
Beyond Substance Use Reduction: The Positive Impact of Mentorship for Addiction Problems (MAP)
*Corresponding Author(s):
Kathlene TracyNew York University School Of Medicine, New York, United States
Tel:+1 212-686-7500,
Email:Kathlene.Tracy@nyumc.org
Abstract
Keywords
Behavioral treatment; MAP; Peer support; Psychiatric symptoms; Quality of life; Substance use
INTRODUCTION
Socially isolated adults are less likely to be receptive to change their addiction behavior [10]. Peer support treatments have shown to be an effective and important resource in the treatment of substance use disorders [8,11]. These services have been beneficial in helping patients achieve a reduction and/or total abstinence from substances [12-14], as well as improving quality of life [15,16]. Impairment to quality of life is a common experience for patients with SUD upon entering treatment and is associated with increased psychiatric symptom severity [15]. Quality of life encompasses general life satisfaction with everyday experiences and events including for example family, social, and employment as well as the importance they hold for the individual [17]. Peer support interventions have also shown a positive effect in reducing psychiatric symptoms [18,19]. Within a systematic review to assess this effect, peer support treatments were associated with symptom reduction and an improvement in functioning for patients, ranging from domains of employment, hospitalizations, social activities, and attitudes about treatment [9]. While the results were overall positive, the review noted limitations and a need for further research. Specifically, there is a need for randomized trials, as their sample was primarily qualitative.
The shift to assess impact on psychiatric and associated symptoms in addition to substance use in research is important as psychiatric symptoms are highly prevalent within the population of patients diagnosed with substance use disorders [20-22]. A meta-analysis conducted found that the comorbidity of depression with alcohol use disorder ranged from 24.3% to 48.5% [23]. Clinical samples have an even higher co-occurrence, ranging from 50-70% [21]. This is higher than in the general population, which has a 20.8% prevalence of mood disorders [24]. Due to this elevated prevalence, the treatment for these patients needs to promote sobriety while working to decrease psychiatric symptoms to create a holistic sense of well-being.
Current literature highlights the effect of substance withdrawal on psychiatric symptoms. During periods of withdrawal, patients are likely to experience an increase in their psychiatric symptoms and a decrease in global functioning [23,25,26]. This makes the onset of treatment a particularly important time. As patients are likely to be suffering from effects of withdrawal and increased psychiatric symptoms upon first entry into treatment, the first 30 days of treatment through two months are crucial as researchers have found that the rate of relapse is at its highest during this timeframe [27-30].
Furthermore, psychiatric symptoms can impede the treatment process and outcome [21-23]. A meta-analysis found that patients with depressive symptoms and a co-occurrence of Alcohol Dependence had an overall poorer prognosis [21]. Findings also show these participants were overall less motivated, less compliant, and displayed a higher craving for alcohol than non-depressed counterparts, as well as a higher and more rapid rate of relapse [21,23].
During initial treatment, there is an increased need for efficacious and supportive treatments for patients as this represents a difficult lifestyle transition [23,26]. Peer-support treatments that place emphasis on the first 30 days are needed where there is an increased vulnerability for patients with Substance Use Disorders who also may suffer from increased psychiatric symptoms. The present study is the first, to our knowledge, aimed at directly addressing the need for interventions targeting initial stages of treatment in order to reduce psychiatric symptom severity and improve participant functioning. First through Mentorship for Alcohol Problems (MAP), a Stage Ia alcohol study conducted during the onset of treatment [13], followed by the current Stage Ib study, Mentorship for Addiction Problems (MAP),which more closely focused on initial treatment with an expansion to include broader substance using populations while assessing the impact on psychiatric and associated symptoms.
