to noise ratio and broad absorption spectrum with very narrow but size-dependent tunable emission spectrum. Because of these features, QDs are ideal for long term tracking and visualization of many molecular events. And this is especially important for the diagnosis of AD and few of their biomarkers in the AD pathology [11].
When it comes to optoelectronic features QDs have unique spectral sharp emission peaks when excited and high quantum efficiency. They provide selective attachment to biological particles in the circulation [6].
When it comes to optoelectronic features QDs have unique spectral sharp emission peaks when excited and high quantum efficiency. They provide selective attachment to biological particles in the circulation [6].
APPLICATION OF DHLA-COATED CDSE/ZNS QUANTUM DOTS
A group of researchers have proved that Dihydrolipoic Acid (DHLA)- coated CdSe/ZnS quantum dots mixed or conjugated with Aß inhibit the fibrillation process. It is also calculated, that the QDs size of about 2.5 nm is the ideal one for the inhibition of the process. Smaller QDs are capable of passing the blood brain barrier more easily which is also an important parameter for the biodiagnostic studies. Also we cannot generalize the concept that some nanoparticles inhibitor promotes the process of fibrillation [8].
Statistical analysis of the TEM images (Figure 2) confirms that QDs are enveloping the fibrils and are mostly concentrated at the ends of the fibrils, where they inhibit the process of elongation. Number of fibrils having length 50-100 nm decreased to 90%. This was the case with the fibrils mixed with QDs, whereas the number of the same fibrils, but this time only conjugated to QDs, dropped to 26%. It is considered, that the reason for the inhibition of the fibrillation process lies in the small size of the nanoparticle and its ability to block the C-terminal end of the fibrils [8].
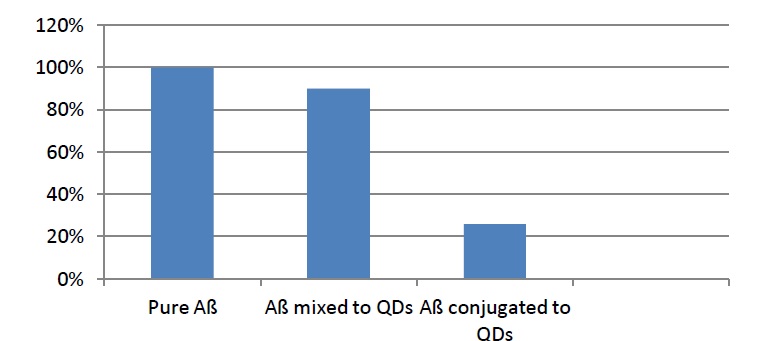
Figure 2: Statistical analysis of the TEM images [7].
The results also showed some very interesting differences between mixed and conjugated fibrils with quantum dots. Samples containing pure Aß have given the following results: short-length fibrils were about 30 nm long and the size around 2 microns was observed for longer fibrils. Aß oligomers mixed with QDs have been in the size range of about 1 micron for long fibrils, but the size for short-length fibrils wasn't significantly different. The greatest difference was detected with the Aß conjugated to QDs, where the thickness of the fibrils was 10 ± 3 nm, contrary to the samples mixed with QDs, which width was 7.7 ± 0.7 nm. Although, both ways showed inhibition in fibrillation process, fibrils conjugated with QDs showed a greater inhibition rate of fibrillation. But this wasn't the case with PEG (Polyethylene Glycol)-coated CdSe/ZnS quantum dots, where no inhibition of the fibrillation process was observed. A possible reason for this result might be that PEG polymer increases the size of QDs and PEG-coated QDs tend to aggregate in buffer solution, because they are less dynamic and less accessible for the Aß monomers there. Changing the ligand of the QDs, we influence the behavior towards the fibrillation process [8].
The whole experiment was done after the incubation time at 37ºC for seven days with three samples (pure Aß, Aß mixed and conjugated with QDs), together with TEM (Transmission Electron Microscopy), AFM (Atomic Force Microscopy) imaging and ThT (Thioflavin T spectroscopic) assay which showed the final results [8].
Besides the statistical analyses, AFM imaging and ThT assay supported the premise of QDs inhibiting the elongation process of Aß oligomers, as it was shown in the TEM images. Emphasizing the most significant result obtained from the analyses and that is QDs conjugated with fibrils decrease their length [8].
It is also important to mention that all the methods and their parameters confirmed the results from TEM images [8].
