Bone Scan in Gastric Cancer Patients
*Corresponding Author(s):
Bandar Idrees AliDepartment Of Surgery, Prince Sultan Military Medical City, Riyadh, Saudi Arabia
Tel:+966555310007,
Email:biaa1003@yahoo.com
Abstract
Background
A bone scan has a very highly sensitive role in the detection of bone metastases. Bone metastasis from stomach cancer occurs only rarely and it is known to have a very poor prognosis. In this study, we aimed to review the incidence, clinical characteristics, and related risk factors for bone metastases in patients with a primary diagnosis of gastric cancer.
Methods
We retrospectively evaluated all patients who were diagnosed with primary gastric cancer and underwent initially staging working up with bone scintigraphy between 2010 and 2014 at Seoul St. Mary’s hospital, The Catholic University of Korea. Total numbers of primary diagnosis of gastric cancer patients were 1589/1721 (92.33%) patients received bone scan as initial staging workup. We further analyzed the patients according to eligibility criteria we created and the incidence of and the risk factors for bone metastases were investigated.
Results
Out of 1589 patients analyzed, bone metastases were confirmed only in 15 patients (0.8 %). The mean age was 59.0 ± 8.6 years (range 24–90) and a majority of patients were male (60%). Dominant histological type either poorly differentiated type or signet ring cancer type and Bormann’s classification type 3 was the majority. The distribution of the gastric tumor in correlation to the upper, middle, and lower third was not clinically variant. The mean tumor size among this group was 3.6 ± 2.3 cm (range 0.6–20 cm). All patients were advanced gastric cancer type clinically and the median follow-up period was 9 months. The incidence of bone metastases was (20%). In (80%) of patients had bone metastases and another site of metastases. Among these, most of the time associated regional lymph node metastases were found. Most patients had multiple bone metastases instead of a single bone lesion. The whole patients of bone metastases were advanced gastric cancers and the most common metastatic site was the whole skeleton, followed by combined vertebra, rib, and scapula. Bone scintigraphy and PET-CT were mostly used together for diagnosing bone metastasis. The serum alkaline phosphatase at the time of diagnosis had increased in only 5 cases (35.71%) and there were clinical symptoms of bone pain in 8 cases (53.0%). Other variables also were not significantly valued like anemia, tumor markers like Carcinoembryonic Antigen (CEA), and Cancer antigen 19-9 (CA19-9). Treatment was given to 14 cases (93.3%) and it was mostly chemo or concomitant chemoradiotherapy.
Conclusion
The preoperative bone scan was positive in 0.8% for bone metastasis in patients with gastric cancer. Suggesting that a whole-body bone scan should not be performed routinely in patients with gastric cancer. A bone scan to be a cost-effective tool may be needed for a selected group of patients i.e. advanced stages of gastric cancer or clinically symptomatic patients. Serum ALK has a poor correlation with early bone metastasis detection.
Keywords
ALK; Bone Metastasis; Gastric cancer; Radionuclide imaging
INTRODUCTION
Gastric Cancer (GC) is the fourth most common cancer diagnosis worldwide in men following lung, prostate and colorectal, and the fifth in women following breast, colorectal, cervical, and lung with an expected incidence of 640,000 and 350,000 cases in 2011, respectively [1]. There are only a few studies have been published in the literature focusing on the onset of bone metastases in GC. Approximately 8% of total cases and 10% of annual cancer deaths worldwide are attributed to GC [2]. Bone is considered one of the most common metastatic sites in many types of cancer like breast, prostate, and lung but rarely in gastric cancer. However, gastric cancer generally metastasizes to the peritoneal membrane, liver, lymph nodes, etc., and it may metastasize to the spleen, adrenalin, ovary, lung, brain, and skin. To improve the overall survival among gastric cancer patients, it is very important to find out the presence or absence of bone metastases as well as distant metastasis during the initial workup. It is usually associated with disseminated vascular coagulation, hemolytic anemia, and other hematological complications, and the prognosis is very poor [3]. Some studies have reported that the patients had a relatively younger age, often had a signet ring cell carcinoma or poorly differentiated adenocarcinoma, and exhibited elevated serum Alkaline Phosphatase (ALP) and/or lactate dehydrogenase levels, but other studies could not identify distinguishing characteristics of BM [4-6]. Although no optimal therapeutic strategy has yet been established for Bone metastasis in gastric cancer, many reports needed in this field to conduct the onset of bone metastases and apply standard treatment guidelines. Moreover, few international guidelines recommend routinely evaluate bone metastasis at the time of diagnosis or during follow-up or pharmacological treatment. Bone metastases in GC are mainly osteolytic, and disruption of bone integrity and resulting in bone pain and pathological fracture. Many reports regarding the value of bone scintigraphy in the initial screening workup for curative gastric cancer have not been well focused. This study aimed to investigate the incidence of and related clinical risk factors for bone metastases during initial workup after the diagnosis of gastric cancer in patients. We also proposed that the use of elevated Alkaline Phosphatase (ALP) levels for the detection of bone metastasis could be helpful to correlate with positive bone scintigraphy findings.
