
Beneficial Effects of Powerinsole® Energy Pad: Investigations with Organ-Specific Cell Cultures
*Corresponding Author(s):
Peter C DartschDartsch Scientific Gmbh, Institute Of Cell Biological Test Systems, Wagenfeld, Germany
Tel:+49 54449801322,
Email:pc.dartsch@dartsch-scientific.com
Abstract
By use of current in vitro methods the effects of a specially designed energy pad, named Powerinsole®, on cultured organ-specific cells were investigated. Two different cell types (connective tissue fibroblasts, cell line L-929 and promyelocytes HL-60 which have been differentiated to functional neutrophils) were studied for the activation of the regenerative and radical-scavenging potential after treatment with the energy pad. Untreated cell cultures served as controls. The first experiments were conducted with a set of anonymized Powerinsole® energy pads with and without activation. When the data clearly pointed out which energy chip was obviously activated and this result was verified by the provider, we then conducted additional experiments.
Powerinsole® energy pad with an active chip was able to increase the recolonization of a cell-free space (= wound closure) by 39.9 ± 12.4 % (mean value ± standard error of the mean) when compared with Powerinsole® with the inactive chip or untreated control. Moreover, Powerinsole® energy pad with an active chip inactivated endogenously generated superoxide anion radicals by 24.0 ± 3.3 % (mean value ± standard deviation). The inactive chip was only effective by 2.5 ± 4.9% (mean value ± standard deviation), which did not differ significantly from untreated controls.
In conclusion, Powerinsole® energy pad has demonstrated its beneficial effects in the in vitro studies presented here and might be useful for the improvement and maintenance of well-being in vivo.
Keywords
Cell culture; Cell metabolism; Cell regeneration; Connective tissue fibroblasts; Energy medicine; Functional neutrophils; Health
INTRODUCTION
For centuries, concepts of life force and healing energy have been out of the field of view among serious scientists. Although the discipline of energy medicine has received more interest during the last years, therapeutic approaches applying energy are still regarded with a great skepticism [1-3]. There are also closely related medical disciplines such as alternative, complementary or integrative medicine which also use different sources of energy for improvement and maintenance of health [4-6].
Prompted by this background, we used current experimental animal-free in vitro methods to investigate the effects of a specially designed energy pad, named Powerinsole®, on organ-specific cells in culture. The energy pad has already shown its potential in a number of well-documented case studies.
MATERIALS AND METHODS
Basic prerequisite for the in vitro experiments
In order to avoid any unwanted influence of the investigator, we decided to test sets of anonymized Powerinsole® energy pads with and without activation for the preliminary studies. When the data clearly pointed out which energy chip was obviously activated and this was verified by the provider, we then conducted additional experiments. The different Powerinsole® energy pads with and without activation were provided for this study by Powerinsole VertriebsgmbH, A-5162 Obertrum, Austria; homepage: www.powerinsole.com.
Powerinsole® energy pad
According to the manufacturer and distributor of Powerinsole®, the energy pad contains a specially designed power chip which is based on principles of biophysics and energy medicine. The mode of operation of Powerinsole® can be best compared to a magnetic resonance therapy. The central part of the Powerinsole® is a power chip, which consists of a flexible printed circuit board including storage medium and several strong and specifically oriented neodymium magnets. Due to the specific position and their movement, the magnets generate a constantly changing mutual magnetic field which generates different frequencies. The energy pad has been shown in well-documented case studies to possess a positive effect on the human body by influencing the energy field of the cells and, thus, increasing cell metabolism and healing process.
Cell regeneration/wound healing assay
The experiments were conducted with connective tissue fibroblasts (cell line L-929, ACC-2; Leibniz Institute DSMZ - German Collection for Microorganisms and Cell Cultures, Braunschweig, Germany) and used in passages 112 to 114. The cells were routinely cultivated in RPMI 1640 culture medium with 10% growth mixture and 0.5% gentamycin and incubated in an incubator at 37°C and an atmosphere of 5% CO2 and 95% air and almost 100% humidity.
The connective tissue fibroblasts were seeded at a density of 50,000 cells/ml into the three individual compartments of Culture-Inserts 3 well (ibidi, Munich, Germany). The compartments of the inserts are separated from each other by 500 µm thick silicone frames and are externally limited by a 700 µm thick silicone wall. Due to its special adhesion area, an insert adheres firmly to the bottom of a culture dish and forms a defined cell-free space (artificial wound), which the seeded cells can recolonize by migration and proliferation. After reaching confluency within 48 hours after seeding, the silicone inserts were removed with tweezers in order to maintain the sharply defined cell-free space between the compartments.
Powerinsole® energy pads with and without activation was placed beneath the cell cultures and the entire culture dish was shielded by several layers of aluminum foil. In order to increase the exposure time as much as possible, only those cell-free spaces that had an original width of 700 µm were evaluated after 36 hours. The cells were then fixed with methanol, stained with azure-eosin-methylene blue solution (Sigma-Aldrich, Deisenhofen, Germany), air-dried and the width of the remaining cell-free spaces was measured by photomicrographs. The experiments were conducted in duplicate and the measurements of the cell-free spaces were done at 10 different locations within one sample.
