
Biomarkers in Psoriasis: A Neurocutaneous Disease
*Corresponding Author(s):
Dra Paola Valeria NandiUniversity Of Buenos Aires Uba, Argentina
Tel:+054 1159368058,
Email:valerianandi72@gmail.com
Abstract
Introduction: Psoriasis is a multifactorial disorder, a polygenic, mediated skin disease by the immune system, with a high rate of psychiatric comorbidity. Invalidates physically, emotionally and socially; with a significant impact on quality of patients' lives.
Patients with psoriasis often experience social stigma. Psoriasis; it can appear at any age, but two peaks have been reported at the beginning: between 20 and 30 years and the second between 50 and 60 years. There are clinical variants of the disease, namely guttate psoriasis, psoriasis erythrodermic, pustular psoriasis and vulgar psoriasis representing more than 90% of the cases. The joints may also be affected. Psoriatic arthritis affects 5-30% of patients with skin disease. Stress is one of the main triggers of psoriasis and has been associated with disease onset and subsequent outbreaks, while outbreaks by themselves they often lead to psychological distress. Inflammation, hyperactivity of the hypothalamic-pituitary-adrenal axis and the activity of the sympathetic nervous system are involved in psoriasis and comorbidity Psychiatric. The treatment of psoriasis is individualized, depending on the severity of the measurable disease of patients; as assessed by questionnaires Standardized. The clinical scales used today to measure the severity of psoriasis are characterized by low reproducibility and high variability among examiners. Therefore there is a real need to identify measurable biomarkers objectively to standardize the evaluation of the severity of psoriasis. The presence of biomarkers in psoriasis and in stress-related pathologies. They can help establish their severity, be useful in monitoring the disease and in therapeutic strategies.
Materials and Methods: I review the path physiological mechanisms that link psoriasis with disorders of the mood. I focus on advances in the discovery of biomarkers of psoriasis, pointing out those that have also been studied in other conditions related to Stress in order to optimize the treatment of patients.
Sources Used: Pub Med; Lilac’s, RIMA, Dermatological Archives, Fitzpatrick Dermatology. Anatomy and physiology of the nervous system (Guyton).
Keywords
DISCUSSION
Psoriasis is one of the main dermatological disorders associated with disorders psychological [1,2]. Studies showed that psoriatic patients have a higher risk of psychiatric comorbidities and suicidal ideas compared to patients who they suffer from other dermatological diseases such as melanoma, and allergic disorders [3]. It was also shown that the quality of life of patients with psoriasis is so impaired like that of patients with cardiovascular disease or cancer.
Psoriasis patients often feel stigmatized, depressed or anxious. Sexual deterioration is more important in the most severely affected patients, and the Alcoholism is more frequent in them than in the general population [4]. Stress is one of the best known triggers of psoriasis has been associated with the appearance of diseases, outbreaks and psychological disorders. Anxiety can occur in psoriatic patients due to disfigurement, Stigmatization or chronic pruritus and in turn can lead to depression. For another on the other hand, psoriasis can be triggered or worsened by psychiatric conditions like depression and anxiety within a vicious circle. It is also related to other mental disorders such as eating disorders, sleep disorders, sexual disorders, substance abuse and dependence, psychosis, bipolar disorder or somatomorphic disorders [5]. Given the accumulated evidence that psoriasis could be associated with ideation Suicidal, studies have shown the relationship between psoriasis and suicide. In a review of eighteen studies with 1,767,583 participants, of which 330,207 were diagnosed with psoriasis, Singh et al., they found that patients with psoriasis were more likely to exhibit suicidal behaviors, to try committing suicide, and committing suicide than patients without psoriasis. Patients with a more serious disease and younger patients have the highest risk [6,7]. Psoriatic patients also experience a lot of psychological stress and, in 40 to 80% of patients, disease activity is influenced by stressful events [8].
Two main factors contribute to the psychosocial impact of psoriasis; stress associated with anticipatory or avoidance coping behavior that is performed to limit the sociocognitive intrusion of psoriasis and the stress resulting from the beliefs of patients or actual experiences of being evaluated by others solely on the base of your skin [9]. Curiously, the psychological burden of psoriasis is not always proportional to the severity of the disease, as measured by the clinical scales available until moment. For this reason, it is recommended that measures that assess morbidity. Psychosocial should also be considered when assessing the severity of psoriasis [10]. The mechanisms through which psychological stress interferes with the appearance of psoriasis or exacerbations and with chronic negative mood disorders are not they know completely. Mechanisms involved are described.
NEUROINMUNOENDOCRINOLOGÍA
Stress has a great impact on the immune response. In the 1920s it noted that psychological factors could influence response parameters immune, however only in 1969 the concept that an integration exists important between the CNS, the endocrine system and the immune response. The great impact that the CNS has on the immune response was defined for the first time in what has been called General Adaptation Syndrome (SGA) that was described in 1978, as a non-specific response of the organism to a stimulus, in which it described three phases characterized by an alarm reaction, a state of resistance and a state of exhaustion respectively. The main biological features are given by the great impact on the gland adrenal, then it was already understood that the endocrine system had a role important in the effector part of the EMS, since hypertrophy was found adrenal; In addition, there was significant thymic atrophy and gastric ulcer. This is how the concept that stress changed the adrenal profile and that through changes in Adrenal hormone release immunological changes occurred.
There are three protagonists
- SNC: which was believed only released neurotransmitters
- Adrenal gland, as the main organ of the endocrine system, responsible for releasing Catecholamines and cortisol
- Immune system: which releases antibodies (LB) and cytokines (LT)
This integration is reinforced when analyzing the close anatomical relationship that it exists between CNS and immune system. From an embryological point of view it is known that the main immune organ that is the thymus, originates from crest cells cardiac neural, where the CNS originates, then the embryological origin of both is very related.
The thymus structure has an organization very similar to that of the CNS, with its neurons and the entire glial system that interconnects them, since there are also cells in the thymus epithelial that connect the entire parenchyma with the lymphocytes that are inside it. If the lymphocyte surface and the ability of this cell to produce a series are analyzed of mediators, of which lymphokines are the most important, from the point of immunological view, it is found that lymphocytes also release neurotransmitters. By therefore the neurotransmitters are not exclusive to the CNS, but there are also them in the immune system. Thus, both lymphocytes and monocytes release adrenocorticotropin, betaendorphine, somatostatin, VIP and a whole series of neurotransmitters that relate it to the CNS.
On the other hand, the histochemical studies that have been carried out to determine whether in the CNS synthesized lymphokines has yielded interesting results. It was shown that Glial cells and neurons produce IL-1, IL-6, IL-12. This establishes a link against any stimulus between the CNS and the immune system, by releasing neurotransmitters, especially cortisol and Corticotrophin Releasing Factor (CRH), which increases cortisol and, in turn, the release of catecholamines and slows the immune system. This is very important because if the immune system is Active will release IL-1, IL-6 and TNF, which reaches the CNS and stimulates it. Therefore, the concept is that the CNS acts on the immune system and vice versa. It allows to explain a series of psychological alterations that occur after processes infectious The immune response is divided into three large groups: response given by LB (antibodies), LT (cellular immunity) and macrophages and polymorphonuclear.
Depending on the response of LT populations, they may be individuals whose T lymphocytes parents are transformed into TH1, thanks to IL-12. TH1 produce IFN? and IL-2, which means that the individual is guaranteed a excellent response against infectious agents, especially intracellular. Instead in the second type of response the T parents go to the TH2 slope which it means that they produce IL-4, IL-10 and basically favor the antibody response.
A higher TH2 response means more chance of being allergic. If you have an answer TH1 is less likely to be allergic, but because it has a good antibacterial defense and antiviral, has a higher risk of generating tissue damage and developing diseases Autoimmune At the CNS level, the microglia cells favor the TH1 response and therefore if there is an infection they will activate, but as there will be a lot of damage there must be a TH2 counterpart, and that's what astrocytes do. The key is the TH1 / TH2 balance. This is important to explain that in the face of stress they release neurotransmitters, which act on the CRH that is in the hypothalamus and directly on the adrenal gland or on the sympathetic ganglia, and produce the release of glucocorticoids and adrenaline. Eleven there is also a direct innervation of the lymphatic tissues by the system peripheral nervous until recently (1990) it was said that stress caused a system depression immune cell by cortisol and catecholamines. This explained a greater risk to infections in an individual with chronic stress.
However, this concept has changed, following the dissection of the immune response (TH1, TH2). Today it is thought that the main effect of corticosteroids on the response Immune is to inhibit the production of IL-12, which in turn inhibits the TH1 response. In other words, the response that is inhibited in acute stress is the TH1 response and an imbalance towards TH2. Inhibition of the immune system is related to a group of divergent pathologies, CNS diseases, asthma, allergy, chronic fatigue, etc.
In the face of acute stress
- Increase in acute phase proteins
- Increase in complement levels, C3, C4.
- Accentuated macrophage stimulation
- Liberation of IL-1, IL-2, IL-6 that cross the blood-brain barrier
In depression there are genetic, environmental factors, such as childhood traumas reinforced by factors of adult life such as stress, work problems that they will act on the release of neurotransmitters and on CRH.