PATIENTS, SUBJECT AND METHODS
Patients
From January 2010 till December 2014, all patients visited Seoul St. Mary’s Hospital, The Catholic University of Korea, Seoul, Korea, were entered into a database of the electronic medical record with the keyword of “Gastric Cancer” were reviewed retrospectively. A total number of 1721 patients were retrieved and reviewed from the system for this study. Among these patients, 1706 patients were excluded from this study because of many reasons. Although 1045 patients have baseline bone scintigraphy for the initial staging, were excluded because they have a clear feature of a benign lesion on bone scan, 473 with concomitant or history of cancer other than gastric cancer, 132 patients had no bone scan during initial staging, and 56 patients with insufficient data. Finally, a total of 15 patients were enrolled in our study (Table. 1). Data were collected for demographic characteristics and clinicopathologic findings (Figure 1). Approval by the Institutional Review Board of the study was taken .
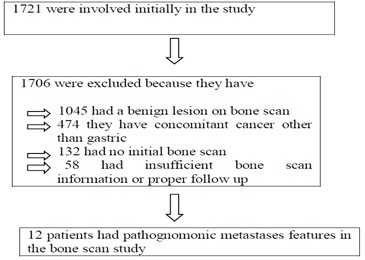 Figure 1: Enrollment and outcomes.
Figure 1: Enrollment and outcomes.
|
No |
Age/Sex |
Histology type |
Borman’s type |
Gastric ca location |
pTNMa |
AJCC 7th stage |
Surgery (Yes/No) |
Metastases site |
Anemia (Hb>10g/dl) |
ALP (44-147IU/L) |
CEA (<5ng/ml) |
CA19-9 (>37ng/ml) |
|
Patient.1 |
55 M |
Signet Ring ca. |
III |
Antrum |
T4aN2M1 |
IV |
No |
Peritoneal carcinomatosis; perigastric, gastrohepatic ligament, left paraaortic space LAP; left clavicle; right scapula |
12.9 |
43 |
51.29 |
2630 |
|
Patient.2 |
61 M |
Intestinal type |
Not available |
Fundus |
T4aN3M1 |
IV |
No |
hepatogastric ligament, splenic hilum, periportal, retroperitoneum, left common iliac, and left the supraclavicular area, rib cage, left scapula, both pelvic bones, sacrum, and T-L vertebrae, the subcutaneous layer of left lower back and left lateral chest wall vertebrae, the |
11.3 |
99 |
38.38 |
101.59 |
|
Patient.3 |
65 M |
Signet ring Ca. |
III |
Cardia |
T3N0M1 |
IV |
Yes |
left 7th rib |
13.9 |
74 |
0.05 |
5.59 |
|
Patient.4 |
41 M |
Signet ring Ca. |
III |
Antrum |
T3N3M1 |
IV |
No |
Metastatic LNs in bilateral supraclavicular areas, mediastinum & left pulmonary hilum, left internal mammary chain, cardiophrenic angle, left anterior peridiaphragmatic area, bilateral retrocrural regions, gastrohepatic ligament area, porta hepatis, peripancreatic area, retroperitoneum, bilateral iliac chains & obturator regions, and left inguinal area, omentum & mesentery, and rectovesical pouch, liver (S7)) perihepatic space, adjacent to hepatic segment 3, bilateral adrenal glands, axial & appendicular skeletons |
11 |
88 |
203.11 |
834.6 |
|
Patient.5 |
48/M |
Intestinal type |
IV |
Antrum to pyloric ring |
T3N3M1 |
IV |