MATERIALS AND METHODS
MAP is a new behavioral treatment that formalizes client-to-client mentorship relationships as an adjunct to standard outpatient substance abuse treatment. It is comprised of selection, training, and supervision procedures to enable successful recovering patients to serve as mentors for clients who are early in the recovery process. MAP mentorship is conceived as an optional module that can be incorporated into professionally run treatment programs based on a wide range of treatment philosophies. Mentees are assigned a mentor upon admission to outpatient substance abuse treatment for 12 weeks. Mentoring activities revolve around assisting mentees in progressing toward individualized treatment goals during 1 hour group mentoring and 1-4 hours of interaction within and outside of the treatment setting per week starting during the first 30 days of treatment when vulnerability to relapse and drop out is high. Mentors underwent mentor training consisting of 1 hour training sessions 2 times per week for 4 weeks prior to mentoring in addition to providing the individual and group mentorship contact to multiple mentees during their 24 week term. The following are the topics covered in the 4 week mentor training period: 1) Overview of MAP, 2) Understanding policy and procedures (e.g., interacting with system), 3) Your role as a Mentor (e.g., boundaries), 4) Helping your mentee maintain sobriety/modified GAS, 5) Maintaining your own sobriety in the process, 6) What to do in a Crisis (e.g., suicidal ideation, homicidal ideation), 7) mental health issues, 8)HIV/infectious disease (ID) risk reduction and 9) Being sensitive to diversity.. Supervision is provided by a professional staff member on an ongoing basis during weekly 1 hour mentor group supervision and ad hoc if needed.
Later Recovery Participants (LRP) and Early Recovery Participants (ERP) with substance use disorders were randomly assigned using an urn randomization program to either MAP plus Treatment as Usual (TAU) or TAU alone. LRP met the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID-I/P) lifetime diagnosis for substance abuse or dependence and were 6 months abstinent from drugs and alcohol [31]. ERP met current SCID-I/P diagnosis for substance abuse or dependence and were actively using substances. There were two cohorts. For participants in the MAP condition, within each cohort, a pool of 4-5 LRP mentors engaged in mentoring activities for 24 weeks until 12-13 recently admitted (within the last 30 days) ERP mentees participated in MAP for 12 weeks. Mentoring activities included weekly contact such as phone calls or meeting in the community/hospital (e.g., getting coffee, sitting in common areas, eating lunch together) to discuss goals, coping skills, and general life experiences. Behavioral and biological measures were conducted at baseline, weekly, monthly, and termination of the study for all participants as well as during the ERP 12 week follow-up phase.
Included in the scope of this paper are the results from the Addiction Severity Index (ASI-Lite) Psychiatric Section, Quality of Life Inventory (QOLI), Substance Clinical Global Impression Scale (SCGI), and General Self Efficacy Scale (GSE). MAP’s positive impact on substance use will be reported in the forthcoming main paper for the study.
The ASI [32] is a standardized, multidimensional, semi-structured, comprehensive clinical interview that provides clinical information important for formulating treatment plans as well as problem severity profiles in six domains commonly affected in substance abuse: Chemical abuse (drug and alcohol), medical, psychiatric, legal, family/social, and employment/support. ASI composite scores were calculated using scoring methodology for psychiatric symptom severity; scores ranged from 0-1, with higher scores indicating greater severity [33]. Internal consistency reliability for the ASI for psychiatric symptom severity in this sample, measured using Cronbach’s alpha, was excellent (standardized alpha=0.81).