The significant fact is that in severe depression there is an overstimulation of CRH which leads to hypercortisolism, and instead of slowing down by feedback, the CRH there is hypertrophy of the adrenal gland in 70% of patients. The neurobiological concept that relates the CNS to the immune response in the Depression is the increase in CRH, which can be found in CSF, and axis activation hypothalamus-pituitary-adrenal.
CRH produces anxiety, fear and regulates the synthesis of neurotransmitters at the peripheral level. Peripheral CRH is part of a system similar to the HHA axis that works locally inside the skin and hair follicles. Either produced locally or by peripheral nerves; CRH can be a key component of the “brain-skin” axis that mediates interactions between the central and peripheral stress response pathways. The association between PASI scores and type 1 CRH Receptor (CRH-R1) was studied in 46 adult patients with psoriasis and 20 healthy controls. The expression of CRH-R1 is determined immunohistochemically in skin biopsies. He found a statistically significant increase in CRH-R1 in the lesions psoriatic, and a correlation between the severity of the disease measured by PASI and the expression of CRH-R1. These results support the role of stress in exacerbations of the disease [11,12]. Another concept that is sustained is the persistent activation of the immune system, basically activated macrophages, with release of IL-1, IL-6, IL-2, TNF that act on CNS and produce prostaglandin E Interleukins synthesized in the brain would be responsible for fever (IL-1), anorexia (IL-1, IL-6), analgesia (IL-2).
It is important because when there are chronic infections the patients are left with a depressive state. There are also depressive states after administering cytokines such as IFN?, TNFα, IL-2. It is possible that the permanent stimulation of the nervous system is a inducing element of depression.
Chronic stress destroys the populations of NK (Natural Killer) cells that destroy the infectious agents, would explain that a depressive patient is susceptible to infections An important aspect is that microglia cells behave as true lymphocytes, presenting antigens and producing IL-2, IFN?, transforming into cells immunological. The counterpart would be made by the astrocytes that become TH2 lymphocytes, slowing the response and preventing severe damage.
Inflammatory cytokines generate imbalances in the synthesis and metabolism of neurotransmitters; serotonin, dopamine, Gamma-Aminobutyric Acid (GABA), glutamate neuroimaging through magnetic resonance imaging with spectroscopy reveal levels high glutamate and low levels of GABA in occipital cortex of subjects with depression. Higher Interferon, through IL-6, IL-1b, TNF interrupts serotonin synthesis and facilitates its degradation.
IFN α and IL-2 stimulate the enzyme Indoleamine 2,3 Dioxygenase (IDO) (enzyme that degrades tryptophan to kinurenine), thereby lowering serotonin levels. The enzyme IDO is also activated by cortisol, that is, hypercortisolemia Keep the symptoms. Kinurenin induces depression by itself.
Kinurenin is transported to the brain where it is metabolized by microglial enzymes to neurotropic components such as quinolinic acid, receptor agonist n-methyl aspartate. Quinolinic acid is synergized with glutamate from activated microglia. Tryptophan and Kinurenin enter the brain by blood-brain barrier through Microvascular endothelial barrier cells.
Tryptophan metabolites can alter the function of CRH and induce depressive symptoms and anxiety (Figure 1), these metabolites also stimulate glutamate receptors [12].
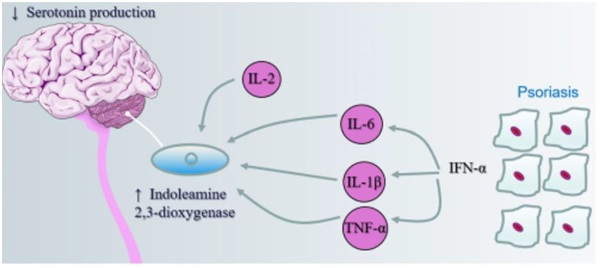 Figure 1: Serotonin production.
Figure 1: Serotonin production.
Activation of sympathetic nervous System
In addition to the neuroendocrine axis, the sympathetic nervous system plays an important role. Anxiety and depression increase the sympathetic tone. The sympathetic system innervates organs lymphoid and promotes inflammation. There is sympathetic positive regulation of proinflammatory cytokines (IL-6, IL-1), greater mobilization of progenitor and hematopoietic cells derived from the bone marrow. Psoriatic patients release norepinephrine in response to stress. Act on receivers alpha adrenergic and stimulates the production of inflammatory cytokines (TNF) and decreases the production of IL-10 (anti-inflammatory) also stimulates the production of fibroblast growth that induces keratinocytic proliferation. Depression, anxiety and chronic stress worsen inflammatory disorders through the hypothalamic-pituitary-adrenal axis with inadequate cortisol levels and through hyperactivity of the sympathetic nervous system.
MELATONINE AND VITAMIN D
Depression is associated with interruptions in melatonin secretion that has a circadian rhythm with increased levels at night and a peak between 2-4 am Melatonin also has immune function; reduces levels of TNF, IL-6, IL-8. It has been recorded that psoriasis patients have lower nocturnal levels of melatonin the decrease in melatonin deregulates the axis and there would be an increase in the hormone Melanocyte Stimulant (MSH). MSH was implicated in depressive symptoms. The decrease in melatonin is also related to the development of the syndrome. metabolic. It is believed that a benefit of phototherapy in psoriasis would be related to the fact that sunlight restores the circadian rhythm of melatonin secretion [12,13]. Vitamin D deficiency is implicated in the pathogenesis of psoriasis and depression. Many studies showed that adequate systemic concentrations of vitamin D3 metabolites allow normal differentiation of keratinocytes, in Psoriasis patients low concentrations of 25 OH D3 were associated with a decrease in circulating regulatory T cell count. So 25OH D3 can act as an immunomodulator and prevent excessive responses TH1, TH17. Absolute or relative deficiency may increase the risk of disorders of the state of encouragement in people [14]. Vitamin D deficiency is also associated with increased concentrations of inflammatory markers and their connection with the development of depression. Vitamin D receptors are found in CNS including structures involved in the control of mood such as hippocampus and prefrontal cortex. Also in Vitamin D brain is involved in the synthesis and release of serotonin and so both the deficiency can alter the function of the serotonergic brain systems and cause depression.
The skin produces more than 95% of systemic vitamin D3. It also generates others biologically active metabolites of vitamin D3 through non-classical pathways; for the both non-classical pathways could be important in the pathogenesis of psoriasis and the depression [15].
NEUROGENIC INFLAMATION
Neurogenic inflammation also intervenes in physiopathogenesis. The inflammation neurogenic and the proliferative process of non-myelinated nerve fibers involved in proliferation and maintenance of psoriatic plaque. Nerve Growth Factor (NGF) is a molecule of the neurotrophin family and could be the initiator and perpetuate cell signaling, by seconds messengers, such as neuropeptides (substance P (SP), Vasoactive Intestinal Peptide (VIP) Calcitonin-Related Gene (CRGP) peptide of said inflammatory process, which would explain Koëbner phenomenon or symmetry in the lesions. Psoriasis is seen as a psychosomatic process. Brain generates signaling neurochemicals that would modulate the peripheral process and vice versa. It would be explained because stress is the main risk factor in psoriasis. Mental illness that produces neuronal stress through mechanisms neurobiological, could generate in the peripheral nerve antiapoptotic mechanisms in genetically vulnerable people. t would also be explained that the same psoriasis produced psychosocial stress, so it would close the pathophysiological circle [15]. Cytokines, chemokines, growth factors, adhesion molecules, neuropeptides and T lymphocytes would appear to act in integrated cell signaling pathways that they would lead to a unique and typical inflammatory and proliferative process. The concept of psychoneuroimmunology and its relationship with psoriasis is relatively new. Farbers et al., have been the first to propose a possible role of neuropeptides in the pathogenesis of psoriasis. On the other hand, activated LTs play a role in the cellular mechanism of the disease. They are related in recent investigations to neurogenic factors in the activation of T cells (psycho neuro imnuno endocrinology) [16,17]. The immune and nervous systems have common recognition molecules between both of them. Two-way communication controlled from the brain (CNS) is mediated by the endocrine system Immunocompetent cells express receptors for several neurohormones and neuropeptides. Psychoneuroimmunological factors that influence proliferative processes and inflammatory associated with psoriasis. Neurogenic inflammation trying to present a pathophysiological hypothesis of relationship of said disease with (emotional) stress that would represent the path of connection of the “mind” with the skin. The cutaneous sensory nervous system in addition to conducting somatosensory impulses can induce a local inflammatory response.
Thomas Lewis., spoke of the “triple response” to injury, that is to say that an injury stimulates the sensory nerves and transmits the impulse through the spinal cord generating antidromic stimulation of connecting fibers that innervate adjacent skin. This process of antidromic stimulation of the dorsal ganglia with vasodilation, Leukocyte exudation and migration is known as: NEUROGENIC INFLAMMATION. It is currently known that neurogenic inflammation is due to the effect of neuropeptides from the endings of the unmyelinated sensory nerves and that this process affects a variety of immune cells through receptors specific for neuropeptides. By radioimmunoassay and / or histochemical staining can determine an important number of neuropeptides (SP, VIP, CGRP) that are the most studied in relation to the inflammatory skin reaction. Normal human skin, SP-positive nerve fibers are in the dermis, epidermis, Meissner corpuscles and around sebaceous glands. SP increases vascular permeability by activity of NK-1 receptors (neurocinin) on postcapillary venules and histamine release by mastocyte cells. CGRP also has a wide distribution in the skin and is one of the most abundant neuropeptides VIP in nerve fibers around arteriolar walls and acini of sebaceous glands. Receptors for neuropeptides in lymphocytes; glucocorticoid receptors, estrogens, androgens, catecholamines and neurotensin in T cells Receptors for cytokines IL-1, IL-3 in brain Stressful events alter SP levels in CNS and peripheral nervous system.