Yes |
metastatic LNs in perigastric area, hepatogastric ligament, celiac axis, SMA, splenic hilum, portocaval, pericaval, aortocaval, left paraaortic areas, T vertebrae, right hepatic lobe |
10.8 |
52 |
1.06 |
52.26 |
|
Patient.6 |
73/F |
Intestinal type |
II |
Antrum, lesser curvature |
sT3N2M0 |
IIIa |
Yes |
LAPs in perigastric and portocaval regions |
11.8 |
56 |
2.47 |
56.85 |
|
Patient.7 |
73/M |
Intestinal type |
Not available |
Cardia |
T4aN3M1 |
IV |
No |
LNs in perigastric, retroperitoneum, both common iliac chains, both adrenal glands, both rib cage, sacrum, left iliac crest, T vertebrae |
9.4 |
64 |
3.74 |
Not done- |
|
Patient.8 |
54/M |
Intestinal type |
Not available |
Antrum |
T4aN3M1 |
IV |
No |
Metastatic LNs in gastrohepatic ligament & perigastric areas, porta hepatis, periportal, portocaval, retrocaval, aortocaval & left paraaortic areas, and possible, Lt. supraclavicular area & posterior cervical space, Carcinomatosis peritonei, liver with tumor thrombi in the portal vein and SMV, C-T-L vertebrae, sacrum, sternum, both rib cages, both pelvic bones, and Lt. femur |
9 |
141 |
451.84 |
16.15 |
|
Patient.9 |
50/F |
Signet ring Ca. |
Not available |
Antrum |
M1 |
IV |
No |
Skull, scapula, ribs, humerus, CTLS spines, pelvis, BM |
8.9 |
1675 |
12.6 |
98.69 |
|
Patient.10 |
63/F |
Signet ring Ca. |
IV |
Body |
cT3N2M0 |
IIIa |
No |
The left paraaortic region, aortocaval region, C-T-L spine, both iliac bones, right pubic bone, right 1st, 3rd, and left 3rd & 5th ribs. |
13.1 |
65 |
13.5 |
1.01 |
|
Patient.11 |
69/F |
Signet ring Ca. |
IIb+IIc |
Body |
T3N3M1 |
IV |
No |
Lt. posterior cervical space, both supraclavicular areas, Rt. internal mammary chain (1st ICS).Lt. highest mediastinum, Rt. retrotracheal area, both perivascular, paratracheal areas, subcarina, Rt. inferior pulmonary ligament, both hila and peri bronchial areas. subphrenic area, perigastric, peri splenic, paraaortic, retrocaval, aortocaval areas, porta hepatis, presacral area. Skull (including facial bone), sternum, bilateral ribs, scapulae, clavicles, CT- L-S vertebrae, bilateral pelvic bones, humeri, and femurs |
8.9 |
276 |
375.46 |
Not done |
|
Patient.12 |
32/F |
Intestinal type |
IIb+IIc |
Body |
T1N0M0 |
IV |
Yes |
none |
12.8 |
47 |
0.53 |
8.27 |
Table1: Patients detailed data and results of procedures.
Bone scintigraphy
The patients have injected IV with approximately 740 MBq of Tc-99m Hydroxy methylene Diphosphonate (HDP). Hydration and frequent urination were encouraged. Two to three hours after the IV injection, anterior and posterior whole-body images were obtained (Siemens E.CAM, Siemens Healthcare Solutions USA, Inc., Deerfield, IL, USA). Two to four pairs of anterior and posterior spot images were additionally obtained. Pinhole images were not routinely performed.