The Quality of Life Inventory (QOLI) is a questionnaire that assesses quality of life through levels of satisfaction and importance within 16 key areas of life including love, work and recreation. It has 32 Likert items that are either on a three-point scale for importance (0,1,2) or a 6-point scale for satisfaction (-3,-2,-1,1,2,3). Score for satisfaction and importance are multiplied for each of the 16 domains and that score is summed across domains to obtain a total score. Higher scores reflect greater levels of satisfaction and importance in areas of quality of life resulting in overall higher ratings of quality of life. Internal consistency reliabilities range from 0.79-0.88 for the life satisfaction scales and from 0.44-0.82 for the objective QOL [34]. In this sample, internal consistency reliability for the QOLI was excellent (standardized alpha=0.87)
The Substances Clinical Global Impressions-Self (SCGI-S) scale was adapted from the CGI for use in multi-center substance abuse clinical trials and evaluates areas of reported use, substance seeking behavior, psychiatric symptoms, physical/medical problems, and maladaptive coping. Each item is rated for overall severity ranging from 1 (no symptoms) to 7 (among the most extreme symptoms) and totaled with higher scores indicating greater severity of substance abuse. The SCGI has been found to be a reliable and valid measure of global functioning, correlates with actual use/related functioning problems, and provides a time efficient outcome measure for short term trials [35]. The internal consistency reliability for the SCGI-S in this sample was excellent (standardized alpha=0.90).
The General Self-Efficacy Scale (GSE) is a psychometric scale assessing for self-efficacy, which is the optimistic beliefs about coping skills and ability to deal with life challenges [36]. The scale consists of 10 items ranked on a 4-point Likert scale ranging from 0 (not at all) to 4 (exactly true). We utilized this scale as one form of assessment of participants’ belief in ability to change circumstances to see if there were any differences in groups that may contribute to their belief in their ability to change substance use. Reliability for the GSE has been tested in 23 countries, with Cronbach’s alphas ranging from 0.76 to 0.90 [36]. In this sample, the internal consistency reliability of the GSE was also excellent (standardized alpha=0.90).
Mean scores for all instruments were compared between treatment arms across study weeks using linear mixed models. Statistical significance was indicated where p
RESULTS
Participant flow
Demographics
|
Characteristic |
All Mentees |
MAP Condition (n=24) |
Control |
p-value* |
|
Age in years, mean (SD) |
45.6 (13.3) |
48.5 (12.1) |
42.7 (14.0) |
0.129 |
|
Sex |
||||
|
Male |
35 (73%) |
18 (75%) |
17 (71%) |
0.745 |
|
Race/ethnicity |
||||
|
White non-Hispanic |
13 (27%) |
6 (25%) |
7 (29%) |
0.624 |
|
Black non-Hispanic |
16 (33%) |
10 (42%) |
6 (25%) |
|
|
Hispanic |
13 (27%) |
5 (21%) |
8 (33%) |
|
|
Other non-Hispanic |
6 (13%) |
3 (13%) |
3 (13%) |
|
|
Education |
||||
|
High school graduate or less |
23 (48%) |
10 (42%) |
13 (54%) |
0.386 |
|
More than high school |
25 (52%) |
14 (58%) |
11 (46%) |
|
|
Currently homeless |
19 (40%) |
11 (46%) |
8 (33%) |
0.376 |
|
Psychiatric disorder** |
||||
|
Mood |
17 (36%) |
10 (42%) |
7 (30%) |
0.423 |
|
Anxiety |
16 (34%) |
10 (43%) |
6 (25%) |
0.181 |
|
Substance use disorder** |
||||
|
Alcohol |
40 (83%) |
21 (88%) |
19 (79%) |
0.701 |
|
Cannabis |
27 (56%) |
12 (50%) |
15 (63%) |
0.383 |
|
Opioid |
12 (25%) |
5 (21%) |
7 (29%) |
0.505 |
|
Cocaine |
21 (44%) |
9 (38%) |
12 (50%) |
0.383 |
|
Days of drug or alcohol use, 30 days prior to intervention, mean (SD) |
4.3 (5.8) |
3.8 (4.6) |
4.9 (6.9) |
0.511 |
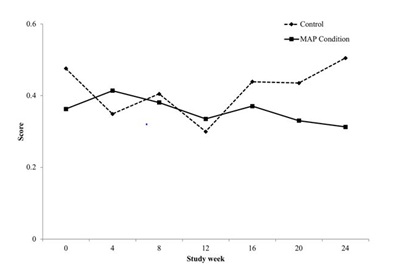
Psychiatric symptoms
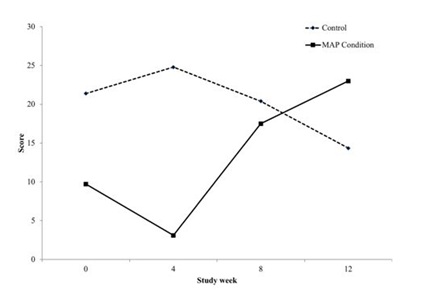
Quality of life
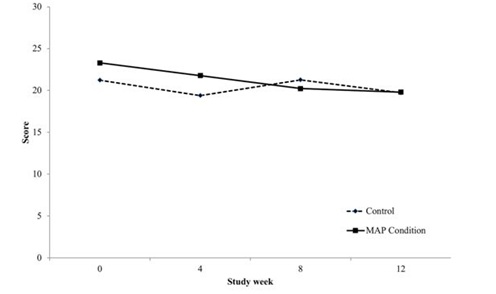
Clinical global impression
General self efficacy
DISCUSSION
