
Beneficial and Mechanistic Effects of a Fibroin Enzymatic Hydrolysate on Memory Impairment Induced by Amyloid Beta in Mice
*Corresponding Author(s):
Sidney J StohsSchool Of Pharmacy And Health Professions, Creighton University Medical Center, Omaha, United States
Tel:+1 2142156655,
Email:sid.stohs9@gmail.com
Abstract
Alzheimer’s disease is characterized by the deposition of amyloid plaques and subsequent formation of neuro-fibrillary tangles in the brain. A lack of safe and effective treatments exists to slow progression and prevents neurodegenerative diseases. In this study, amyloid beta (AB)(2 μg/3 μL) was injected (intrahippocampal) into mice to induce memory loss to investigate the beneficial effects of a silk fibroin enzymatic hydrolysate (FEH) with an average molecular weight of 2700 Daltons on memory and learning impairment which were assessed using the Morris water maze, passive avoidance, and Y-maze tests. Protein expression of BDNF-associated and p75 apoptotic signaling pathways were determined to investigate mechanistic effects of FEH. Oral FEH supplementation (10 mg/kg) significantly ameliorated AB-induced memory and learning impairment with higher doses producing small, additional increases. FEH treatment increased the levels of brain-derived neurotrophic factor (BNDF) and reduced the levels of pro-inflammatory cytokines (tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and IL-6) in brain hippocampal tissues of AB-treated mice.FEH stimulated the BDNF/tropomyosin receptor kinase B/phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin/postsynaptic density protein 95 pathway and suppressed the expression of proteins of the p75 apoptotic signaling pathway, including tumor necrosis receptor-associated factor 6, B-cell lymphoma 2 (Bcl2)/Bcl2-associated X protein, caspase-3, and nuclear factor-kappa B (NF-κB) in the brain hippocampus of amyloid beta-administered mice. These results support previous human studies indicating the potential of FEH supplementation to prevent memory and learning impairment, and provide data concerning its underlying mechanism of action.
Keywords
Amyloid beta plaque; Fibroin enzymatic hydrolysate; Neurodegenerative diseases; Neurofibrillary tangles; Memory and learning
ABBREVIATIONS
AB: Amyloid Beta
FEH: Fibroin Enzymatic Hydrolysate
BDNF: Brain-Derived Neurotrophic Factor
DO: Donepezil
TNF-α: Tumor Necrosis Factor-Alpha
IL: Interleukin
Ach: Acetylcholine
AchE: Acetyl cholinesterase
(NF-κB): Nuclear Factor-kappa B
(TrkB): Tropomyosin receptor Kinase B
(PI3K): Phosphatidylinositol 3-Kinase
(AKT): Protein Kinase B
(P-AKT): Phosphorylated Protein Kinase B
(mTOR): mammalian Target Of Rapamycin
[cAMP]: cyclic Adenosine Monophosphate
(CREB): Response Element-Binding Protein
(JNK): c-Jun N-Terminal Kinase
(p-ERK 1/2): phosphorylated Extracellular Signal-Regulated Kinase ½ P75
(TRAF6): TNF Receptor-Associated Factor 6
(Bcl2): B-Cell Lymphoma 2
(Bax): Bcl2-associated X protein
(PSD 95): Postsynaptic Density Protein 95
INTRODUCTION
Alzheimer's disease is the most common cause of dementia in the elderly, and is characterized by the loss of memory and other cognitive abilities [1]. One of the major distinguishing pathological features and biomarkers of Alzheimer’s disease is extracellular deposition of Amyloid Beta (AB) peptide into plaques in the brain [2]. Amyloid beta is a peptide with an average of 36-43 amino acids. Accumulation of amyloid plaques enhances the formation of neuro-fibrillary tangles which are associated with the loss of neurons by neuronal inflammation, oxidative stress and apoptosis [3].
In a study by Peng et al., [4] AB was shown to decrease cortical Brain-Derived Neurotrophic Factor (BDNF) mRNA levels in mice. Since BDNF plays a critical role in neuronal survival and function, it is important to understand this AB-induced BDNF reduction to elucidate the mechanisms of Alzheimer's disease pathogenesis [5,6]. BDNF stimulates the activation and phosphorylation of proteins in the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway by binding to the receptor Tropomyosin Receptor Kinase B (TrkB) with high affinity, inducing the phosphorylation of the transcription factor cyclic Adenosine Monophosphate [cAMP] response element-binding protein (CREB) [6]. However, BDNF also binds to the p75 neurotrophin receptor with low affinity, mediating apoptosis via the c-Jun N-Terminal Kinase (JNK) or Nuclear-Factor Kappa B (NF-kB) cascades [6,7]. Thus, BDNF plays a variety of roles in neuronal survival and is one of the most important biomarkers for memory and learning disorders including Alzheimer's disease [5-7].