Image interpretation and review
The images and reports of all patients involved in this study were agreed for bone scintigraphy scoring as follow:
- 0: negative
- 1: bone metastasis - less likely
- 2. Either benign or bone metastasis.
- 3.Bone metastasis - more likely
Two clinicians have independently reviewed all the data and they were blind to each other score. Then, all results were submitted to a senior radiologist for final scoring. Cases with equivocal findings were submitted to a second radiologist for review (Table 2) and PET–CT or MRI used to validate the bone scan finding if labeled as a score of a final interpretation was achieved by consensus.
|
No |
Age/Sex |
Bone lesion type |
Number of bone Metastases |
Site of bone metastases |
Diagnostic modality |
Bone pain |
Modality of bone Mets treatment |
|
|
|
|
|
Patient.1 |
55/M |
MIXED |
2 |
Left clavicle; right scapula |
PET/CT |
No |
CTx. |
… |
… |
|
… |
|
Patient.2 |
61/M |
MIXED |
multiple |
The rib cage, left scapula, |
Bone scan, MRI, PET/CT |
Yes |
Palliative RTx/ CTx |
|
… |
|
|
|
Patient.3 |
65/M |
MIXED |
1 |
7th rib |
Bones can PET/CT |
No |
Surgery |
|
… |
… |
|
|
Patient.4 |
41/M |
OSTEBLASTIC |
multiple |
Bilateral 4th ribs, humeri, femurs, acetabula, iliac and ischial bones |
Bones can PET/CT |
Yes |
CTx, RTx |
|
… |
|
|
|
Patient.5 |
48/M |
OSTEOLYTIC |
1 |
T vertebrate |
Bone scan, PET/CT |
No |
CTx |
… |
… |
|
… |
|
Patient.6 |
73/F |
MIXED |
0 |
none |
Bone scan, PET/CT |
No |
None |
… |
… |
|
|
|
Patient.7 |
73/M |
OSTEBLASTIC |
multiple |
both rib cage, sacrum, left iliac crest, T vertebrae |
Bone scan, PET/CT |
Yes |
None |
… |
… |
… |
… |
|
Patient.8 |
54/M |
MIXED |
multiple |
C-T-L vertebrae, sacrum, sternum, both rib cages, both pelvic bones, and Lt. femur skull scapula ribs humerus |
Bone scan, PET/CT MRI |
Yes |
CTx |
… |
… |
|
|
|
Patient.9 |
50/F |
MIXED |
multiple |
CTLS spines pelvis, the whole skeleton |
Bone scan, PET/CT |
Yes |
CTx |
… |
…. |
… |
|
|
Patient.10 |
63/F |
MIXED |
multiple |
C-T-L spine, both iliac bones, right pubic bone, right 1st, 3rd and left 3rd & 5th ribs |
Bone scan, PET/CT MRI |
Yes |
CTx |
… |
… |
… |
|
|
Patient.11 |
69/F |
OSTEBLASTIC |
multiple |
the skull (including facial bone), sternum, bilateral ribs, scapulae, clavicles, C-T-L-S vertebrae, bilateral pelvic bones, humeri and femurs |
Bone scan, PET/CT MRI |
Yes |
CTx |
|
… |
… |
|
|
Patient.12 |
32/F |
OSTEOLYTIC |
none |
none |
Bone scan, PET/CT |
Yes |
none |
… |
… |
|
|
Table 2: Patients detailed data and results of procedures.
RESULTS
In our reviewed data, we showed that the bone metastases were found rarely, and only in about 0.8 % of patients who initially worked up for gastric cancer? The patient and tumor clinicopathological features of gastric cancer cases giving rise to bone metastasis of the study population are shown in table 1. The mean age was 59.0 ± 8.6 years (range 24-90) and most patients were male (60%). Dominant histological type either poorly differentiated type or signet ring cancer type. And Broman’s classification type 3 was the majority. Distribution of gastric tumor in correlation to upper, middle, and lower third was not differently observed. The mean tumor size among this group was 3.6 ± 2.3 cm (range 0.6–20 cm). All patients were advanced gastric cancer type clinically and the median follow-up period was 9 months.