Early in treatment when individuals may be struggling with psychiatric symptoms associated with withdrawal, having a treatment that could help with better management of these symptoms can potentially improve outcomes and increase likelihood of initial and sustained abstinence. In addition, individuals who have co-occurring psychiatric conditions can also benefit and potentially achieve improved substance use disorder outcomes with treatments that positively impact psychiatric symptoms. As a result, there is a need to develop treatments that not only target reduction of substance use, but also better manage psychiatric and associated symptoms.
Mentorship for Addiction Problems (MAP), a new peer to peer supportive treatment which targets providing support during the initial phases of treatment, was associated with improved psychiatric and associated symptoms. Psychiatric ASI composite scores steadily decreased for participants in the MAP intervention across the entire study participation both within active treatment and during follow-up while they increased for those participants in standard treatment alone.
During the active phase of the intervention, overall severity of functioning improvement was associated with involvement in MAP. Total clinical global impression severity ratings, which consider not only substance, but psychiatric and associated symptoms in overall ratings, decreased steadily for those participants in the MAP intervention during the active treatment phase, but fluctuated for those in treatment as usual alone.
Although substance use has multiple negative effects on the individual using, there are also immediately reinforcing effects of physical sensation or engagement within a social structure. When an individual stops using they are void of this reinforcement to feel a sense of pleasure. In addition, often they have burnt the bridges with non-substance based relationships that may have been relied on in the past for pleasure, so they have lost social connection outside of using based relationships. Therefore, it is critical to develop treatments that also create natural reinforcements within the individual’s life. For example, the mentoring activities are instrumental in creating a space for social and fun experiences that do not include substance use. During active treatment, quality of life significantly increased for those within MAP, but decreased for those in treatment as usual alone. These quality of life improvements shown in MAP may be associated with improvements in psychiatric symptoms. Impairment to quality of life has been found to be a common experience for patients with SUD upon entering treatment and has been shown to be associated with increased psychiatric symptom severity [15]. Future studies further investigating this relationship are warranted.
In further attempts to understand our results, we chose to measure self-efficacy to begin to examine the participant’s confidence in their ability to succeed in making change happen in their life, which may contribute to the participant’s overall ability to manage changes in substance use. Confidence in ability to change substance use behavior has been noted as an important outcome to assess addiction interventions [38]. There were no significant differences between our treatment groups.
It should be noted that this is a Stage Ib study. Similar to other Stage I studies, there are limitations due to sample size, which may impact ability to detect statistically significant differences between groups. Additionally, the number of patients with opioid abuse, in particular, is relatively small (22%) and the effects of MAP on people with addiction who are on replacement therapy, such as buprenorphine is unknown [39]. However, beyond reduction of substance use discussed in the main paper, even with these limitations, MAP shows much promise in improving management of psychiatric and associated symptoms among those early in substance use disorder treatment. In addition, it may offer a foundation to begin to develop other areas of one’s life to offer a valuable competing reinforcement to substance use.