There are no investigations that have measured the local release of neuropeptides since sensory nerves of the skin after stressful stimuli in animals or humans, Therefore they are hypotheses. Neuropeptides are difficult to measure because of their rapid metabolization. Keratinocyte hyperproliferation (“brand” of psoriasis) could be caused by VIP, SP, CGRP that synergize. VIP and SP have a mitogenic effect of leukotriene B4 on human keratinocytes SP degrades and increases the number of mast cells CGRP mitogenic endothelial cell (angiogenesis) increased adhesion of peripheral leukocytes to the endothelium especially Lymphomononuclear can be a product of the signaling of SP and CGRP. SP also induces the Expression of Adhesion Molecules (ELAM-1) on vessels dermal, causing neutrophil chemotaxis and stimulating the synthesis of IL-2 in cells T, inducing the secretion of IL-1 by keratinocytes. SP induces positive regulation of E-selectin expression.
Neurogenic Growth Factor (NGF) generates neural proliferation and positive regulation of neuropeptides. In tissues with psoriasis, keratinocytes express high levels of NGF and increased NGF receptors in lesions NGF is mitogenic of keratinocytes protecting them from apoptosis, recruits mast cells promoting its degranulation. All early events in a developing injury. It also activates LT and recruits cellular inflammatory infiltrates. Schell, et al., observed that NGF induces in keratinocytes the expression of a chemokine (cc chemokine) RANTES (regulatet upon activated normal T cell expressed and secretect) which is chemotactic for memory CD4 T cells. An analogue of vit D3, tacalcitol inhibits the production of RANTES and IL-8 in cultured epidermal keratinocytes. Also inhibition of RANTES by estradiol.
Circulating RANTES levels are high in patients with psoriatic arthritis. Therefore NGF, via chemokines, mast cells, lymphocytes generates response inflammatory In psoriasis there would be increased expression of NGF in keratinocytes, generating inflammatory response, nerve proliferation and increase in neuropeptides which in turn due to its proinflammatory effect it induces keratinocyte proliferation and in turn the expression of NGF generating a vicious circle in predisposed people. Patients without psoriasis the expression of NGF is 3 to 4 times less x mm 2 of the epidermis Stressful events raise blood NGF levels and mRNA synthesis of NGF in hypothalamus we could say that NGF plays a leading role in the development of isomorphic lesions. And is responsible for the exacerbation of outbreaks clarifying the role of inflammation neurogenic, with the influence of the CNS on emotional stress reactions and mental diseases in the pathogenesis of psoriasis can open a new way of treatment (drugs that block inflammatory processes induced by neuropeptides) Cerebral neurotrophin (BDNF) Brain-Derived Neurotrophic Factor would be symmetrical at Peripheral NGF. Involved in neuronal plasticity and has functions in the skin, inducing apoptosis in the subpopulation of normal basal keratinocytes but not in psoriasis. Over expression of TNF (inflammatory overstimulation) decreases the secretion of BDNF. They would generate nuerochemical cascades of excitatory amino acids (glutamate) by inhibiting apoptosis which would generate clones of immortal cells (similar to proliferations neoplastic) [18].
LIFE QUALITY MEASUREMENT SCALES
The treatment is individualized according to the measurable severity of the disease the patients. The area of Psoriasis and the Severity Index (PASI) are considered in the currently the gold standard for assessing disease activity.
This score takes into account the intensity of the erythema, the thickness of the lesion and the scale in the four regions of the body, namely the head and neck, trunk, upper and lower limbs, and the percentage of affected area. When evaluating the severity of the disorder and choose the treatment, the doctor should also consider the perception of the disease by patients. There are currently several tools for measuring the quality of life. The Dermatology Life Quality Index (DLQI) is a self-reported questionnaire of ten elements, work, studies, personal relationships and treatment. The DLQ1 score varies from zero, with no effect on the subjects’ life, to 30 effect extremely large in the lives of subjects [19].
The Psoriasis Life Stress Inventory (PLSI) is a fifteen-item questionnaire what it measures is psychosocial stress associated with everyday events [20]. The Psoriasis Disability Index (PDI) consists of fifteen questions specific to psoriasis that address disability in daily activities, the employment, personal relationships, leisure and treatment.
The Quality of Life Index (PSORIQol) of psoriasis is an instrument designed to clinical practice and trials that assess the ability of individuals to meet their needs [21]. The accumulated disability in the life course (CLCI) [19]. Unlike the conventional measures and scales that monitor evaluations at a time or specific period of the patient’s life in a transversal way, the CLCI takes into account the cumulative impact that psoriasis causes longitudinally throughout life, interfering with the maximum potential development of the patient and with his vital perspective if the psoriasis would not have existed It emerged from the work of an international committee composed of professionals working in the field of dermatology, psychology, psychometry; providing a boarding Multidisciplinary for patients. It is based on the analysis of the mechanisms and interconnections that influence the trajectory vital of the patient with a chronic disease; analyzing the way the factors protectors and risk factors intervene longitudinally in the course of the disease.
The ultimate goal is to achieve a better understanding of the overall progressive impact of the psoriasis, contributing to identify the most vulnerable patients against this cumulative deterioration and therefore facilitating treatment decisions more appropriate and individually adjusted to each patient. It is a concept of new introduction in psoriasis that arises from the affectation Longitudinal and cumulative factors that cause known factors such as: the stigmatization associated with psoriasis as well as the various physical comorbidities and Psychopathological. The CLCI incorporates aspects as important as coping strategies, the external factors (perceived supports of the environment) and personality styles that they would play the role of modulating or protective factors of vulnerability to the CLCI. The interaction between these factors would explain the variability that exists in the experience of the disease of each patient. The CLCI would be the cumulative result of the balance between A) stigmatization load and physical and psychological comorbidities; B) coping strategies and C) the factors external modulated by the patient’s personality style. The relative weight of each one of the elements in each patient would explain the interindividual differences in the impact of psoriasis in patients with skin involvement of similar intensity and we will allow to determine the vulnerability of each patient to the impact and deterioration caused by psoriasis. This accumulated affectation that progressively deteriorates different areas exerts a influence on the vital development of psoriasis patients, decreasing their personal fulfillment and vital expectations, and even influencing decision making transcendental (Major Life-Changing decisions - MLCD) that will mark your identity and personal, professional, social and family development. The patient with psoriasis feels that his life would be substantially different without the impact of this chronic and visible disease (Figure 2)
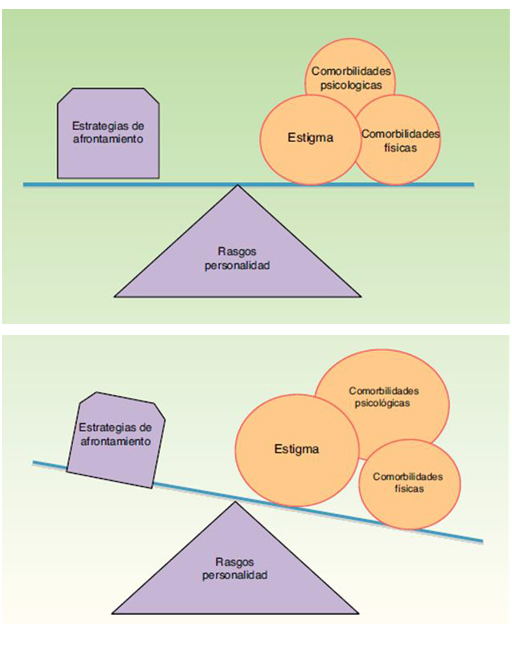 Figure 2: Personality traits.
Figure 2: Personality traits.
The progressive deterioration that accumulates throughout life can determine the inability to achieve your personal goals, altering your life path, with what the reality he experiences differs from what his life could have been in the absence of psoriasis. Studies conducted by Bhatti, et al., in which patients consider psoriasis they have been influenced by important decision making, 66% report that they have influenced the career choice, 58% in employment choice, 50% in relationships personal, 44% in the academic area, 22% in the decision to have children, 20% in the early retirement.
Many of these decisions occur in critical stages of life which implies greater risk that disability affects decision making and therefore has more CLCI risk. If it starts at a later age less vulnerability to modify the quality of life. What difference does the CLCI concept make with respect to the already known Qol? The answer would be in the dynamic, global and absolutely individual and inherent sense. To each individual. Gives broad and holistic perspective, deepens the progressive longitudinal nature, Cumulative and often irreversible deterioration caused by the disease. While the quality of life has three dimensions: life, physical, social; the CLCI incorporates additional aspects to integrate them into a global vision. Physical, psychological comorbidities and stigmatization shape the concept of CLCI and manifests itself in many of the areas of the patient’s life, social, sexual, socioeconomic habits. Low income can lead to eating habits inadequate (promote obesity) and low adherence to treatments. Patients with ineffective coping strategy and limited or absent social support they will accuse any difficulty more severely.