The incidence of bone metastases was 20%. In 80% of the patients, they had combined bone metastases and another site of metastases. Among these, most of the time associated regional lymph node metastases was found. 4 patients 28.57% had palliative surgery due to bleeding or obstruction complication. Other variables were not significantly valued like anemia, Alkaline phosphatase (ALK), tumor markers like Carcinoembryonic Antigen (CEA), and Cancer Antigen 19-9 (CA19-9). Table 2 showed the clinicopathological characteristics of bone metastasis. In bone lesion type, the mixed type was most predominantly found (53.3%). Several bone metastases at the time of diagnosis were multiple sites in most of the patients by (73.3%). Although the site of bone metastases was not remarkable, combined vertebra, rib, and scapula or whole skeleton were observed most of the time. In (60.0%) of patients, the diagnostic modality was one scan + PET-CT. Regarding clinical symptoms of presence or absence of bone pain was not different among patients.
Bone Scan
A bone scan was performed using 99mTc-hydroxymethylenediphosphonic acid (HDP, Mallinckrodt, and St. Louis, MO, USA). The dose of 99mTc-HDP was 1295 MBq (= 35 mCi). Whole-body bone scan images were acquired using a dual-head gamma camera (Forte, ADAC-Philips, Holt, MO, USA) equipped with low energy highresolution collimator 3 hours post 99mTc-HDP injection.
Interpretation of Bone PET and Bone Scan
A positive finding for BM was defined as the presence of an abnormally high bony uptake, which is not associated with typical degenerative, traumatic, or peri-articular lesions [7]. Diagnostic accuracies were also analyzed using the Receiver Operating Characteristic (ROC) curve analysis by utilizing a 4-point grading system; definite, probable, less likely, and no evidence of BM. The Consensus was reached by two nuclear medicine physicians to call a lesion BM. A gold standard for BM was either the presence of typical findings compatible with BM in at least 2 imaging studies among MRI, 18F-FDG PET/CT or 131I whole-body scan, or the presence of a clinical progression causing a change of treatment plan during at least a one-year follow-up. Those who had at least one proven BM lesion were considered BM positive patients regardless of the presence of any false positive BM findings.
Note:- PET = positron emission tomography.
DISCUSSION
In the present study, we showed that Bone metastases occurred at a significantly higher incidence in patients with multiple numbers of regional metastatic lymph nodes and advanced tumor stage and depth. As with most other tumors, bone metastases developed in multiple sites rather than the solitary site, and even in the more aggressive form of involvement of the whole-body skeleton. Bone metastasis is a rare condition in GC and is clinically underestimated. Mori et al. investigated 719 cases of malignant tumors among 2240 consecutive autopsies [8] at Tokyo Medical and Dental University. These included 176 cases of GC of which 28 cases (15.9%) exhibited metastasis in bone, the third-most common site of GC metastases. The metastatic rate in the liver and lungs was 34.7% and 31.3%, respectively. Consistent with these findings, Yoshikawa and Kitaoka and Yamamura et al., showed that the metastatic rate in bone from curatively resected GC cases were high among autopsy cases but were comparatively low in clinical practice at a rate of 1.2-1.4% [9,10]. Also, in support of these findings, Maeyama et al. reported the rate of bone metastasis in clinical practice and autopsy as 0.7% and 17.6%, respectively. Choi et al., evaluated bone metastasis from GC by bone scintigraphy [11]. They investigated 234 bone scans from a total of 1776 GC patients. The 234 patients were classified according to their original clinical stage rather than by standard stage and were identified as having the advanced-stage disease. Of these cases, 106 (45.3%) had metastatic bone lesions. The findings discussed above suggest that asymptomatic bone metastasis is underestimated as examination by bone scintigraphy is not a routine clinical practice. It is also possible that peritoneal dissemination or liver metastasis masks the clinical manifestation of bone metastasis. For these reasons, the rate of bone metastasis in clinical cases may be higher than expected.