Study with TiO2-coated quantum dots pointed out that these kinds of nanoparticles promote the fibrillation process by becoming nucleation centers. Using tyrosine fluorescence spectra for the same three samples, only now coated with tyrosine, the intensity of emission was high, which directly implied on the promotion of the fibrillation process. Researchers think that this effect happened because of the two possible reasons: 1. Tyrosine moiety interacts with the QDs. The over coated ZnS shell offers Zn ions that give tyrosine the chance to interact with QDs. 2. FRET (Förster Resonance Energy Transfer) mechanism between the donor (tyrosine moiety) and the acceptor (QDs), because of the overlap between the QDs absorption spectrum and tyrosine emission spectrum. Calculated Förster distance was less than 60 Å, which is the critical distance for energy transfer and the indirect proof that QDs are present very near to fibrils [8].
Statistical analysis of the TEM images (Figure 2) confirms that QDs are enveloping the fibrils and are mostly concentrated at the ends of the fibrils, where they inhibit the process of elongation. Number of fibrils having length 50-100 nm decreased to 90%. This was the case with the fibrils mixed with QDs, whereas the number of the same fibrils, but this time only conjugated to QDs, dropped to 26%. It is considered, that the reason for the inhibition of the fibrillation process lies in the small size of the nanoparticle and its ability to block the C-terminal end of the fibrils [8].

Figure 2: Statistical analysis of the TEM images [7].
The results also showed some very interesting differences between mixed and conjugated fibrils with quantum dots. Samples containing pure Aß have given the following results: short-length fibrils were about 30 nm long and the size around 2 microns was observed for longer fibrils. Aß oligomers mixed with QDs have been in the size range of about 1 micron for long fibrils, but the size for short-length fibrils wasn't significantly different. The greatest difference was detected with the Aß conjugated to QDs, where the thickness of the fibrils was 10 ± 3 nm, contrary to the samples mixed with QDs, which width was 7.7 ± 0.7 nm. Although, both ways showed inhibition in fibrillation process, fibrils conjugated with QDs showed a greater inhibition rate of fibrillation. But this wasn't the case with PEG (Polyethylene Glycol)-coated CdSe/ZnS quantum dots, where no inhibition of the fibrillation process was observed. A possible reason for this result might be that PEG polymer increases the size of QDs and PEG-coated QDs tend to aggregate in buffer solution, because they are less dynamic and less accessible for the Aß monomers there. Changing the ligand of the QDs, we influence the behavior towards the fibrillation process [8].
The whole experiment was done after the incubation time at 37ºC for seven days with three samples (pure Aß, Aß mixed and conjugated with QDs), together with TEM (Transmission Electron Microscopy), AFM (Atomic Force Microscopy) imaging and ThT (Thioflavin T spectroscopic) assay which showed the final results [8].
Besides the statistical analyses, AFM imaging and ThT assay supported the premise of QDs inhibiting the elongation process of Aß oligomers, as it was shown in the TEM images. Emphasizing the most significant result obtained from the analyses and that is QDs conjugated with fibrils decrease their length [8].
It is also important to mention that all the methods and their parameters confirmed the results from TEM images [8].
Study with TiO2-coated quantum dots pointed out that these kinds of nanoparticles promote the fibrillation process by becoming nucleation centers. Using tyrosine fluorescence spectra for the same three samples, only now coated with tyrosine, the intensity of emission was high, which directly implied on the promotion of the fibrillation process. Researchers think that this effect happened because of the two possible reasons: 1. Tyrosine moiety interacts with the QDs. The over coated ZnS shell offers Zn ions that give tyrosine the chance to interact with QDs. 2. FRET (Förster Resonance Energy Transfer) mechanism between the donor (tyrosine moiety) and the acceptor (QDs), because of the overlap between the QDs absorption spectrum and tyrosine emission spectrum. Calculated Förster distance was less than 60 Å, which is the critical distance for energy transfer and the indirect proof that QDs are present very near to fibrils [8].
PHOTOSTIMULATION AND QDS
Scientists are trying a new technique by stimulating the cortical neurons with natural light conjugated with QDs. This is a very promising technique, mostly because of its non-invasive properties, which do not include any genetically or chemical manipulation. It is proven that optically excited QDs can perturb the electrochemical equilibrium of a cell membrane [12]. Aß aggregates in AD induce the process of depolarization of the cell membrane [9]. By using CdSe QDs we could inhibit this event, because of their unique optoelectronic features mentioned above. QDs size, about 3-4 nm, is appropriate for crossing the cell membrane and it has also a protein range size. They can be activated and deactivated by turning on and off the excitation light [12].