On the contrary, those with positive coping and good social network will minimize the impact. I would explain that people with mild psoriasis can show a condition Serious psycho-emotional and vice versa. The role of so-called coping strategies is a key modulating factor in the CLCI concept that will be influenced by the coping style of each patient, together with their beliefs about psoriasis: concept and symptom (identity), etiology (causes), consequences of psoriasis, duration and stages and their treatment, and perception of the degree of control over the disease and its outbreaks. These strategies in turn conditioned by the personality of each one. Patients with greater perception of disease control and with adequate emotional expression are those that showed better levels of health compared to patients with passive and avoidant coping. They also showed that the interval of time between relapses was longer in patients with high levels of sense of coherence (personality style that facilitates problem solving in a way adaptive to stressful situations that patients with low levels of this parameter) Passive or negative coping strategies, such as fatalism, denial, hopelessness and helplessness, as well as repression of emotions have been associated with a greater presence of symptomatology and emotional disorders. These maladaptive strategies can relieve anxiety and distress levels in a first, but they are counterproductive in the long term. For example: avoiding social situations will prevent anticipatory anxiety but that longitudinally it would cause social disconnection and isolation. Avoidance also creates difficulty in adherence to treatment of the condition. Dermatological accentuating social isolation and generating a vicious circle. The weight of these factors would be endorsed by cognitive behavioral psychotherapy that added to the dermatological treatment facilitates a modification in the perception that the patient has of his illness; provides adequate coping strategies and decreases the presence of psychopathology improving the clinical severity of psoriasis. The interaction between factors such as psychopathology, physical comorbidities and Stigmatization feelings of each patient are contrasted with the strategies of coping with the disease and personality styles, conditioning a result final. All these factors are not stable over time but may vary depending on of the patient's vital moment generating a greater or lesser vulnerability to the risk of accumulated deterioration of psoriasis.
That is why it may happen that a mild psoriasis manifests stigmatization experiences, box of intense anxiety and that there is not enough family or social support triggering a serious risk of CLCI.
The full development of the CLCI concept would make it possible to overcome the specific fixed image and Incomplete offered by quality of life questionnaires that analyze how factors such as stigmatization or physical and psychic comorbidities, measures traditionally in a transversal way, they generate a cumulative, longitudinal and progressive preventing its potential vital development. It also helps us determine the role they play as modulating factors in this accumulated disability coping strategies (adaptive or maladaptive) and external factors such as family and social support for these patients. By way of analogy will allow us to read the psychosocial and personal “black box” conditioned by the psoriasis of each person. That is, to what extent psoriasis has contributed to making that individual the person that is at that time. If we had access to the series chained and unique psychosocial events to which psoriasis has led in a specific individual, it seems likely that we could establish the importance of a proactive attitude from the point of view of both medical treatment and psychological techniques, individual or group, to improve the coping of the disease [22]. At this point the great limitation of the CLCI becomes evident. A question remains pending fundamental…
How to determine what parameters to use?
There is no validated instrument to quantify or value the concept. Although there are some proposals in this regard, the awareness of dermatologists regarding the CLCI concept and its implications will contribute to identify and understand the Vulnerability presented by patients, addressing early corresponding risk factors, and adapt the therapeutic strategies generating greater adherence and therapeutic compliance.
This better adhesion and the strengthening of the link between dermatologist and patient will allow generate adaptive coping strategies against the disease and provide early psychological assistance when necessary. Since the reliability of the scores are uncertain, and that there is great variability and low reproducibility between doctors, scientists are trying to identify biomarkers that can Objectively evaluated to standardize the measurement of the severity of psoriasis [23].
BIOMARKERS
A biomarker is a characteristic that is measured and objectively evaluated as a Indicator of normal biological processes, pathogenic processes or responses Pharmacological to a therapeutic intervention [24]. In clinical practice, they can be used as diagnostic tools, for disease staging, as prognostic indicators or to control the clinical response after an intervention. It can also help you understand the pathogenesis of various diseases or develop new therapies. In the future, the biomarkers could play a central role in personalized therapy, since they could help identify patients who will not respond to a particular treatment or they could have adverse reactions. In addition, biomarkers have played a very important role in understanding the pathogenesis of psoriasis and have facilitated the development of biological therapies [25]. Several molecules have been studied as possible biomarkers in psoriasis being the most important soluble biomarkers, biomarkers associated with tissue, oxidative stress markers, genetic markers and subsets of cells (Table 1).
|
Biomarker |
Expression |
|
Serum |
|
|
CRP |
|
|
ESR |
 |
|
Haptoglobn |
 |
|
C3,C4 complement proteins |
 |
|
Fibrihogen |
 |
|
Soluble P-selecton |
 |
|
VEGF |
 |
|
TGF-β1 |
 |
|
TMP-1 |
 |
|
MMP-1 |
 |
|
HBD-2 |
|
|
S100A8/A9 |
 |
|
S100A12 |
 |
|
IFN-γ |
 |
|
TNF-α |
 |
|
IL-6 |
 |
|
IL-8 |
 |
|
IL-12 |
 |
|
IL-18 |
 |
Table1: Biomarkers table.
SOLUBLE BIOMARKERS
Protein C Reagent and other Inespective Indicators of Inflammation
Since psoriasis is a chronic inflammatory disease. Rocha- Pereira, et al., evaluated the extent of the inflammatory response in mild and severe psoriasis by measurement of the levels of PCR, fibrinogen, ESR (erythrocyte globular), haptoglobin and complement proteins C3 and C4. The authors showed that all Psoriasis patients had higher levels of CRP than controls. 97% of psoriasis patients also had higher levels of haptoglobin that controls. Fibrinogen complement proteins, ESR, and C3 and C4 were also significantly lower in patients with inactive psoriasis than in patients with active psoriasis Coimbra et al., [26]. He studied PCR as a potential monitor for psoriasis vulgaris. The authors found that CRP levels correlate with PASI and concluded that PCR could be used to assess the severity of psoriasis and control the response to tartamiento [27]. CRP levels were also measured in patients with psychological disorders. In a study conducted in 80 patients, elevated CRP levels were associated with depression and anxiety. A study of 73,131 participants showed that elevated levels of CRP were associated with a high risk of psychological stress and depression. Platelet P-selectin was also proposed as a possible biomarker for psoriasis. Platelet P-selectin is a platelet cell adhesion molecule which, after platelet stimulation, translocates to the plasma membrane and It acts as a receptor for monocytes and neutrophils. Garbaraviciene, et al., investigated the P-selectin platelet in patients with psoriasis and patients with other disorders inflammatory skin and concluded that the level of platelet P-selectin increases by psoriasis patients and correlates with the severity of psoriasis measured by PASI the authors also demonstrated that there is a strong correlation between Platelet-expressed P-selectin and soluble P-selectin that can be easily measured through routine measurements and concluded that plasma P-selectin could be used as a biomarker in psoriasis. High levels of platelet P-selectin and norepinephrine and delay were also identified. in recovery in depressive and anxious patients who were exposed to a task of acute psychological stress.