Many theories reported regarding the mechanisms of bone metastases in gastric cancer patients. Maehara et al., investigated bone micrometastasis using a monoclonal anti-cytokeratin antibody and they found that 9 (20%) of 45 EGC cases examined had cytokeratin-positive cells in the bone marrow at the time of primary surgery [12]. These findings suggested that the presence of micrometastatic cells in the bone marrow was closely related to angiogenesis in the primary tumor. Macadam et al., showed in multivariate analysis of risk factors revealed that bone marrow cytology was a significant factor for recurrence and death. While investigations on bone marrow micrometastasis by immunocytochemistry may play a role in predicting disease recurrence, at present it cannot be clinically applied to detect bone metastasis [13]. Lehnert et al. used light and transmission electron microscopy to investigate lymph and blood capillaries of human gastric mucosa and found that the upper and middle levels of the lamina propria of the gastric mucosa contained no lymph capillaries. The mucosa has a rich supply of blood capillaries, many of which are adjacent to the basal lamina of gastric glands and surface epithelium. He suggests that the low incidence of lymph node metastases in the early mucosal GC might be explained by the rarity of lymph vessels in the mucosa and that blood-borne metastases in recurrent EGC might be related to the rich vascularity of the gastric mucosa [14]. Rino et al. reported five EGC cases without vascular invasion (v0) resulting in bone metastasis and concluded that high-risk factors for bone metastasis were accompanying ulceration, lymph node metastasis, and distant metastasis to other organs [15]. GC might metastasize to the bones through the vertebral vein system as suggested by Batson [16]. Yamamura et al. reported that GC with bone metastasis resulted in the invasion of the lymphatic vessels more frequently than the invasion of venules [17]. They also speculated that GC might metastasize to the bone through the thoracic duct. The latter is supported by the higher rate of lymph node metastasis in those cases with bone metastasis compared to that for EGC as a whole. For advanced cancer that has invaded adjacent organs, cancer cells may invade a fairly large vein, such as those found in the vertebral vein system outlined by Seto et al. [18]. Most cases of bone metastasis do not show liver metastasis, most venous drainage from the stomach is via the portal vein, and many cases of bone metastasis are associated with lymph node metastasis, suggesting that the mechanisms underlying bone metastasis involve lymphatic channel into the systemic circulation. Ell reviewed skeletal imaging of metastatic diseases and identified MRI as sensitive in detecting bone marrow involvement [19]. Despite this finding, bone scintigraphy continues to be the modality of choice because of its simplicity, low cost, and ability to screen the entire body [19]. Choi et al., evaluated bone metastasis from GC by Tc-99m MDP imaging [20]. They investigated 234 bone scans of GC patients. One hundred and six patients (45.3%) had bone scan abnormalities that qualified as metastatic bone lesions.
The most frequent metastatic sites were the spine (66%), the ribs (59%), pelvis (43%), femur (30%), and skull (22%) [20]. The least frequent metastatic sites were the shoulder girdle (17%), such as the scapula and clavicle, sacroiliac joint (7.2%), humerus (6.0%), sternum (4.2%), and tibia (3.0%) [20]. On the other hand, roentgenographic evaluation of bone metastases has limited value because symptoms caused by bone metastases frequently occur before abnormal imaging. Bone metastases were diagnosed by bone scintigraphy in 13 out of 24 cases described in our review. Laboratory data may be helpful in the diagnosis of bone metastasis. Although tumor markers do not play an important role, many cases with bone metastasis show elevated serum Alkaline Phosphatase (ALP). In the study by Choi et al., ALP was elevated in 64% of 106 patients with bone metastasis [20]. Seto et al., also found that ALP was significantly elevated in GC cases with bone metastasis compared to those without [18]. Once cancer disseminates to the bone marrow, Disseminated Intravascular Coagulation (DIC) may occur. Clinically, patients show a tendency to bleed that is confirmed on a combination of laboratory data for platelet count and fibrin degradation products. Several chemotherapy regimens for bone metastasis of GC have been reported as case reports. Kobayashi et al., and Hironaka et al., demonstrated that sequential methotrexate and 5-fluorouracil chemotherapy resulted in a high rate of alleviation (80% and 89%, respectively) of DIC caused by bone metastasis from GC [21,22]. Limitations of this study include its retrospective design, a small number of patients although the initial study group was large [23], and the highly positive bone metastases cases in the bone scan were not confirmed with pathologic diagnosis although the additional radiological tool was used to confirm the finding in bone scintigraphy study. In this study, we assessed initially bone metastases as a screening modality by a sensitive examination tool, i.e. technetium bone scintigraphy. We showed above during this report the strategy and grading system for bone scintigraphy reading. When the findings are highly suspicious for bone metastases in technetium bone scintigraphy, additional radiological tools were used, either CT, MRI, PET-CT to differentiate inflammatory bone lesions from bone metastasis, and finalize the findings which were not conclusive.