Similar research was done in the field of motor dysfunctions in dementia. It was the first study to introduce a novel type of Aß-induced cytotoxicity. A reversible depolarization besides the influence of oligomers aggregates, originates from increased Na+-influx to muscle fibers trough TTX (Tetrodotoxin)-sensitive Na+-channels [13]. Aß concentration from 10-6 to 10-8 lead to slow significant and reversible depolarization of the skeletal muscle membrane in the resting potential state. These concentrations were derived from the experiment with the frog skeletal muscle fibers According to these findings, some scientists are referring to AD as a disorder of plasma membrane [10,13].
It is important to say that Aß-induced depolarization is concentration dependent and reversible [13].
Aß oligomers lead to hyperexcitability, due to the induced membrane depolarization and this hyperexcitability causes cognitive deficits in early and mid stages of AD. This state of neurons, influenced by the event of hyperexcitability, probably occurs before the cell death, that Ca-influx induces [9].
Together with the usage of quantum dot films it is possible to govern the opening/closing of ion channels, by which the events such as depolarization and hyper polarization can be controlled. Quantum dots provide us with better understanding of neural behavior, because the same cells function and communicate through electrical signaling. With the excitation of dots through light, we actually excite electrons inside the QDs. And if the voltage change of the membrane within this event is high enough, action potential is generated. Action potential is the signal of communication between neurons. This is the leading premise of all experiments made in the field of Photostimulation [12,14].
Excited QDs produce a temporary electric dipole. This dipole moment triggers an electric field and with enough strength it can stimulate the opening of ion channels. Dipole moment occurs because of the charge separation between the electron and hole in the exciton of the QD (Figure 3) [14].
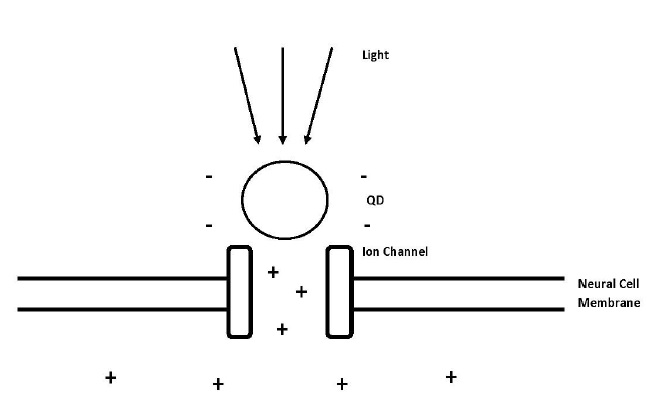
Figure 3: Process of induced depolarization using quantum dots and light energy. The light excites electrons inside the quantum dots and because of that they are producing electric dipole moment, which opens the voltage-gated ion channel and lets the positively charged ions flow into the cell and change the action potential of the neural cell [12].
In one of these experiments, the mentioned statements above were confirmed, together with the usage of CdSe films and probes. Events such as hyperpolarization or depolarization were observed through patch clamp recording with the results of Na+ and K+ activated channels [12].
The delivery of QDs to the human brain tissue wouldn't be a problem, thanks to the possibility of the modification of QDs surface and attachment of many molecules that can selectively bind the dots on the specific neural cells [12]. The selectivity here is a very important feature, due to the binding to the proteins and receptors on the surface of the cell. They are ideal for the changing of membrane action potential, because all the receptors and proteins on its surface are in a nanometer range scale [12].
Nanoparticles were firstly used only for imaging, but their unique optoelectronic features are far beyond this type of their usage. Today they are widely used in photovoltaics, lasers and light emitting diodes [14].
When it comes to the component of light and its delivery to the brain, the solution would be the retina because it naturally absorbs light [12].
In order to get the most convenient and satisfied results when going through this type of QD application, we have to come across few limitations: the strength of the dipole moment, the size of the nanocrystals and its separation distance from the ion channel [14].
Similar research was done in the field of motor dysfunctions in dementia. It was the first study to introduce a novel type of Aß-induced cytotoxicity. A reversible depolarization besides the influence of oligomers aggregates, originates from increased Na+-influx to muscle fibers trough TTX (Tetrodotoxin)-sensitive Na+-channels [13]. Aß concentration from 10-6 to 10-8 lead to slow significant and reversible depolarization of the skeletal muscle membrane in the resting potential state. These concentrations were derived from the experiment with the frog skeletal muscle fibers According to these findings, some scientists are referring to AD as a disorder of plasma membrane [10,13].