Vascular Endotelial Growth Factor (VEGF)
VEGF is a proangiogenic factor that is found in high levels in psoriasis. Is responsible for the increase in dermal vascularization, which is specific to plaque psoriatic one study measured the concentrations of VEGF and VEGF soluble receptors (sVEGF R1 and sVEGF R 2) in patients with psoriasis before and after treatment. The scientists found that VEGF levels correlated with activity of the disease measured by PASI and that the treatment of psoriasis led to a reduction of serum VEGF levels. They also discovered that high levels of sVEGF R1 could be an indicator of clinical improvement [28]. Chen, et al., performed a meta-analysis in which they included studies investigating the Effect of narrowband UVB treatment on serum levels of VEGF and IL-8 in patients with psoriasis. They included 13 studies with a total of 400 patients with Psoriasis and 221 controls. The authors discovered that psoriatic patients had significantly higher levels of VEGF than controls before treatment with NB-UVB (phototherapy) and that VEGF levels decreased significantly after of treatment Serum IL-8 levels were not significantly different between psoriatic patients and controls before treatment, but decreased significantly after treatment with NB-UVB. The authors concluded that VEGF and IL-8 are sensitive markers to evaluate the response to treatment. VEFG is currently considered a possible target for new therapies for psoriasis, as some authors demonstrated that a monoclonal antibody against VEFG, determined the complete remission of psoriasis in a patient treated for cancer of colon [29]. Another study conducted on a mouse model of psoriasis showed that the blockage systemic VEGF-A by a monoclonal antibody determined the normalization of the epidermal proliferation, decreased the size of dermal vessels and reduced cells inflammatory. However it is necessary to continue investigating. The role of VEGF and the Brain-Derived Neurotrophic Factor were also studied. (BDNF) in the pathogenesis of mental disorders. VEGF and BDNF are involved in neurogenesis and synaptic plasticity. Long-term stress determines changes permanent in brain structures. Animal studies showed that the stress is associated with low levels of BDNF and VEGF in the brain and with characteristic behaviors of depression [30]. Transforming Growth Factor (TGF-β) Deregulation of TGF-β especially the TGF-β1 isoform was reported in patients with psoriasis over expression of TGF-β1 in keratinocytes induces inflammation in various skin diseases. One study evaluated the association between TGF-β1 and TGF-β2 in scales and plasma of psoriatic patients and disease activity. The authors found a correlation between plasma TGF-β1 and the severity of the disease, measured by PASI. The same group of authors also measured the concentrations of TGF-β1 and TGF-β2 in psoriatic patient before and after treatment to assess whether they could be Indicators of treatment efficacy. The scientists found a correlation between plasma concentrations of TGF-β1 and disease activity in patients with severe psoriasis (PASI> 15) and concluded that TGF-β1 should be considered a biomarker of disease activity during treatment. Other authors also found higher levels of TGF-β1 in the plasma of psoriatic patients than in controls and a correlation with the extent of disease. Meki, et al., analyzed the correlation between serum VEGF, TGF-β1, and the oxide nitric and disease severity in patients with psoriasis and found that all could be recognized as markers of the severity of psoriasis. For the therefore, some authors consider that TGF-β1 in plasma, Tissue Inhibitors of Metalloproteinases- (TIMP) 1, Matrix Metalloproteinase (MMP) and IL-18 must measured in psoriatic patients for superior results. Other authors studied the association between cytokines and depression in a model animal and found that rats exposed to chronic mild stress for four weeks showed behavioral changes similar to depression and expression altered cytokines in the brain, with high rates of proinflammatory cytokines (IL-1B, TNF-α, IL-6) and reduced levels of TGF-β and IL-10 Antimicrobial Peptides: Human Beta Defensin 2 and Catelicidine. Antimicrobial peptides play an important role not only in killing pathogenic microorganisms, but also to modify the inflammatory responses of the Guest. Human Defensin 2 (HBD-2) and cathelicidin (LL-37) increase in psoriasis in response to proinflammatory and type 1 cytokines and are probably responsible of the low rate of psoriatic skin infection [31]. It is also believed that they could play a role in the pathogenesis of psoriasis. The studies showed that the high number of genomic copies of HBD-2 is associated with a increased risk of psoriasis Jansen, et al., showed that serum HBD-2 levels were correlate with the severity of the disease in patients with psoriasis and concluded that HBD-2 could be a useful biomarker for disease activity. Similar results were found by Jin, et al., who analyzed the correlation between the HBD-2 and disease activity in psoriatic patients treated with the inhibitor of the Janus Kinase (JAK) and concluded that HBD-2 could be a biomarker for monitor the response to treatment. Other authors consider it a biomarker for skin pathology driven by IL-17A. The role of Neutrophil Extracellular Traps (NET) in the induction of HBD-2 production in psoriatic plaques.
Psychological stress was associated with low levels of antimicrobial peptide expression Epidermal in mice and an increased risk of developing skin infections. The Antimicrobial peptide expression is regulated by glucocorticoid mechanisms and b-adrenergic. S-100 PROTEIN S-100 proteins are a large family of low molecular weight dimeric proteins characterized by the presence of two calcium binding sites. They have activity proinflammatory, antimicrobial and chemotactic S100A7 (psoriasin), S100A8 (calgranulin), S100A9 (calgranulin B) and S100a12 (calgranulin c) increase notably in psoriasis lesions [32]. Several scientists investigated these proteins to find out if they could be reliable biomarkers in psoriasis Benoit, et al., measured the levels of S100A8 and 100A9 in patients with psoriasis and controls healthy and found significantly higher concentrations of S100A8 and S100A9 in the serum of psoriatic patients. The levels were higher in patients with a most serious disease (PASI> 15). Anderson, et al., measured serum levels of S100A7 and specific psoriasin autoantibodies in patients with vulgar psoriasis. The authors they found that even though the S100A7 was over expressed in injuries psoriatic skin, serum levels of S100A7 were reduced. The specific psoriasin autoantibodies were also not significantly high. Therefore, the authors concluded that S100A7 is not a serum biomarker promising for psoriasis. This result, however, was not found by other authors. Wilsmann-Theis, et al., analyzed the expression of S100A7, S100A8, S100A9 and S100A12 in the skin and serum of psoriatic patients and compared it with those found in patients with dermatitis atopic and lichen ruber and in healthy controls. The authors found that all studied proteins were highly expressed in the serum and skin of the patients with active psoriasis Serum levels of S100A7 and S100A12 were closely associated with activity of the disease in psoriatic patients, and serum levels of S100A8, S100A9 and S100A12 decreased after treatment with etanercept. The authors concluded that levels of S100A12 are correlated with disease activity and therapeutic response and is the most significant marker among S100 proteins [33].
CITOCINES
Cytokines have a fundamental role in the pathogenesis of psoriasis, especially those produced by dendritic cells (IL-18, IL-20, TNFα, IL-23) produced by TH1 cells (IFN ?, IL-2, TNF α) all these cytokines are biomarkers Potential for psoriasis. Cutaneous damage induces the production of keratinocyte derived peptides that have chemo attractants and immunomodulatory effects on dendritic cells and T cells. Macrophages act as antigenic presenters and produce cytokines (TNF α) Plasmocytes: IFN α, myeloid cells release nitric oxide generating vasodilation Cutaneous Myeloid cells when stimulated by IFNα, TNFα, IL-1b, IL-6, secrete IL-12 and IL-23. These cytokines act in the activation and differentiation of naive T cells to T helper, TH1 and TH17. 28 TH1 in turn secret: TNF / IFN / IL-2 and TH17: IL-17a / IL-17f, IL-22, also cytokines Chemotactic and S100 keratinocytes are part of the pathogenesis together with TNF and IFNα NK cell (natural killer) the inflammatory cytokines IL-22 (TH) and IL-20 (keratinocyte / dendritic cell) increase in psoriasis causing hyper proliferation of keratinocytes. A vicious circle ensues where inflammatory cytokines from keratinocytes activated act on innate and adapted immune cells and support the cascade inflammatory Neutrophils gather in the epidermis forming microabscesses with IL-17a and it is characteristic of psoriasis. From the complex interaction between immune cells and cytokines in pathogenesis, the TNFα / IL-12 / IL-23 and IL-17 stand out as key cytokines and targets for biological treatments In a job done in Norway; Silje Solberg, et al., they included 40 patients with vulgar psoriasis in treatment with infliximab (anti TNF) ustekinumab (anti IL-12/23) secukinumab (anti IL-17 a) etanercept (anti TNF). Blood was collected at the start of treatment and intra treatment. PASI / IMC / DLQ1 was controlled Cytokines were measured using Luminex technology
Results: PASI / DLQ1 71% / 65% respectively. Those who received infliximab and secukinumab had better parameters, somewhat lower with etanercept and ustekinumab. Cytokines such as IL-22, IL-7, IL-18, IFN? were found in most sera; IL-2 and IL-21 in ¼ of the samples Pro-inflammatory markers: IL-1 a and b, IL-6, IL-12, IL-17 a, IL-18, IL-22, TNF α, IFN ?, IFNα involve 4 times higher risk of severe psoriasis, PASI> 10, with increases in IL-17 a for 1pg / nl.
Pro-inflammatory cytokines (IL-1 b, IL-6, IL-12, IL-23, TNF and IFN α) induces in different differentiation stages of TH1 TH17 cells.The levels of TNF / IFN ?, IL-2, IL-17 a could be predicted from the cytokine levels mentioned above in the pathogenesis. The interaction of T cells and IL-2 is important for tolerance and immunity.
PASI positively correlated with IL-2 and one of the functions of IL-2 would be to facilitate the differentiation of antigen presenting T cells into effector T cells and of memory. It also correlated IL-2 with DLQ1 positively.
IL-12 and IL-23 differentiate virgin T cells in TH1 and TH17 and share a subunit common p 40 (ustekinumab white) the active IL-12 is heterodimeric P70, composed of p40 and single p35 subunit. The decrease in IL-12 p70 during follow-up was associated with a lower chance of achieve PASI 90. IL-5 correlates with a good TH2 response due to the restitution of TH1 / TH2 balance.
IL-5, IL-10, IL-22, GM-CSF correlates effectively to treatment. Arican, et al., measured serum levels of IFN-?, TNFα, IL-6, IL-8, IL-12, IL-17, IL-18, in patients with psoriasis and in healthy controls. The authors found levels significantly higher IFN ?, TNF α, IL-6, IL-8, IL-12 and IL-18 in patients with psoriasis. IL-17 levels were slightly higher in psoriatic patients, but the difference was not statistically significant. The authors also found a correlation between IFN?, IL-12, and IL-18 and clinical severity and activity of the disease measured by PASI.
Pietrzak, et al., measured serum levels of IL-18 in patients with psoriasis and in subjects healthy and found elevated levels of IL-18 in psoriatic patients, as well as a correlation between this cytokine and disease activity. Flisiak, et al., They obtained similar results. In addition, the authors discovered that the combined measurement of IL-18 and TGF-β 1 could be a biomarker of the activity of the psoriasis.