There is a lack of effective treatments to slow the progression and prevent neurodegenerative diseases [8]. Cholinesterase inhibitors are used in the treatment of dementia including Alzheimer’s disease [9]. Donepezil (DO) is a commonly used cholinesterase inhibitor worldwide, but has numerous side effects including nausea, diarrhea, vomiting, muscle cramps and heart disease [9,10]. Thus, there is a great need to identify novel therapies that focus on the cascade of events which lead to the accumulation of toxic amyloid aggregates [11].
A previous study has shown that a silk Fibroin Enzymatic Hydrolysate (FEH) given orally to healthy humans for three weeks dose-dependently increased the memory quotient, learning gradient, retrieval efficiency and number of words remembered [12].The current study investigated the effect of FEH on AB-induced memory and learning impairment in mice. This study aimed to provide additional evidence of the beneficial effects of silk peptides on memory and learning impairment and to identify the mechanisms underlying these effects, evaluating the involvement of the BNDF signaling pathway.
MATERIALS AND METHODS
FEH treatment and amyloid beta administration
The study was conducted in the Brain Research Laboratory, Department of Oriental Medicinal Sciences, Kyung Hee University, Seoul 02447, Republic of Korea, and the experimental protocol was approved May 29, 2019 by the Kyung HeeUniversity’s Institutional Animal Care and Use Review Committee. FEH were obtained from Sunbio Corporation (Long Beach, CA,USA) which had been prepared by protease hydrolysis and consists of polypeptides with molecular weights of between 500 to 5000 Daltons [12]. The major amino acids found in the peptide components include glycine (42.5 %), alanine (28.2 %) and serine (9.4 %). Amyloid Beta (AB) and Donepezil (DO) were procured from Sigma Chemical Co. The molecular weight of AB was 4514 g/mol.
C57BL/6M mice were obtained from Searon-bio (Uiwang-si, Korea) and were housed 8 animals per cage (temperature of 22 ± 2°C, humidity of 55 ± 5%, and a 12-h light/dark cycle), fed an AIN 93G diet and allowed water ad libitum for an adaptation period of 7 days.
The animals were randomly assigned into five groups with 8 mice per group: normal control, Amyloid Beta (AB) control (single bilateral intrahippocampal [IHP] injection of amyloid beta at 2 μg/3 μL), Donepezil [DO] (positive control, IHP injection of AB 2 μg/3 μL + DO 2 mg/kg, p.o.), FEH 10 (IHP injection of AB 2 μg/3 μL + FEH 10 mg/kg b.w., p.o.), FEH 50 (IHP injection of AB 2 μg/3 μL + FEH 50 mg/kg b.w,, p.o.) and FEH 150 (IHP injection of AB 2 μg/3 μL + FEH 150 mg/kg b.w,, p.o.). DO is an acetylcholine esterase inhibitor that has been shown to enhance memory and learning in rodents, and was used as a positive control [13] All animals were treated daily with FEH or DO for three weeks at which time the memory and learning abilities of the mice were tested, and the animals were sacrificed by cervical dislocation.
Morris water maze
The water maze was a circular pool (180 cm in diameter and 45 cm in height) that was filled with water (23 ± 1°C, 25 cm in depth). The studies were conducted on all animals in each group according to the procedure of Hagaki et al., [14] and summarized as follows. Visual cues were placed on the edge of the pool. The pool was divided into four quadrants and the escape platform was placed in the middle of one quadrant, 1.0 cm below the surface of the water. Treatments were administered for a total of 4 weeks.
Mice were trained for 5 days with 4 training sessions per mouse located in different regions of the pool. During training, the time taken for the mouse to locate the platform (the escape latency) was measured within 60 s. If a mouse failed to reach the platform within 60 s, it was guided to the platform. On the sixth day, a probe test was conducted whereby mice were allowed to swim freely for 90 seconds in the water tank in which the platform had been removed. Behavioral experiments were conducted for a total of 9 days. The escape latency and the time spent in the target quadrant were analyzed using Smart System 3.0 video tracking software (Eurofins Panlab Inc.,St. Charles, MO USA).