Assay for generation of endogenous oxygen radicals
The basic principle of the test assay has been already described in detail elsewhere [7]. This cell-based test assay uses the formation of intracellular superoxide anion radicals of phagocytic cells (functional neutrophils) as a model to investigate the efficacy of Powerinsole® with and without an active energy chip to neutralize endogenously generated oxygen radicals.
Human promyelocytes (cell line HL-60, ACC-3, ECACC 98070106; Leibniz Institute DSMZ - German Collection for Microorganisms and Cell Cultures, Braunschweig, Germany) were routinely grown in RPMI 1640 with 10 % growth mixture and 0.5 % of gentamycin and incubated at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Subcultures were differentiated to functional neutrophils by the addition of 1.5% dimethylsulfoxide to the culture medium for 6 days. Then, cells are capable of undergoing an oxidative or respiratory burst upon phorbol ester stimulation [8,9].
The promyelocytes were cultured separately in the incubator during the differentiation process to functional neutrophils under the direct influence of Powerinsole® energy pads with and without an active chip. The chips were directly plced beside the culture flasks containing the cells and were wrapped in several layers of alumium foil to avoid any mutual interactions between the samples.
After 6 days, the cells were collected by centrifugation (5 min at 190 x g), washed twice with phosphate-buffered saline by resuspending and centrifugation and were finally resuspended in phosphate-buffered saline with calcium and magnesium containing 10 mM glucose. Cell suspensions were added to the reaction mixture consisting of phosphate-buffered saline with 30 mM glucose, phorbol 12-myristate 13-acetate and WST-1 as tetrazolium dye (all from Sigma-Aldrich, Deisenhofen, Germany). The course of superoxide anion radical inactivation as generated by the stimulated functional neutrophils was monitored by cleavage of the dye as a differential measurement of the optical density at 450 and 690 nm for 40 min at 37°C using a BioTek ELx 808 ELISA. Independent experiments were conducted in triplicate on different days with each experiment having four single measurements.
STATISTICAL ANALYSIS
Statistical analysis of the measurement data was done by using the parameter-free two-sided Wilcoxon-Mann-Whitney test and assumed as significant at p ≤ 0.05.
RESULTS AND DISCUSSION
As depicted in figure 1, the recolonization of the cell-free space by Powerinsole® with an active chip was significantly faster than with Powerinsole® with the inactive chip. When summarizing the collected data from the duplicate experiments, the fibroblast cultures exposed to Powerinsole® with the active chip showed an increased recolonization/wound closure by 39.9 ± 12.4% (mean value ± standard error of the mean) when compared with Powerinsole® with the inactive chip or untreated control, respectively. Despite the high deviations in the measurement data, the increase in wound closure was statistically significant (p ≤ 0.05).
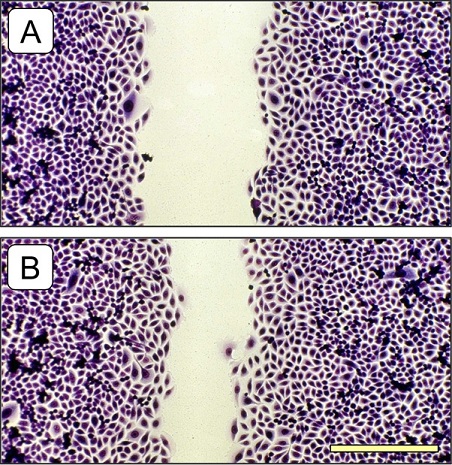
Figure 1: Photomicrograph of the recolonization of a cell-free space (initial width 700 µm) by cultured connective tissue fibroblasts, which had been incubated for 36 hours with Powerinsole® with an inactive chip (A) and Powerinsole® with an active chip (B). The promoting effect of wound closure in (B) can be clearly recognized in this direct comparison. Olympus IX50 inverted microscope with planachromate 10x at bright field illumination with an Olympus E-10 digital camera at 4 megapixel resolution. The scaling bar in the lower right represents 200 µm.
In the experiments on cell regeneration/wound healing as conducted in this study, especially the granulation phase was simulated. This phase is characterized in vivo by an increased proliferation and migration of connective tissue fibroblasts from the surrounding tissue to close the defect [10,11]. The data demonstrate that Powerinsole® is able to promote the cell regeneration/wound healing process by stimulating migration and proliferation of connective tissue fibroblasts. From this point of view, the statements of the manufacturer that Powerinsole® is able to promote cell regeneration/wound healing can be verified on the cellular level. However, this fact is quite hard to understand when you are looking only on this feature alone. The data become much more feasible when considering also the following data of superoxide anion radical scavenging.