High levels of cytokines were also identified in disorders related to stress. A study in 38 medical students showed that stress Psychological was associated with an increase in TNFα, IL-6, receptor antagonists IL-1 (IL-1Ra), IFN? and IL-10. High levels of IFN ? and IL-6, TNF-α and IL-1Ra were identified in those students who perceived stress as high when they were exposed to stressful event and high levels of IFN-? and low levels of IL-10 and IL-4 were identified in those with high anxiety.
Other studies found that acute stress is associated with elevated levels of IL-6 and IL-1b Cytokine levels were also measured in psoriatic patients exposed to stress psychological.
Mastrolonardo, et al., measured salivary levels of IL-1b in 25 patients with psoriasis and 50 controls that were exposed to a standardized stressful procedure. The authors found that at the beginning of the study the average levels of IL-1b were higher in patients with psoriasis than in controls. After the stressful event, the level of IL-1b increased in the control group but not in the psoriasis group.
The scientists concluded that psoriatic patients might have an answer Defective immune system to adrenergic stimuli. A study conducted in Mice with psoriasis assessed the influence of sleep loss on levels of inflammatory cytokines the authors found elevated levels of cytokines pro-inflammatory IL-1b, IL-6, IL-12 (Figure 3) and decreased levels in the anti-inflammatory cytokine IL-10 and concluded that sleep loss is associated with exacerbations of the disease [34].
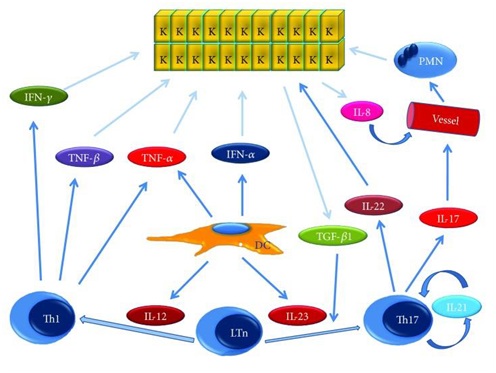 Figure 3: Mecanismo molecular en la psoriass: Description general. Vea el texto para la explication.
Figure 3: Mecanismo molecular en la psoriass: Description general. Vea el texto para la explication.
DC=celulas dendriticas, IFN-α = interferon- α, IFN-γ, IL-8 = interlucina-8, IL-12 = interlucina-12, IL-17 = interlucina-17, IL-21 = interlucina-21, IL-21, IL-22 = interlucina-22,IL-23 = interlucina 23, K=queratinocito, LTn = linfocito T virgenes, PMN = polmorfonucleares, Th1 = T helper 1, Th17 = T helper 17, TGF-β 1=transformate factor de crecimiento beta1, TNF-α = factor de necrosis tumoral α y TNF-β factor de necrosis tumoral β
CARCINOMA OF ANCIENT SCAMOUS CELLS 2
The Squamous Cell Carcinoma Antigen (SCCA) is a member of the family of serine protease inhibitors and serves as a serological marker for carcinomas of advanced squamous cells of the cervix, lung, head and neck, vulva and esophagus. However, elevated levels of SCCA are also identified in disorders. inflammatory as psoriasis and atopic dermatitis.
Two SCCA proteins have been identified: SCCA 1 and SCCA 2. SCCA 2 inhibits the G cathepsins of chymotrypsin-like and kinase-like proteins mast cells. In a prospective cross-sectional study, conducted in 123 patients with psoriasis and 25 healthy controls, serum SCCA 2 levels were measured and compared with PASI Significantly higher levels of SCCA 2 were found in patients psoriatic than in healthy controls, elevated levels of SCCA 2 in the lesional skin in comparison with non-lesional skin of psoriatic patients. It was also found that IL-17 and IL-22 increase the production of SCCA 2, and the authors therefore concluded that SCCA 2 could be a useful biomarker in psoriasis, reflecting an inflammation of helper type [17].
BIOMARKERS ASSOCIATED TO THE FABRIC
KERATINA: Keratins are a family of cytoskeleton proteins. Are the main structural proteins of the vertebrate epidermis? As the Keratinocytes migrate from the basal layer to the cornified layer, express different Keratins, Therefore, keratin 5 (K5) and K 14 are normally expressed in the layer baseline, while K1 and K10 are expressed in the suprabasal layers of differentiation K6 and K16 normally occur in the outer root sheath of hair follicles and the nail bed and are not found in the intrafolicular epidermis. However, in psoriasis and other disorders, K1 and K10, which are markers of terminal differentiation, they are replaced by K6 and K16, which are markers of hyperproliferation IL-1 has an important role in the activation of keratinocytes and the synthesis of K6 [35] Bhawan, et al., showed that K16 expression is observed in the uninjured skin of psoriatic patients and concluded that K16 could be a marker of preclinical psoriasis and could help identify people who could develop the disease.
Fransen, et al., flow cytometry was used to identify different subpopulations epidermal the authors found correlations between mild psoriasis and cells K10 + K6 + and moderate psoriasis and K10 + K6 + cells They also discovered that psoriasis treatment is associated with an increase in K10 + K6 + cells and a decrease in K10 + K6 + cells in mild and severe forms of the illness.
Conexina: The connexins are binding proteins. Connection 30 and connection 26 they are regulated more in psoriatic skin and have been associated with hyperproliferation epidermal
KERATINOCIT HYPERPROLIFERATION MARKERS
Bcl-2 family proteins have an important role in apoptosis, some members of this family promote apoptosis and others inhibit it. It has been observed that keratinocytes of psoriasis plaques are more resistant to apoptosis than normal keratinocytes. It is believed that some members of the Bcl-2 family, especially Bax and Bak proapoptotic proteins and antiapoptotic proteins Bcl-2 and Bcl-x are involved in the pathogenesis of psoriasis. The increase of levels of all these proteins in the skin of psoriatic patients supports the idea that they play a role in epidermal hyperplasia. Thermal Shock Proteins (HSPs) are a large family of proteins that have several important functions, one of which is the protection of cells against apoptosis HSPs can inhibit the activity of Bap-2 proapototic proteins.
Kakeda, et al., analyzed the expression of HSP80 in normal-looking skin, lesions of psoriasis and psoriatic patients treated with ustekinumab and concluded that HSP90 was regulated in more in psoriatic lesions and regulated in less after Ustekinumab treatment.
The p53 protein plays an important role in the control of cell proliferation and apoptosis Baran et al., detected elevated levels of p53 protein in psoriatic skin compared to non-lesion skin taken from psoriatic patients and samples of skin taken from healthy volunteers. The authors concluded that the increase in p53 expression could be the result of the agencies’ attempt to counteract proliferation Ki67 is a marker of cell proliferation and, therefore, is found in levels high in psoriatic lesions. Some authors showed that the expression of Ki67 It decreases after treatment. The studies support the idea that all the proteins mentioned above could serve as biomarkers in psoriasis.
OTHER BIOMARKERS
Oxidative stress markers
Oxidative Stress (OS) represents the imbalance between oxidants and antioxidants in favor of the oxidants. The skin is the interface between the human body and the environment and, for therefore; it is an important objective for oxidative stress. The reactive species of Oxygen (ROS) can be caused by external factors such as smoking, lightning UV, microorganisms or air pollution or endogenous factors such as oxidation reactions and reduction of normal cellular metabolism. Endogenous antioxidants such as Superoxide Dismutase (SOD), Catalase (CAT), Glutathione Peroxidase (GP), vitamin c, vitamin e, carotenoids, thiol Antioxidants, etc., normally eliminate or mitigate the effects of ROS. When the antioxidant capacity is exceeded, the resulting operating system causes damage Oxidative of lipids and membrane proteins and DNA [36]. TH1 cytokines and TH2 cytokines involved in the pathogenesis of psoriasis generate ROS. TNF- a, for example, activates the Nicotine Adenine Dinucleotide Pathway Oxidases (NAPDH) and, therefore, increases ROS. On the other hand, reduce antioxidants the increase in the production of oxygen metabolites is a known feature of psoriasis.