Passive avoidance test
This test was conducted according to the procedure of Malikowsha et al., [15] on all 8 animals in each of the five groups. After three weeks of treatment, mice were placed in a 250 mm X 250 mm shuttle box with a small dark chamber and a bright chamber (Scitech Korea Inc, Seoul, Republic of Korea). In summary, the guillotine door was opened and after the mouse entered the dark compartment, the door closed automatically. In the dark compartment, the mouse received a low-intensity electric shock (75V, 0.5 mA 50 Hz) to induce fear conditioning. After 24 hours, the experimental animal was placed in the bright chamber, and the time taken for it to enter the dark chamber was measured and quantified.
Y-maze test
The Y-maze test was conducted according to the procedure of Kraeuter et al., [16] on all 8 of the animals in each of the five groups. The Y-maze (350 mm X 80 mm) consisted of three plastic arms at a 120º angle from each other. After three weeks of treatment, each mouse was randomly placed in one arm and allowed to move freely for 10 min. Alternations were defined as sequential entries into each of the three arms. The spontaneous alternation percentage was calculated as the percentage of actual alternations among the total number of possible arm entries.
Measurement of brain acetylcholine, acetyl cholinesterase, BNDF and cytokines (tumor necrosis factor-alpha, interleukin-1β and interleukin-6) levels
Hippocampal brain samples were obtained from all mice in each group, and ELISA kits were used for determination of Ach and AchE (BioVision Inc., Milpitas, CA USA); BNDF (MyBioSource Inc., San Diego, CA USA); TNF-α, IL-1β and IL-6 (R&D Systems, Minneapolis, MN USA) according to the manufacturer’s instructions.
Western blot Analyses
Hippocampal brain tissues from all mice was placed in radio immune precipitation assay buffer (Sigma-Aldrich Korea, Seoul, Republic of Korea) and centrifuged for 20 min at 124,000 × g at 4°C. The proteins were resolved using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE, 10%) and transferred onto a polyvinylidene difluoride membrane. After incubation with blocking buffer for 1 hour, membranes were incubated overnight at 4°C with primary antibodies against BDNF, TrkB, PI3K, p-PI3K, AKT, phosphorylated AKT (p-AKT), mTOR, postsynaptic density protein 95 (PSD95), phosphorylated extracellular signal-regulated kinase 1/2 (p-ERK 1/2), P75, TNF receptor-associated factor 6 (TRAF6), B-cell lymphoma 2 (Bcl2), Bcl2-associated X protein (Bax), Caspase-3, NF-kB, phosphorylated NF-kB (p-NF-kB) and β-actin (Cell Signaling Technology, Beverly, MA, USA). The membranes were then incubated for 2 hours with a secondary antibody (Cell Signaling Technology) at room temperature. The immunoreactive protein bands were detected using enhanced chemiluminescence western blotting detection reagents (Ez-Capture II, ATTO, Tokyo, Japan), andCS Analyzer 3.0 software (ATTO) was used for quantification.
Immunohistochemistry
Hippocampal brain tissues of all mice in each group were fixed in 10% buffered formalin and embedded in paraffin. The paraffin blocks were cut into 4 μm-thick sections, and sections were stained with rabbit anti-BNDF antibody (Abcam, Cambridge, UK). Image analysis was performed by microscope to detect color changes.
Statistical analysis
All data were expressed as mean ± standard deviation (SD) and statistical analyses were conducted using one-way ANOVA and t-tests using SPSS software (SPSS PASW Statistics 23.0, SPSS Inc. Chicago, IL, USA). Duncan’s multiple range test was used to analyze differences between groups. A P-value < 0.05 was considered statistically significant.
RESULTS
Effect of FEH on Amyloid Beta (AB)-induced memory impairment
The Morris water maze test was used to measure the effect of FEH on AB-induced memory impairment in mice. Figure 1A provides representative tracking maps to reach the target under the various experimental conditions. AB-administration via a single bilateral Intra-Hippocampal [IHP] injection doubled the escape latency time (Figure 1B) while significantly (P<0.05) decreasing time spent in the target quadrant (Figure 1C) compared to the normal placebo control mice. However, oral administration of the positive control DO and FEH to AB-treated mice significantly ameliorated memory and learning impairment compared to the AB only control group (P < 0.05) (Figures 1B,1C). A small FEH dose response effect was observed, but the differences were not significant.