It is important to say that Aß-induced depolarization is concentration dependent and reversible [13].
Aß oligomers lead to hyperexcitability, due to the induced membrane depolarization and this hyperexcitability causes cognitive deficits in early and mid stages of AD. This state of neurons, influenced by the event of hyperexcitability, probably occurs before the cell death, that Ca-influx induces [9].
Together with the usage of quantum dot films it is possible to govern the opening/closing of ion channels, by which the events such as depolarization and hyper polarization can be controlled. Quantum dots provide us with better understanding of neural behavior, because the same cells function and communicate through electrical signaling. With the excitation of dots through light, we actually excite electrons inside the QDs. And if the voltage change of the membrane within this event is high enough, action potential is generated. Action potential is the signal of communication between neurons. This is the leading premise of all experiments made in the field of Photostimulation [12,14].
Excited QDs produce a temporary electric dipole. This dipole moment triggers an electric field and with enough strength it can stimulate the opening of ion channels. Dipole moment occurs because of the charge separation between the electron and hole in the exciton of the QD (Figure 3) [14].

Figure 3: Process of induced depolarization using quantum dots and light energy. The light excites electrons inside the quantum dots and because of that they are producing electric dipole moment, which opens the voltage-gated ion channel and lets the positively charged ions flow into the cell and change the action potential of the neural cell [12].
In one of these experiments, the mentioned statements above were confirmed, together with the usage of CdSe films and probes. Events such as hyperpolarization or depolarization were observed through patch clamp recording with the results of Na+ and K+ activated channels [12].
The delivery of QDs to the human brain tissue wouldn't be a problem, thanks to the possibility of the modification of QDs surface and attachment of many molecules that can selectively bind the dots on the specific neural cells [12]. The selectivity here is a very important feature, due to the binding to the proteins and receptors on the surface of the cell. They are ideal for the changing of membrane action potential, because all the receptors and proteins on its surface are in a nanometer range scale [12].
Nanoparticles were firstly used only for imaging, but their unique optoelectronic features are far beyond this type of their usage. Today they are widely used in photovoltaics, lasers and light emitting diodes [14].
When it comes to the component of light and its delivery to the brain, the solution would be the retina because it naturally absorbs light [12].
In order to get the most convenient and satisfied results when going through this type of QD application, we have to come across few limitations: the strength of the dipole moment, the size of the nanocrystals and its separation distance from the ion channel [14].
NANOTOXICOLOGY
Nanoparticles are extremely small microscopic particles that could cause potential hazards for the living organisms. A number of potential pathologies could be caused by nanoparticles, like that in respiratory, cardiovascular and gastrointestinal system [3]. Because of this problem, with the use of nanoparticles and growing funding for many researches of this type, a new discipline in science arose and that is nanotoxicology [15]. Also there is a lack of standardize protocols for testing their possible toxic impact on biologic systems [16].
The essence for these health concerns lies in the fact, that nanoparticles are able to enter the human body through several ports, including first the most common and that is the respiratory system, via the lungs, from which point it enters the blood stream, and by that action it affects the organs. Also nanoparticles can enter the central nervous system directly through the axons of olfactory pathway or through the olfactory bulb [6].
QDs toxicity depends on several factors: their size, concentration, charge, outer coating bioactivity (capping material and functional groups), oxidative photolytic and mechanical stability. QD physicochemical properties are the most important feature to understand, when it comes to their eventual toxicity, where QDs stability plays a crucial role, since it easily degrades itself under photolytic and oxidative conditions, realizing toxic substances in the organism [16].
Some studies have shown that the toxicity of the QDs is size and concentration related and that CdSe quantum dots are toxic and aren't suitable for medical purposes, but with the ZnS coating it was proven a significant decrease in toxicity in cell culture [15].
The main idea with the usage of ZnS coating was to reduce the leakage of Cd into the bloodstream and its possible aggregation in tissues, which is very toxic for living organisms. Selenium is an essential metal but small concentrations of the ionic form (Se2-) are very toxic. Zn and Cd are in the same group of the periodic table (IIb), as well as Se and S (VIa). Because of this schedule, an ionic replacement is easily possible (Zn with Cd, S of with Se). This mechanism was proposed by Derfus et al. (2004). But later, Kloepfer et al., stated that cadmium leaks nevertheless the presence of the ZnS coating and has suggested that the coating actually decreases the stability of QDs and causes an even greater toxicity [17].