Several studies measured markers of oxidative stress in patients with psoriasis. Malondialdehyde (MDA), SOD, CAT, total bilirubin, bilirubin concentrations Direct, uric acid, apolipoproteins and paraoxonase 1 (PON 1) were measured at 100 patients with psoriasis and in 100 controls. The authors found levels significantly higher in MDA in patients with psoriasis than in healthy. As well found a significant decrease in the activity of SOD, CAT and PON1, all Very important antioxidants MDA is the final product of lipid peroxidation whose serum levels are correlate with the degree of lipid peroxidation of the tissue. MDA and oxide levels Nitric (NO) were measured in the plasma of psoriatic patients and healthy controls, and the MDA levels were measured in lesion tissue and non-lesion patients were psoriatic. He found significantly higher plasma levels of MDA and NO in patients psoriatic compared to controls. MDA tissue levels were higher in psoriatic lesions than in non-lesion tissues. No correlation found between tissue levels of MDA and NO and disease activity. Oxidized Low Density Lipoproteins (Ox.LDL) also increase in tissue psoriatic, and anti-Ox-LDL antibodies increase in patients’ plasma psoriatic these are considered markers of cardiovascular involvement in patients with psoriasis [37]. Some authors also proposed urinary biomarkers of oxidative stress. The Nitrate, MDA, and 8-hydroxyideoxyguanosine (8-OHDG) levels were measured in samples of urine of psoriatic patients and patients with atopic dermatitis. Nitrate levels and 8-OHDG in the urine was significantly increased in patients with psoriasis. Patients with atopic dermatitis had elevated nitrate levels. The severity of psoriasis and atopic dermatitis correlated with the urinary level nitrate Pruritus and chronic idiopathic urticaria have also been associated with levels elevated 8-OHDG. Urinary levels of vitamin C and lipoperoxides (substances Thiobarbituric acid reagents, TBARS) were also measured in psoriatic patients. Vitamin C levels were lower in patients with psoriasis than in controls healthy and, among psoriasis patients, those with a more serious disease they had lower levels than those with psoriasis in remission. The levels of lipoperoxides were higher in psoriatic patients than in those controls, the most severely affected patients have the highest levels.
Several authors analyzed the effect of different therapies on oxidative stress in psoriasis patients. The results are contradictory, some authors report high levels of antioxidants after treatment and others do not report changes significant. Biological therapies such as infliximab and etanercept, however, ROS and CRP levels appear to decrease, while the levels of antioxidants [38].
Genetic Markers
Psoriasis is a disorder of genetic transmission. The mode of inheritance is not understood all right. The risk of developing psoriasis is 41% in individuals whose parents are affected and 14% when only one is affected. There is also a concordance of psoriasis of up to 73% in monozygotic twins [39]. Psoriasis has been associated with several alleles of Human Leukocyte Antigen (HLA) such as HLA-Cw6, HLA-B37, HLA-B13, HLA-B57, HLA-Cw1, HLA-DR7, HLA-DQ9. The Strongest relationship is between psoriasis and HLA-Cw6. Patients with this allele they are more likely to have early onset psoriasis. Some authors consider that patients who have a family history of psoriasis, the onset of the disease before age 40 and the expression of HLA-Cw6 have type 1 psoriasis, While patients without a history of the disease, the onset of psoriasis after age 40, and the lack of HLA expression has type 2 psoriasis. However, this classification is not generally accepted due to frequent overlap. Genome association studies identified at least 13 major loci of susceptibility to psoriasis. The susceptibility to psoriasis 1 (PSORS 1) is the locus of most important susceptibility, and is found on chromosome 6p21. Allen, et al., found an association between PSORS 1 and early onset psoriasis, defined such as psoriasis that occurs before the age of 50. According to the authors, identifying the association with PSORS 1 could be a better way to discriminate between type 1 and type 2 psoriasis [40]. New susceptibility loci for psoriasis have been identified, including RUNX3, TAGAP, and STAT3 involved in the regulation of the function of T cells, DDX58 that participate in IFN-mediated antiviral responses, ZC3H12C with a role in macrophage activation, and CARD14 and CARM1 involved in NF-a signaling B. Sequence variants in IL-23 receptor genes have been shown (IL-23R) and IL-12B have a role in the pathogenesis of chronic epithelial inflammation. Other genes associated with psoriasis are the genes that encode 313 protein from zinc finger (ZNF313), protein 1 that interacts with TNFAIP3 (TN1P1) and protein 3 induced with TNF-a (TNFAIP3) within the nuclear factor pathway (NF-) Kb [41]. Micro RNAs are small non-coding RNA molecules that participate in the RNA silencing and in the post-transcriptional regulation of genetic expression. MIR-21, MIR-31, etc. would be involved in the inflammatory process. His presence in blood makes them promising biomarkers for diagnosis, prognosis or treatment response [39,41].
SUB-ASSEMBLY OF CELLS
TH1, TH17, TH22 cells have an important role in the pathogenesis of psoriasis and can be detected in psoriatic lesions. Kagami and colab. Used the cytometry of flow to identify and quantify these cells between the circulating CD4 + cells of the patients with psoriasis and found that TH1 cells and TH22 cells increase in Plasma of psoriatic patients. They also found that circulating levels of TH1 and TH17 decreased after treatment with infliximab [42]. Circulating Natural Killer (NK) T cells are found in higher percentages in patients with cutaneous psoriasis than in controls and patients with psoriatic arthritis. Cell subsets were also measured in psoriasis patients exposed to acute psychosocial stress. In a study in 23 patients with psoriasis and 25 healthy controls that were exposed to a standardized laboratory stressor, the CD4 + cell number was significantly higher in psoriatic patients than in controls and CD3 + / CD5 + decreased significantly in psoriatic patients than in controls According to the authors, these changes could explain the role of the factors stressors in the activation of psoriatic rash. Despite promising results, more studies are required to support the use of subsets of cells as biomarkers in psoriasis.
CORTISOL LEVEL
Since more than half of psoriatic patients declare that the disease is seen aggravated by psychological stress, some authors evaluated the role of cortisol in the exacerbation of the disorder. A study in 40 patients with psoriasis and 40 controls who were exposed to acute psychological stressors found that psoriasis patients had lower levels of salivary cortisol at the start of the study and lower serum cortisol levels after exposure to stress and than levels were particularly low in those psoriatic patients, stress influenced The evolution of the disease. Other authors examined the influence of daily stressors on levels of cortisol in patients with psoriasis and found that high levels of factors stressors were associated with low cortisol levels and they were predictive factors of exacerbation of disease since other authors did not find a correlation between cortisol level and outbreaks of psoriasis, more studies are needed to support the role of the HPA axis in the psoriasis [24].
BIOMARKERS IN THE PSORIASSIC ARTHRITIS
Studies that psoriatic arthritis is not diagnosed in patients with vulgar psoriasis, probably due to the heterogeneity of this condition, but also to the lack of systemic biomarkers. Therefore, several authors tried to identify biomarkers for psoriatic arthritis that could improve the diagnosis of this disease. A study in 52 patients with psoriasis (26 of those who had psoriasis alone and 26 with psoriasis and psoriatic arthritis) and 26 controls aimed to identify serum biomarkers for psoriatic arthritis and psoriasis. The authors found higher levels of CRP, Osteoprotegerin (OPG) and highly sensitive MMP-3 in patients with psoriasis and psoriatic arthritis compared to patients with psoriasis alone and concluded that these could be biomarkers for arthritis psoriatic in patients with psoriasis. PCR is a sensitive marker of inflammation. OPG is a marker of periostitis, and the formation of new bone and MMP-3 is a enzyme that plays a role in the destruction of bones and cartilage. Other authors showed that the levels of S-calprotectin (S100A8 / S100AP) and PCR they are significantly higher in patients with psoriatic arthritis than in controls healthy.
S-Calprotectin seems to reflect the best burden of tissue disease than CRP and is a potential biomarker for psoriatic arthritis.
Cytokine levels were also measured in patients with psoriatic arthritis. The IL-1 receptor antagonist levels (IL-1ra) correlate with the severity of joint disease IL-6 is also significantly higher in patients with psoriasis and psoriatic arthritis. than in patients with psoriasis alone. Biomarkers of synovial and synovial fluid were also investigated in patients with psoriasic arthritis. The levels of TNF-α, IL-6, IFN-?, IL-1b are elevated in the synovium of those patients a connection between psoriatic arthritis and the cell pathway was also described natural killers with respect to genetic biomarkers, HLA-Cw6 is associated with both the psoriasis of the skin as with psoriatic arthritis. HLA-B27 is associated with arthritis psoriatic and is an indicator of a more serious disease, more often associated with enthesitis, dactylitis and peripheral and axial joint damage. HLA-B39 is associated with progression in early illness. HLA-B22 is associated with a lower risk of progression [24,43].
CONCLUSION
Psoriasis is a chronic skin disease mediated by the immune system with a high rate of psychiatric comorbidity, which is often not recognized.
Beyond the negative consequences of mood such as depression and anxiety in the patient's quality of life, the evidence suggests that these conditions they can worsen the severity of psoriatic disease. The mechanisms behind this relationship are not fully understood, but the inflammation seems to be a key feature that links psoriasis with disorders of the mood; and the physiological modulators of this inflammation, including the axis hypothalamic-pituitary-adrenal and the sympathetic nervous system demonstrate changes in psychopathology that can be contributory.
Cyclic disruptions in the secretion of melatonin (sleep hormone) are also observed in both depression and psoriasis, and with an anti-inflammatory activity and known antioxidant, could represent a contributing factor to both pathologies and other comorbidities such as diabetes, cardiovascular disease.
The association between psoriasis and psychiatric comorbidity with the deficit deserves to be highlighted of vitamin D.
Understanding the complexities of the biological mechanisms at play will be key to optimize the management of patients with psoriasis and concomitant depression or anxiety.
The recognition of psychiatric comorbidity in a first step is imperative for Treat these patients as a whole.
Gravity measurements have uncertain, subjective and low reliability reproducibility that is why representative markers are sought in both pathologies.