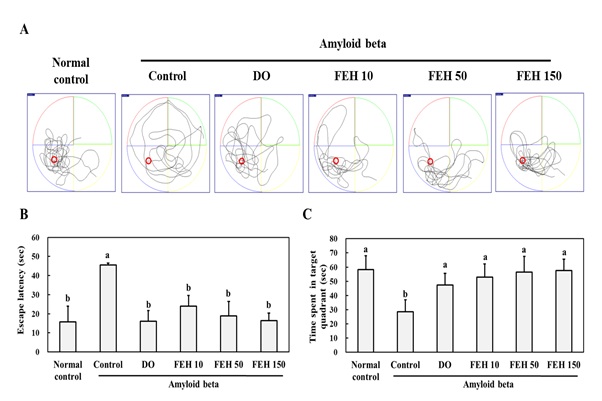 Figure 1: Effect of Fibroin Enzymatic Hydrolysate (FEH) on the Morris water maze test results of AB-treated mice.
Figure 1: Effect of Fibroin Enzymatic Hydrolysate (FEH) on the Morris water maze test results of AB-treated mice.
The mice were divided into the following treatment groups: normal control, AB-treated control, DOES positive control, and FEH-treated. (A)-Representative tracking maps to reach the target; (B)-Escape latency time (sec); and (C)-Time spent in target quadrant (sec). Data are presented as mean ± SD (n=8). Values with non-identical superscript letters indicate significant differences (P<0.05) as determined by Duncan’s multiple range test.
Passive avoidance and Y-maze tests were also performed. The administration of AB significantly decreased the step-through latency in the passive avoidance test (Figure 2A) and the spontaneous alteration percentage in the Y-maze test (Figure 2B). Administration of oral FEH daily for three weeks significantly decreased the effects of AB on these two tests. Again, a small FEH dose response effect was observed, but the differences between doses were not significant.
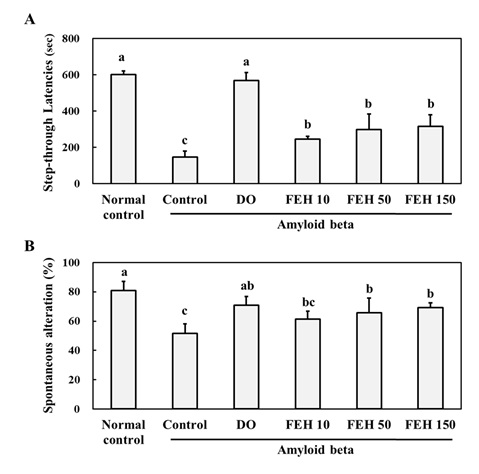 Figure 2: Effect of Fibroin Enzymatic Hydrolysate (FEH) on: (A)-passive avoidance test, and (B)-Y-maze test in AB-treated mice.
Figure 2: Effect of Fibroin Enzymatic Hydrolysate (FEH) on: (A)-passive avoidance test, and (B)-Y-maze test in AB-treated mice.
The mice were divided into the following treatment groups: normal control, AB-treated control, DO positive control, and FEH-treated (3 doses). Values are presented as mean ± SD (n=8). Values with non-identical superscript letters indicate significant differences (P<0.05) as determined by Duncan’s multiple range test.
Effect of FEH on AB-induced BNDF reduction and inflammation in the brain
The administration of AB significantly (P<0.05) decreased Ach and BDNF levels and increased AchE levels in the brain (hippocampus) (Figure 3A-C). Oral administration of DO and FEH to AB-treated mice did not affect Ach levels (Figure 3A), but significantly increased BNDF (Figure 3B) and decreased AchE (Figure 3C) levels in the hippocampal tissue (P < 0.05).
The administration of AB also induced significant (P<0.05) increases in the levels of the inflammatory cytokines IL-1β TNF-α and IL-6 (Figure 3D-F) in brain hippocampus compared to the normal placebo control group. However, oral administration of DO and FEH resulted in significant decreases in the levels of IL-1β, TNF-α and IL-6 compared to the AB control group (P < 0.05). FEH dose-response effects were observed for the three cytokines.