Although biochemically incompatible and toxic cadmium telluride nanoparticles are ideal for the use in nanotechnology, because of their physical similarities to proteins that would block the fibrillation process and because of their familiarity with the compound. Nanoparticles may be toxic to living organisms, because of their ability to induce release of proinflammatory mediators, which cause inflammatory response and damage of organs [7]. The ideal nanoparticle would be biodegradable and nontoxic [2].
The question whether QDs actually cause AD, is still a controversial subject and there is no scientific proof for it. But another question remains unanswered and that is what happens with the remaining nanoparticles after the drug introduction? Do they affect the brain [2]?
Many studies must be carried out for the use of nanoparticles in humans, because known experiments were made only on animal models and in vitro and the effect on human system is difficult to evaluate from these kinds of researches [15]. For example, in 2013 researchers showed that QD-based imaging conducted on a monkey model showed no adverse effects, giving the fact that the QDs accumulated themselves in lymph nodes, bone marrow, spleen and liver up to 3 months after injection [18].
It is necessary to make the following analyses for better understanding of potential toxicity of nanoparticles, and that are: analysis of the effect of physicochemical properties on cell bioavailability, uptake and bioprocessing [15].
The essence for these health concerns lies in the fact, that nanoparticles are able to enter the human body through several ports, including first the most common and that is the respiratory system, via the lungs, from which point it enters the blood stream, and by that action it affects the organs. Also nanoparticles can enter the central nervous system directly through the axons of olfactory pathway or through the olfactory bulb [6].
QDs toxicity depends on several factors: their size, concentration, charge, outer coating bioactivity (capping material and functional groups), oxidative photolytic and mechanical stability. QD physicochemical properties are the most important feature to understand, when it comes to their eventual toxicity, where QDs stability plays a crucial role, since it easily degrades itself under photolytic and oxidative conditions, realizing toxic substances in the organism [16].
Some studies have shown that the toxicity of the QDs is size and concentration related and that CdSe quantum dots are toxic and aren't suitable for medical purposes, but with the ZnS coating it was proven a significant decrease in toxicity in cell culture [15].
The main idea with the usage of ZnS coating was to reduce the leakage of Cd into the bloodstream and its possible aggregation in tissues, which is very toxic for living organisms. Selenium is an essential metal but small concentrations of the ionic form (Se2-) are very toxic. Zn and Cd are in the same group of the periodic table (IIb), as well as Se and S (VIa). Because of this schedule, an ionic replacement is easily possible (Zn with Cd, S of with Se). This mechanism was proposed by Derfus et al. (2004). But later, Kloepfer et al., stated that cadmium leaks nevertheless the presence of the ZnS coating and has suggested that the coating actually decreases the stability of QDs and causes an even greater toxicity [17].
Although biochemically incompatible and toxic cadmium telluride nanoparticles are ideal for the use in nanotechnology, because of their physical similarities to proteins that would block the fibrillation process and because of their familiarity with the compound. Nanoparticles may be toxic to living organisms, because of their ability to induce release of proinflammatory mediators, which cause inflammatory response and damage of organs [7]. The ideal nanoparticle would be biodegradable and nontoxic [2].
The question whether QDs actually cause AD, is still a controversial subject and there is no scientific proof for it. But another question remains unanswered and that is what happens with the remaining nanoparticles after the drug introduction? Do they affect the brain [2]?
Many studies must be carried out for the use of nanoparticles in humans, because known experiments were made only on animal models and in vitro and the effect on human system is difficult to evaluate from these kinds of researches [15]. For example, in 2013 researchers showed that QD-based imaging conducted on a monkey model showed no adverse effects, giving the fact that the QDs accumulated themselves in lymph nodes, bone marrow, spleen and liver up to 3 months after injection [18].
It is necessary to make the following analyses for better understanding of potential toxicity of nanoparticles, and that are: analysis of the effect of physicochemical properties on cell bioavailability, uptake and bioprocessing [15].