Several of the biomarkers studied to assess the severity of psoriasis also they have been identified in stress-related disorders. While not yet found an ideal biomarker for psoriasis, there is more and more evidence supporting the role potential of VEGF, TGF-B1, HBD-2 and IL-18 in assessing the severity of disease. In addition PCR, platelets, p-selectin, TNF-α, IL-6 could help evaluate both the skin disease as the associated psychopathology. Since biomarkers have the potential to help identify new molecules key that play an important role in the pathogenesis of the disease, setting in this way new treatment goals or better markers of the disease severity and response to treatment; bookmark search ideals continues.
REFERENCES
- Wolff K, Gold Smith, Katz S et al. (2012) The Fitzpatrick Dermatology in general medicine.
- Dalgard FJ, Gieler U, Tomas-Aragones L, Lien L, Poot F, et al. (2015) The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol 135: 984-991.
- Pompili M, Innamorati M, Forte A, Erbuto D, Lamis DA, et al. (2017) Psychiatric comorbidity and suicidal ideation in psoriasis, melanoma and allergic disorders. Int J Psychiatry Clin Pract 21: 209-214.
- Fortune DG, Richards HL, Griffiths CE (2005) Psychological factors in psoriasis: Consequences, mechanisms, and interventions. Dermatol Clin 23: 681-694.
- Ferreira BI Abreu JL Reis JP, Figueiredo AM (2016) Psoriasis and associated psychiatric disorders: a systematic review on ettiopathogenesis and clinical correlations. J Clin Aesthet Dermatol 9: 36-43.
- Singh S, Taylor C, Kornmehl H, Armstrong AW (2017) Psoriasis and suicide: A systematic review and a meta-analysis. J Am Acad Dermatol 77: 425-440.
- Geberg A, Hansen PR, Gislason GH, Skov L, Mallbris L (2016) Risk of self-harm and nonfatal suicide attempts, and completed suicide in patients with psoriasis: a population-based cohort study. Br J Dermatol 175: 493-500.
- Basavaraj KH, Navya MA, Rashmi R (2011) Stress and quality of life in psoriasis: An update International Journal of Dermatology 50: 783-792.
- Fortune DG, Main CJ, O'Sullivan TM, Griffiths CE (1997) Assessing illness-related stress in psoriasis: the psychometric properties of the Psoriasis Life Stress Inventory. J Psychosom Res 42: 467-475.
- Kimball AB, Jacobson C Weiss S, Vreeland MG, Wu Y (2005) The psychosocial burden of psoriasis. Am J Clin Dermatol 6: 383-392.
- Moynihan J, Rieder E, Tausk F (2010) Psychoneuroimmunology: The example of psoriasis. Giornale Italiano di Dermatologia e Venereologia 145: 221-228.
- Miller AH, Haroon E, Felger JC (2017) Therapeutic implications of brain-immunity interactions. Treatment in the Translation. Neuropychopharmacology 42: 334-339.
- Patel N, Nadkarni A, Cardwell LA, Vera N, Frey C, et al. (2017) Psoriasis, Depression, and Inflammatory Overlap: A Review. Am J Clin Dermatol 18: 613-620.
- Pietrzak D, Pietrzak A, Grywalska E, Kici?ski P, Roli?ski J, et al (2018) Serum concentrations of IL-18 and 25 OH D 3 correlate with the severity of the depression in men with psoriasis. PLoS One 13(8): 0201589.
- Harvima IT1, Viinamäki H, Naukkarinen A, Paukkonen K, Neittaanmäki H, et al. (1993) Association of cutaneous mast cells and sensory nerves with psychic stress in psoriasis (1993) Psychotherapy and psychosomatics. 60: 168-176.
- Connor CJ, Liu V, Fiedorowicz JG (2015) Exploring the physiological link between psoriasis and state disorders cheer up. Research and practice in dermatology Page no: 11.
- Cemil BC, Canpolat F, Yilmazer D, Eskio?lu F, Alper M (2012) The association of PASI scores with CRH-R1 expression in patients with psoriasis. Arch Dermatol Res 304: 127-132.
- J. Calvo et al. (2005) Neurogenic inflammation in psoriasis: a new pathophysiological hypothesis. Rev Psychiatry Fac Med Barma 32: 120-132.
- Finlay AY, Khan GK (1994) Dermatology Life Quality Index (DLQI)--a simple practical measure for routine clinical use. Clin Exp Dermatol 19: 210-216.
- Lewis VJ, Finlay AY (2005) Two decades experience of the Psoriasis Disability Index. Dermatology 210: 261-268.
- Sarkar R, Chugh S (2016) General measures and quality of life issues in psoriasis. Indian Dermatology Online Journal 7: 481-488.
- Ros S, Puig L, Carrascosa JM (2013) Accumulated disability in the life course; the psoriasis scar in the life of patient Dermo-Syphiliographic Proceedings.
- Molteni S, Reali E (2012) Biomarkers in the pathogenesis, diagnosis, and treatment of psoriasis. Psoriasis: Objectives and Therapy 2: 55-66.
- Biomarkers Definitions Working Group (2001) Biomarkers and surrogate endpoints: preferred Definitions and conceptual framework. Clin Pharmacol Ther 69: 89-95.
- Ayala-Fontánez N, Soler DC, McCormick TS (2016) Current knowledge on psoriasis and autoimmune diseases. Psoriasis (Auckl) 6: 7-32.
- Rocha-Pereira P, Santos-Silva A, Rebelo I, Figueiredo A, Quintanilha A, et al. (2004) The inflammatory response in mild and severe psoriasis Br J Dermatol 150: 917-928.
- Coimbra S, Oliveira H, Reis F, Belo L, Rocha S, et al. (2010) C-reactive protein and leucocyte activation in psoriasis vulgaris according to severity and therapy. J Eur Acad Dermatol Venereol 24: 789-796.
- Flisiak I, Zaniewski P, Rogalska-Taranta M, Chodynicka B (2012) Effect of psoriasis therapy on VEGF and its soluble receptors serum concentrations. .J Eur Acad Dermatol Venereol 26: 302-307.
- Akman A, Yilmaz E, Mutlu H, Ozdogan M (2009) Complete remission of psoriasis after therapy with bevacizumab for colon cancer Clin Exp Dermatol 34: 202 - 204.
- Nowacka M, Obuchowicz E (2013) BDNF and VEGF in the pathogenesis of stress-induced affective diseases: an insight from experimental studies. Pharmacological reports 65: 535-546.
- Vachatova S, Andrys C, Krejsek J, Salavec M, Ettler K, et al. (2016) Metabolic syndrome and selective inflammatory markers in psoriatic patients. J J Immunol Res 2016: 8.
- Morizane S, Gallo RL (2012) Antimicrobial peptides in the pathogenesis of psoriasis. J Dermatol 39: 225-230.
- Wilsmann-Theis D, Wagenpfeil J, Holzinger D, Roth J, Koch S, et al. (2016) Among the S100 proteins, S100A12 is the most significant marker for psoriasis disease activity. J Eur Acad Dermatol Venereol 30: 1165-1170.
- Mommers JM, van Rossum MM, van Erp PE, van De Kerkhof PC (2000) Changes in keratin 6 and keratin 10 (co-)expression in lesional and symptomless skin of spreading psoriasis. Dermatology 201: 15-20.
- Jiang S, Hinchliffe TE, Wu T (2015) Biomarkers of An Autoimmune Skin Disease--Psoriasis. Genomics Proteomics Bioinformatics 13: 2224-233.
- Zhou Q, Mrowietz U, Rostami-Yazdi M (2009) Oxidative stress in the pathogenesis of psoriasis. Free Radic Biol Med 47: 891-90.
- Sikar Aktürk A, Özdo?an HK, Bayramgürler D, Çekmen MB, Bilen N, et al. (2012) Levels of nitric oxide and malondialdehyde in plasma and tissue of patients with psoriasis. Journal of the European Academy of Dermatology and Venereology 26: 833-837.
- Bacchetti T, Campanati A, Ferretti G, Simonetti O, Liberati G (2013) Oxidative stress and psoriasis: the effect of the factor inhibitor treatment α antitumor necrosis. British dermatology magazine 168: 984-989.
- Timis TL Orasan RI (2018) Understanding psoriasis: Role of miRNAs. Biomed Rep 9: 367-374.
- Allen MH, Ameen H, Veal C, Evans J, Ramrakha-Jones VS, et al. (2005) The main psoriasis susceptibility locus, PSORS1, is not a risk factor for late onset psoriasis. J Invest Dermatol 124: 103-106.
- Ryan C, Korman NJ, Gelfand JM, Lim HW, Elmets CA (2014) Research on gaps in psoriasis: opportunities for future studies. J Am Acad Dermatol 70: 146-167.
- Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A (2010) Circulating Th17, Th22 and Th1 cells increase in psoriasis. Journal of Investigative Dermatology 130: 1373-1383.
- Tampa M, Sarbu MI, Mitran M-I, Mitran C-I (2018) Pathophysiological mechanisms and the search for biomarkers in psoriasis. A stress related illness. Disease Markers Pg no: 1-14.
Citation: Nandi DPV (2019) Biomarkers in Psoriasis: A Neurocutaneous Disease. J Clin Dermatol Ther 5: 035.
Copyright: © 2019 Dra Paola Valeria Nandi, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