As shown in figure 2, Powerinsole® with the active chip caused a marked inhibition of superoxide anion radical generation by 24.0 ± 3.3% (mean value ± standard deviation). The inactive chip was only effective by 2.5 ± 4.9 % (mean value ± standard deviation), which did not differ significantly from untreated controls. The scavenging of superoxide anion radicals by Powerinsole® with the active chip was statistically significant (p ≤ 0.01).
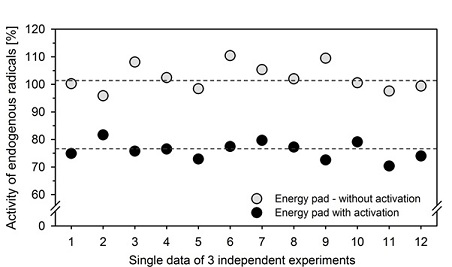
Figure 2: Inactivation of endogenously formed superoxide anion radicals by Powerinsole® energy pad with and without an active chip. The untreated control was set as 100%. The single measurement data of three independent experiments with 4 samples each are depicted. It can be seen that Powerinsole® with the inactive chip had no effect compared to the untreated control, but Powerinsole® with the active chip caused a statistically significant inactivation of superoxide anion radicals generated in the course of an oxidative burst by about 24%. Dashed lines represent mean values for Powerinsole® with and without an active chip.
Our body constantly reacts with oxygen as part of the energy producing processes of cells [12]. As a consequence, most reactive oxygen species come from endogenous sources as by-products of normal and necessary metabolic reactions. Such as energy generation from mitochondria or the detoxification reactions involving the liver cytochrome P-450 enzyme system [13,14]. An overload of endogenous radicals occurs during complicated wound healing or inflammatory processes [15-17]. Therefore, the inactivation of endogenously generated reactive oxygen radicals seems to be a good tool for the reduction of local oxidative stress and unwanted tissue damage [18].
In conclusion, Powerinsole® energy pad has demonstrated its beneficial effects in the experimental animal-free studies presented here. The energy pad is able to promote the cell regeneration/wound healing process as well as the inactivation of endogenously generated reactive oxygen radicals. Both effects might be useful for the improvement and maintenance of well-being in vivo.
REFERENCES
- Rubik B (2002) The biofield hypothesis: its biophysical basis and role in medicine. J Altern Complement Med 8: 703-717.
- Gulmen FM (2004) Energy medicine. Am J Chinese Med 32: 651-658.
- Oschmann JL (2015) Energy Medicine – E-Book: The Scientific Basis. 2nd edition, Elsevier Health Sciences, New York.
- Jobst KA (2004) Science and healing: from bioelectromagnetics to the medicine of light. J Altern Complement Med 10: 1-3.
- Feinstein D, Eden D (2008) Six pillars of energy medicine: clinical strengths of a complementary paradigm. Altern Ther 14: 44-54.
- Wisneski LA, Anderson L (2009) The Scientific Basis of Integrative Medicine. 2nd edition, CRC Press, Boca Raton, USA.
- Dartsch PC (2006) TIIOS – a sensitive and cell-based test assay for the screening of biologically active substances for their antioxidant potential. Innov Food Technol 32: 72-75.
- Teufelhofer O, Weiss RM, Parzefall W, Schulte-Hermann R, Micksche M, et al (2003) Promyelocytic HL60 cells express NADPH oxidase and are excellent targets in a rapid spectrophotometric microplate assay for extracellular superoxide. Toxicol Sci 76: 376-383.
- Peskin AV, Winterbourn CC (2000) A microtiter plate assay for superoxide dismutase using a water-soluble tetrazolium salt (WST-1). Clin Chim Acta 293: 157-166.
- Martin P (1997) Wound healing - aiming for perfect skin regeneration. Science 276: 75-81.
- Singer AJ, Clark RAF (1999) Cutaneous wound healing. N Engl J Med 341: 738-746.
- Droge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82: 47-95.
- Kuhn MA (2003) Oxygen Free Radicals and Antioxidants: An overview of how antioxidants protect the body from disease. Am J Nurs 103: 58-62.
- Lenaz G, Bovina C, Formiggini G, Parenti Castelli G (1999) Mitochondria, oxidative stress, and antioxidant defences. Acta Biochim Pol 46: 1-21.
- Halliwell B (1994) Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet 344: 721-724.
- Bergamini CM, Gambetti S, Dondi A, Cervellati C (2004) Oxygen, reactive oxygen species and tissue damage. Curr Pharm Des 10: 1611-1626.
- Nathan C (2002) Points of control in inflammation. Nature 420: 846-852.
- Ward PA, Warren JS, Johnson KJ (1988) Oxygen radicals, inflammation, and tissue injury. Free Radic Biol Med 5: 403-408.
Citation: Dartsch PC (2020) Beneficial Effects of Powerinsole® Energy Pad: Investigations with Organ-Specific Cell Cultures. J Med Stud Res 3: 016.
Copyright: © 2020 Peter C Dartsch, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