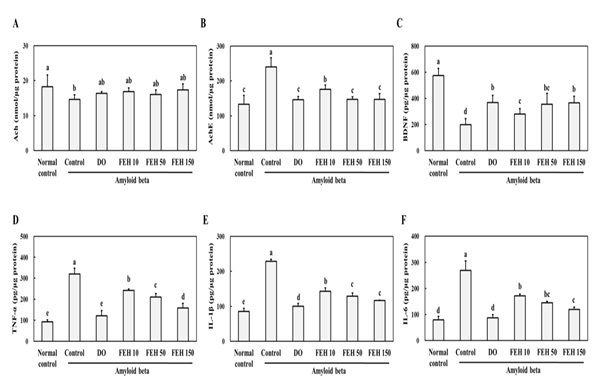 Figure 3: Effect of Fibroin Enzymatic Hydrolysate (FEH) on:(A)-Acetylcholine [Ach], (B)-Acetyl cholinesterase [AChE], (C)-Brain-Derived Neurotrophic Factor [BDNF], (D)-tumor necrosis factor-alpha [TNF-α], (E)-Interleukin-6 [IL-6], and (F) -Nterleukin-1β [IL-1β] levels inhippocampal brain tissue from AB-treated mice.
Figure 3: Effect of Fibroin Enzymatic Hydrolysate (FEH) on:(A)-Acetylcholine [Ach], (B)-Acetyl cholinesterase [AChE], (C)-Brain-Derived Neurotrophic Factor [BDNF], (D)-tumor necrosis factor-alpha [TNF-α], (E)-Interleukin-6 [IL-6], and (F) -Nterleukin-1β [IL-1β] levels inhippocampal brain tissue from AB-treated mice.
The mice were divided into the following treatment groups: normal control, AB-treated control, DOES positive control, and FEH-treated. Western blotting was used to evaluate the levels in mouse brains. Values are presented as mean ± SD (n=8). Values with non-identical letters represent significant differences (P<0.05) as determined by Duncan’s multiple range test.
Effect of FEH on the BDNF/TrkB/PI3K/AKT/mTOR/PSD95 pathway in AB-administered mice
Protein expression of components of the TrkB/PI3K/Akt/mTOR/PSD9 pathway was assessed by western blotting to elucidate the possible molecular mechanisms underlying the protective effects of FEH (Figure 4). The results demonstrated that AB-administration decreased BDNF protein expression in the brain hippocampus, similar to the results shown in Figure 2C. In addition, AB-administration inhibited the protein expression of the TrkB/PI3K/Akt/mTOR/PSD9 pathway in the hippocampus. However, oral administration of DO and FEH increased the protein expression of all components of the TrkB/PI3K/Akt/mTOR/PSD9 pathway in AB-treated mice (P < 0.05).
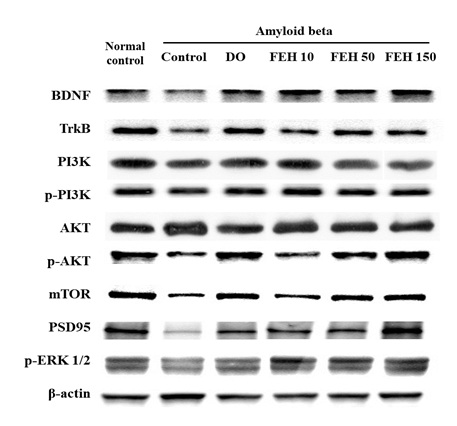 Figure 4: Effect of Fibroin Enzymatic Hydrolysate (FEH) on hippocampal protein express of the BDNF/TrkB/PI3K/AKT/mTOR/PSD95 pathway in AB-treated mice.
Figure 4: Effect of Fibroin Enzymatic Hydrolysate (FEH) on hippocampal protein express of the BDNF/TrkB/PI3K/AKT/mTOR/PSD95 pathway in AB-treated mice.
Mice were divided into the following treatment groups: normal control, AB-treated control, DO positive control, and FEH-treated (3 doses). Western blotting was used to evaluate the levels of each protein in mouse brain. The Figure is representative of the data from random animals in each experimental group.
Effect of FEH on the p75 apoptotic signaling pathway in AB-administered mice
The binding of BDNF to the p75 neurotrophin receptor causes a reduction in Trk receptor signaling and plays an important role in apoptosis (6). Thus, the protein expression of the p75 apoptotic signaling pathway including TRAF6, Bcl2/Bax, Caspase-3 and NF-kB in the brain hippocampal tissue of amyloid beta-administered mice were determined (Figure 5). AB-administration increased the protein expression of the p75 apoptotic signaling pathway, while oral administration of DO and FEH resulted in significant decreases in protein expression compared to the AB-treated control group (P < 0.05).