CONCLUSION
Nanotechnology as an emerging new technology, gives us great insights on possible solutions when it comes to curing Alzheimer's disease and many other clinical states. The usage of quantum dots in this subject is a truly fascinating finding, which opens completely new perspectives in the treatment of the most common form of dementia. In this review paper are shown accessible researches and their results, and the information about the progress of the same. Unique properties of QDs are the ones, who play a major role in the development of nanotechnology in the field of medicine. To light come their optoelectrical and imaging features. It is proven that quantum dots are able to inhibit the fibrillation process, which leads to the development of AD. Particularly the usage of CdSe/ZnS QDs, and them decreasing the size of fibrils is discussed in this paper. Representing a way better solution, than commercial fluorescent dyes, these QDs are capable to influence the electrical capacity of cell membranes. In the case of AD, quantum dots manage to reduce the process of depolarization of the membrane in conjunction with natural light. This state of the membrane is induced by the high concentrations of Aß oligomers. With this treatment, any case of chemical and genetic manipulation is avoided. As other nanoparticles, QDs have also the ability to cross the brain blood barrier, which is essential for a successful drug delivery and action. But there are some disadvantages too. Scientists around the world are discussing the toxicity of nanoparticles that occurs because of the in-compatibility of particles with the organisms. Every experiment with QDs was done only on animals and it is still unknown how the remaining nanoparticles concentration affect the human system, in this case the brain. But there is no doubt that the usage of quantum dots in the treatment and detection of the disease, would lead to a completely new era of nanotechnology in medicine.
REFERENCES
- Cobarrubia ER (2013) Alzheimer's Disease and Nanotechnology.
- King's College London (2015) The global impact of dementia. Alzheimer's Disease International, King's College London, England.
- Mahajan G (2011) Fighting Alzheimer's disease with nanotechnology. Honolulu, Hawaii.
- Morais MGDe, Martins VG, Steffens D, Pranke P, Costa JAV (2014) Biological Applications of Nanobiotechnology. Journal of Nanoscience and Nanotechnology 14: 1007-1017.
- Nazem A, Mansoori GA (2011) Nanotechnology for Alzheimer's disease detection and treatment. Insciences J 4: 169-193.
- Lugo K, Miao X, Rieke F, Lin LY (2012) Remote switching of cellular activity and cell signaling using light in conjunction with quantum dots. Biomed Opt Express 3: 447-454.
- Thakur G, Micic M, Yang Y, Li W, Movia D, et al. (2011) Conjugated Quantum Dots Inhibit the Amyloid ß (1-42) Fibrillation Process. International Journal of Alzheimer’s Disease. Pg no: 15.
- Blanchard BJ, Stockwell BR, Ingram VM (2002) Eliminating membrane depolarization caused by the Alzheimer peptide Aß(1–42, aggr.). Biochemical and Biophysical Research Communications 293: 1204-1208.
- Mukhamedyarov MA, Grishin SN, Yusupova ER, Zefirov AL, Palotas A (2009) Alzheimer's beta-amyloid-induced depolarization of skeletal muscle fibers: implications for motor dysfunctions in dementia. Cell Physiol Biochem 23: 109-114.
- Lukiw WJ (2013) Alzheimer's Disease (AD) as a disorder of the plasma membrane. Front Physiol 4: 4-24.
- Winter JO, Gomez N, Korgel BA, Schmidt CE (2005) Quantum dots for electrical stimulation of neural cells. Proceedings of SPIE - The International Society for Optical Engineering 5705: 235.
- Shilo M, Sharon A, Baranes K, Motiei M, Lellouche JP, et al. (2015) The effect of nanoparticle size on the probability to cross the blood-brain barrier: an in-vitro endothelial cell model. J Nanobiotechnology 13: 19.
- Zherebetskyy D, Scheele M, Zhang Y, Bronstein N, Thompson C, et al. (2014) Hydroxylation of the surface of PbS nanocrystals passivated with oleic acid. Science 344: 1380-1384.
- Fakruddin M, Hossain Z, Afroz H (2012) Prospects and applications of nanobiotechnology: a medical perspective. Journal of Nanobiotechnology 10: 31.
- Seeman P, Seeman N (2011) Alzheimer's disease: ß-amyloid plaque formation in human brain. Synapse 65: 1289-1297.
- Sattler KD (2010) Handbook of Nanophysics: Nanomedicine and Nanorobotics. CRC Press, Boca Raton,, Florida, USA.
- Winnik FM, Maysinger D (2013) Quantum dot cytotoxicity and ways to reduce it. Acc Chem Res 46: 672-680.
- Hardman R (2006) A Toxicologic Review of Quantum Dots: Toxicity Depends on Physicochemical and Environmental Factors. Environ Health Perspect 2: 165-172.
Citation: Devedži? A (2017) Beauty of Fine Dots-Detection and Treatment of Alzheimer's Disease Using CdSe/ZnS Quantum Dots. J Nanotechnol Nanomed Nanobiotechnol 4: 016.
Copyright: © 2017 Anida Devedzic, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Journal Highlights
© 2026, Copyrights Herald Scholarly Open Access. All Rights Reserved!