ACKNOWLEDGEMENT
REFERENCES
- Tracy K, Wallace SP (2016) Benefits of peer support groups in the treatment of addiction. Subst Abuse Rehabil 7: 143-154.
- Swarbrick M, Tunner TP, Miller DW, Werner P, Tiegreen WW (2016) Promoting health and wellness through peer-delivered services: Three innovative state examples. Psychiatr Rehabil J 39: 204-210.
- van Der Horst M, Coffé H (2011) How friendship network characteristics influence subjective well-being. Soc Indic Res 107: 509-529.
- Resnick SG, Rosenheck RA (2008) Integrating peer-provided services: A quasi-experimental study of recovery orientation, confidence, and empowerment. Psychiatr Serv 59: 1307-1314.
- Lee HJ, Szinovacz ME (2016) Positive, negative, and ambivalent interactions with family and friends: associations with well-being. J Marriage Fam 78: 660-679.
- Davidson L, Chinman M, Sells D, Rowe M (2006) Peer support among adults with serious mental illness: A report from the field. Schizophr Bull 32: 443-450.
- Dickerson F, Savage CL, Schweinfurth LA, Goldberg RW, Bennett M, et al. (2016) The experience of peer mentors in an intervention to promote smoking cessation in persons with psychiatric illness. Community Ment Health J 52: 416-423.
- Rowe M, Bellamy C, Baranoski M, Wieland M, O’connell MJ, et al. (2007) A peer-support, group intervention to reduce substance use and criminality among persons with severe mental illness. Psychiatr Serv 58: 955-961.
- Davidson L, Chinman M, Kloos B, Weingarten R, Stayner D, et al. (1999) Peer support among individuals with severe mental illness: A review of the evidence. Clin Psychol 6: 165-187.
- Zhang MWB,Tran BX, Nguyen HLT, Le HT, Long NH, et al. (2017) Using online respondent driven sampling for Vietnamese youths’ alcohol use and associated risk factors. Health Inform Res 23: 109-118.
- Bassuk EL, Hanson J, Greene RN, Richard M, Laudet A (2016) Peer-delivered recovery support services for addictions in the United States: A systematic review. J Subst Abuse Treat 63: 1-9.
- Dobkin PL, De CM, Paraherakis A, Gill K (2002) The role of functional social support in treatment retention and outcomes among outpatient adult substance abusers. Addiction 97: 347-356.
- Tracy K, Burton M, Miescher A, Galanter M, Babuscio T, et al. (2011) Mentorship for Alcohol Problems (MAP): A peer to peer modular intervention for outpatients. Alcohol 47: 42-47.
- Havassy BE, Hall SM, Wasserman DA (1991) Social support and relapse: Commonalities among alcoholics, opiate users, and cigarette smokers. Addict Behav 16: 235-246.
- Pasareanu AR, Opsal A, Vederhus JK, Kristensen O, Clausen T (2015) Quality of life improved following in-patient substance use disorder treatment. Health Qual Life Outcomes 13:35.
- Donovan D, Mattson ME, Cisler RA, Longbaugh R, Zweben A (2005) Quality of life as an outcome measure in alcoholism treatment research. J Stud Alcohol Suppl 15: 119-139.
- Frisch B, Clark MP, Rouse SV, Rudd MD, Paweleck JK, et al. (2005) Predictive and treatment validity of life satisfaction and the quality of life inventory. Assessment 12: 66-78.
- Chinman M, George P, Dougherty RH, Daniels AS, Ghose SS, et al. (2014) Peer support services for individuals with serious mental illnesses: Assessing the evidence. Psychiatr Serv 65: 429-441.
- Vally Z, Abrahams L (2016) The effectiveness of peer-delivered services in the management of mental health conditions: A meta-analysis of studies from low- and middle-income countries. Int J Adv Couns 38: 330-344.