The expression of BDNF in the mouse brain hippocampus was also determined immunohistochemically. AB-administration suppressed BDNF expression, while oral administration of DO and FEH to AB-treated animals increased BDNF expression in hippocampal tissues (Figure 6).
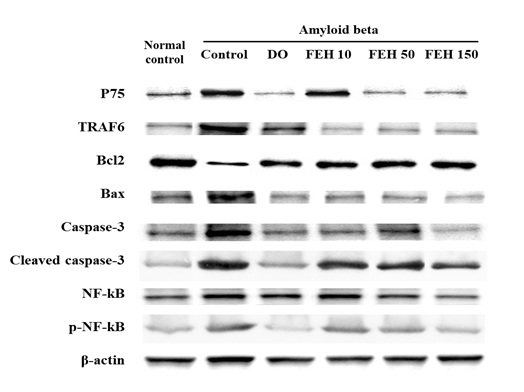 Figure 5: Effect of Fibroin Enzymatic Hydrolysate (FEH) on protein expression of the p75 apoptotic signaling pathway in hippocampal brain tissue from AB-treated mice.
Figure 5: Effect of Fibroin Enzymatic Hydrolysate (FEH) on protein expression of the p75 apoptotic signaling pathway in hippocampal brain tissue from AB-treated mice.
DISCUSSION
The accumulation of amyloid plaques in the brain plays an important role in the pathogenesis of Alzheimer's disease and results in synaptic dysfunction, synaptic loss, and neuronal apoptosis [17]. The intra-hippocampal injection of AB into the brains of mice induces the development of memory and learning impairment [18-20]. In this study, we investigated the effect of FEH on AB-induced memory and learning impairment to provide further evidence of the beneficial effects of FEH and to identify the mechanisms underlying these effects.
AB-administration induced the development of memory and learning impairment as determined by the Morris water maze, passive avoidance and Y-maze tests (Figures 1 & 2). AB-induced memory impairment was associated with the production of pro-inflammatory cytokines (IL-1β, TNF-α and IL-6) in the brain hippocampus (Figure 3) confirming that AB induces the production of neuro-inflammatory molecules which contribute to the pathogenesis of neurodegenerative diseases including Alzheimer’s [18-20].
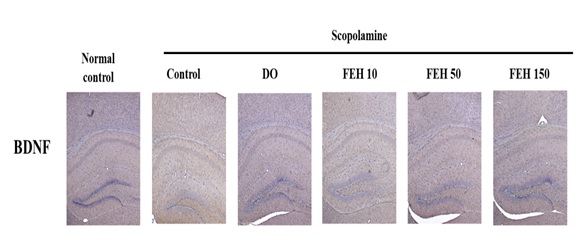 Figure 6: Effect of fibroin enzymatic hydrolysate (FEH) on BDNF expression in hippocampal brain tissue from AB-treated mice.
Figure 6: Effect of fibroin enzymatic hydrolysate (FEH) on BDNF expression in hippocampal brain tissue from AB-treated mice.
The mice were divided into the following treatment groups: normal control, AB-treated control, DO positive control, and FEH-treated (3 doses). The expression of BDNF in the mouse brain was evaluated immunohistochemically. The Figure is representative of the data from random animals in each experimental group.
Pro-inflammatory cytokines attenuate NF-κB-dependent microglial phagocytosis, thereby hindering efficient plaque removal by resident microglia [20]. These researchers therefore suggested the potential use of anti-inflammatory therapies for the treatment of Alzheimer's disease [20]. More recently, Agnihotri and Aruoma [21] reviewed the roles of inflammatory processes and oxidative stress on neurodegenerative diseases, and the beneficial effects of nutrigenomics and personalized health management. Theresults of the current study demonstrated the inhibitory effects of FEH treatment against memory and learning impairment and pro-inflammatory cytokine production (Figures 1-3) Furthermore, it provided evidence for a relationship between AB, inflammatory factors and memory and learning, and the beneficial effects of FEH.
Murer et al., [22] studied the brains of individuals with Alzheimer’s disease and demonstrated that neurons containing neurofibrillary tangles did not contain BDNF-immunoreactive material, suggesting a neuroprotective action of BDNF. BDNF is a neurotrophic molecule with pleiotropic effects on the nervous system [22,23]. The injection of AB significantly decreased BDNF expression in mouse brain hippocampal tissue, but BDNF increased in hippocampal tissue from AB-treated mice that received FEH daily (Figure 6).
BDNF-associated pathways were investigated to elucidate the possible molecular mechanisms underlying the inhibitory effects of FEH on memory and learning impairment. BDNF binds with high affinity to its target receptor TrkB, triggering the activation of three signaling cascades, namely the PI3K/Akt/mTOR, Ras/ERK and phospholipase C gamma pathways [24-27]. These pathways are related to the ability of BDNF to activate the transcription factor CREB which further stimulates the expression of BDNF and is associated with hippocampal neurogenesis [26,27]. AB-administration suppressed the expression of proteins in the BDNF/TrkB/PI3K/AKT/mTOR/PSD95 pathway in mouse brain (Figure 4). However, FEH treatment stimulated the p-PI3K/p-AKT/mTOR/PSD95 pathway and the expression of p-ERK in AB-treated mice.
BDNF can also bind to the p75 neurotrophin receptor, a member of the tumor necrosis factor family of receptors, with low affinity to mediate diverse cellular processes including neurite outgrowth, synaptic plasticity and inflammation and cell death [28]. By BDNF stimulates the pro-survival NF-kB and pro-apoptotic Jun kinase signaling cascades [28]. In our study, AB injection increased the expression of proteins in the p75 apoptotic signaling pathway including TRAF6, Bcl2/Bax, Caspase-3 and NF-kB (Figure 5). However, FEH treatment suppressed protein expression in the p75 apoptotic signaling pathway. These results suggest that FEH inhibited memory and learning impairment through stimulation of the BDNF/TrkB/PI3K/AKT/mTOR/PSD95 pathway and inhibition of the p75 apoptotic signaling pathway.
The results of this study provided evidence of the beneficial effects of FEH as well as information regarding the mechanisms underlying these effects via stimulation of BDNF expression in the hippocampus. The results support the use of FEH in the treatment of memory and learning impairment.
FUNDING
This study was funded by NI & Pharm Inc., Seoul, Republic of Korea
CONFLICTS OF INTEREST
JKN is an employee of NI & Pharm Inc. All other authors declare no conflicts of interest.
COMPETING INTERESTS
JKN played no role in the analysis of the data and the writing of the manuscript. The study was conducted independent of the funding agency.
AUTHOR CONTRIBUTIONS
All authors contributed to the study design. DEM, JY, JL, SYC and SM conducted the study. All authors were involved in the writing of the manuscript.
REFERENCES
- Katzman R (1993) Education and the prevalence of dementia and Alzheimer's disease. Neurology 43: 13-20.
- Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, et al. (1993) Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Natl Acad Sci USA 90: 9649-9653.
- Bronzuoli MR, Iacomino A, Steardo L, Scuderi C (2016) Targeting neuroinflammation in Alzheimer's disease. J Inflam Res 9: 199-208.
- Peng S, Garzon DJ, Marchese M, Klein W, Ginsberg SD, et al. (2009) Decreased brain-derived neurotrophic factor depends on amyloid aggregation state in transgenic mouse models of Alzheimer's disease. J Neurosci 29: 9321-9329.
- Song X, Liu B, Cui L, Zhou B, Liu W, et al. (2017) Silibinin ameliorates anxiety/depression-like behaviors in amyloid β-treated rats by upregulating BDNF/TrkB pathway and attenuating autophagy in hippocampus. Physio. Behav 179: 487-493.
- Cunha C, Brambilla R, Thomas KL (2010) A simple role for BDNF in learning and memory? Front Mol Neurosci 3:1.
- Robinet C, Pellerin L (2010) Brain-derived neurotrophic factor enhances the expression of the monocarboxylate transporter 2 through translational activation in mouse cultured cortical neurons. J Cere. Blood Flow Metab 30: 286-298.
- Hane FT, Robinson M, Lee B, Bai O, Leonenko Z, et al. (2017). Recent progress in Alzheimer’s disease research, Part 3: diagnosis and treatment. J Alzheimer’s Dis 57: 645-665.
- Albert MS, Blacker D (2006) Mild cognitive impairment and dementia. Ann Rev Clin Psychol 2: 379-388.