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, et al. (2004) Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: Results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry 61: 807-816.
- Lejoyeux M, Lehert P (2011) Alcohol-use disorders and depression: Results from individual patient data meta-analysis of the acamprosate-controlled studies. Alcohol Alcohol 46: 61-67.
- Hides L, Samet S, Lubman DI (2010) Cognitive Behaviour Therapy (CBT) for the treatment of co-occurring depression and substance use: Current evidence and directions for future research. Drug Alcohol Rev 29: 508-517.
- Conner KR, Pinquart M, Gamble SA (2009) Meta-analysis of depression and substance use among individuals with alcohol use disorders. J Subst Abuse Treat 37: 127-137.
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, et al. (2005) Lifetime prevalence of age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry 62: 593-602.
- Shu JE, Lin A, Chang G (2015) Alcohol withdrawal treatment in the medically hospitalized patient: A pilot study assessing predictors for medical or psychiatric complications. Psychosomatics 56: 547-555.
- Conner KR, Pinquart M, Holbrook AP (2008) Meta-analysis of depression and substance use and impairment among cocaine users. Drug Alcohol Depend 98: 13-23.
- Maisto SA, Pollock NK, Cornelius JR, Lynch KG, Martin CS (2003) Alcohol relapse as a function of relapse definition in a clinical sample of adolescents. Addict Behav 28: 449-459.
- Cornelius JR, Maisto SA, Pollock NK, Martin CS, Salloum IM, et al. (2003) Rapid relapse generally follows treatment for substance use disorders among adolescents. Addict Behav 28: 381-386.
- Ramo DE, Brown SA (2008) Classes of substance abuse relapse situations: A comparison of adolescents and adults. Psychol Addict Behav 22: 372-379.
- Ouimette P, Coolhart D, Funderburk JS, Wade M, Brown P J (2007) Precipitants of first substance use in recently abstinent substance use disorder patients with PTSD. Addict Behav 32: 1719-1727.
- First MB, Spitzer RL, Gibbon M, Williams JBW (2002) Structured clinical interview for DSM-IV-TR axis I disorders, research version, Patient Edition (SCID-I/P). Biometrics Research, New York, USA.
- Mclellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, et al. (1985) New data from the addiction severity index. Reliability and validity in three centers. J Nerv Ment Dis 173: 412-423.
- McGahan P, Griffith J, McLellan AT (1986) Composite scores from the addiction severity index: Manual and computer software. Veterans Administration Press1992:142-153.
- Lehman AF, Ward NC, Linn LS (1982) Chronic mental patients: The quality of life issue. Am J Psychiatry 139: 1271-1276.
- Tracy K, Baker S, LoCastro J, Mezinskis J, Simon S, et al. (1999) The Substance Clinical Global Impression (SCGI) scale: Measuring global functioning in substance related clinical trials. Problems Drug Depend 180: 169.
- Schwarzer R, Jerusalem M (1995) Generalized Self-Efficacy scale. In: Weinman J, Wright S, Johnston M (eds.). Measures in health psychology: A user’s portfolio. Causal and control beliefs 35-37.
- SAS Institute Inc (2011) Base SAS® 9.3. Procedures guide: Statistical procedures. SAS Institute Inc, North Carolina, USA.
- Zhang MW, Fang P, Ho RC (2016) Global outreach and user preferences of a smartphone application developed for drinkers. Technol Health Care 24: 495-501.
- Ho RCM, Chen KY, Broekman B, Mak A (2009) Buprenorphine prescription, misuse and service provision: A global perspective. Adv Psychiatric Treat 15: 354- 363.
Citation: Tracy K, Wachtel L, Goldmann E (2018) Beyond Substance Use Reduction: The Positive Impact of Mentorship for Addiction Problems (MAP). J Alcohol Drug Depend Subst Abus 4: 009.
Copyright: © 2018 Kathlene Tracy, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