- Grutzendler J, Morris JC (2001) Cholinesterase inhibitors for Alzheimer's disease. Drugs 61: 41-52.
- Khan A, Corbett A, Ballard C (2017) Emerging treatments for Alzheimer’s disease for non-amyloid and non-tau targets. Expert Rev Neurother 17: 883-895.
- Kang YK, Lee BY, Bucci LR, Stohs SJ (2018) Effect of a fibroin enzymatic hydrolysate on memory improvement: a placebo-controlled, double-blind study. Nutrients 10:
- Shin CY, Kim HS, Cha KH, Won DH, Lee JY, et al. (2018). The effects of donepezil, an acetylcholinesterase inhibitor, on impaired learning and memory in rodents. BiomolTher (Seoul) 26: 274-281.
- Higaki A, Mogi M, Iwanami J, Min L-J, Bai H-Y, et al. (2018) Predicting outcome of Morris water maze test in vascular dementia mouse model with deep learning. PLoS ONE 13: 0191708.
- Malikowsha N, Salat K, Podkowa A (2017) Comparison of pro-amnesic efficacy of scopolamine, biperiden, and phencyclidine by using passive avoidance task in CD-1 mice. J PharmacolToxicol Methods 86: 76-80.
- Kraeuter AK, Guest PC, Sarnyai Z (2019) The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. Pre-Clinical Models 1916: 105-111.
- Wirths O, Multhaup G, Bayer TA (2004) A modified beta-amyloid hypothesis: intraneuronal accumulation of the beta-amyloid peptide--the first step of a fatal cascade. J Neurochem 91: 513-20.
- Morris G, Berk M, Walder K, Maes M (2015) Central pathways causing fatigue in neuro-inflammatory and autoimmune illnesses. BMC Med Feb. 6: 13.
- Amor S, Puentes F, Baker D, van der Valk P (2010) Inflammation in neurodegenerative diseases. Immunology 129: 154-169.
- Koenigsknecht-Talboo J, Landreth GE (2005) Microglial Phagocytosis Induced by Fibrillar β-Amyloid and IgGs Are Differentially Regulated by Proinflammatory Cytokines. J Neurosci 25: 8240-8249.
- Agnihotri A, Aruoma OI (2020) Alzheimer's Disease and Parkinson's Disease: A Nutritional Toxicology Perspective of the Impact of Oxidative Stress, Mitochondrial Dysfunction, Nutrigenomics and Environmental Chemicals. JAmer Coll Nutr 39: 16-27.
- Murer MG, Boissiere F, Yan Q, Hunot S, Villares J, et al. (1999) An immunohistochemical study of the distribution of brain-derived neurotrophic factor in the adult human brain, with particular reference to Alzheimer's disease. Neuroscience 88: 1015-1032.
- Hu Y, Russek SJ (2008) BDNF and the diseased nervous system: a delicate balance between adaptive and pathological processes of gene regulation. J Neurochem 105: 1-17.
- Yoshii A, Constantine-Paton M (2010) Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev Neurobiol 70: 304-322.
- Numakawa T, Odaka H, Adachi N (2018) Actions of Brain-Derived Neurotrophin Factor in the Neurogenesis and Neuronal Function, and Its Involvement in the Pathophysiology of Brain Diseases. Int J Mol Sci 19: 3650.
- Wan D, Xue L, Zhu H, Luo Y (2013) Catalpol Induces Neuroprotection and Prevents Memory Dysfunction through the Cholinergic System and BDNF. Evid Based Complement Alternat Med 2013: 134852.
- Mullen LM, Pak KK, Chavez E, Kondo K, Brand Y, et al. (2012) Ras/p38 and PI3K/Akt but not Mek/Erk signaling mediate BDNF-induced neurite formation on neonatal cochlear spiral ganglion explants. Brain Res 2: 25-34.
- Bamji SX, Majdan M, Pozniak CD, Belliveau DJ, Aloyz R, et al. (1998) The p75 neurotrophin receptor mediates neuronal apoptosis and is essential for naturally occurring sympathetic neuron death. J Cell Biol 140: 911-923.
Citation: Yun J, Lee J, Cha S-Y, Maeng S, Noh JK, et al. (2020) Beneficial and Mechanistic Effects of aFibroin Enzymatic Hydrolysate on Memory Impairment Induced by Amyloid Beta in Mice. J Altern Complement Integr Med 6: 127.
Copyright: © 2020 Jeongmoon Yun, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