CONCLUSION
Although it had a very poor prognosis, the incidence of bone metastasis from GC is very rare. It seems that the higher stage and poorly differentiated type of gastric cancer, the higher chance for bone metastases. It is not well understood the mechanisms of underlying bone metastasis arising from gastric cancer, but most likely involve the lymphatic channels. Some cases give rise to late bone metastasis after surgery. It can be considered that bone metastasis arising from EGC is a rare condition and as a result, adjuvant chemotherapy is not recommended. In the postoperative follow-up of EGC cases that are histologically less differentiated and display involvement of lymph nodes, recurrences as bone metastasis should be considered. Elevation of ALP can be used to detect bone metastasis and bone scintigraphy are recommended for its diagnosis.
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest.
FUNDING
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sector. All authors accept full responsibility for the work.
REFERENCES
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. (2011) Global cancer statistics. CA Cancer J Clin 61: 69-90.
- Guggenheim DE, Shah MA (2013) Gastric cancer epidemiology and risk factors. J Surg Oncol 107: 230-236.
- Yoshikawa K, Kitaoka H (1983) Bone metastasis of gastric cancer. Jpn J Surg 13: 173-176.
- Lee H, Lee WW, Park SY, Kim SE (2016) F-18 Sodium Fluoride Positron Emission Tomography/Computed Tomography for the Detection of Thyroid Cancer Bone Metastasis Compared with Bone Scintigraphy. Korean J Radiol 17: 281-288.
- Crivellari D, Carbone A, Sigon R, Buonadonna A, Cannizzaro R, et al. (1995) Gastric cancer with bone marrow invasion at presentation: case-report and review of the literature. Tumor 81: 74-76.
- Choi CW, Lee DS, Chung JK, Lee MC, Kim NK, et al. (1995) Evaluation of bone metastases by Tc-99m MDP imaging in patients with stomach cancer. Clin Nucl Med 20: 310-314.
- D’Angelica M, Gonen M, Brennan MF, Turnbull AD, Bains M, et al. (2004) Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg 240: 808-816.
- Lehnert T, Erlandson RA, Decosse JJ (1985) Lymph and blood capillaries of the human gastric mucosa. A morphologic basis for metastasis in early gastric carcinoma. Gastroenterology 89: 939-950.
- Mori W, Adachi Y, Okabe H, Ohta K (1963) An analysis of 755 autopsied cases of malignant tumors -A statistical study of their metastasis. Gan No Rinsho 9: 351-374.
- Abramson DI (1962) Blood vessels and lymphatics. New York: Academic Press 531-556.
- Yamamura Y, Kito T, Yamada E (1985) Clinical evaluation of bone and bone marrow metastasis of gastric carcinoma. Jpn J Gastroenterology Surg 18: 2288-2293.
- Maehara Y, Hasuda S, Abe T, Oki E, Kakeji Y, et al. (1998) Tumor angiogenesis, and micrometastasis in the bone marrow of patients with early gastric cancer. Clin Cancer Res 4: 2129-2134.
- Macadam R, Sarela A, Wilson J, MacLennan K, Guillou P (2003) Bone marrow micrometastases predict early post-operative Recurrence following surgical resection of oesophageal and gastric carcinoma. Eur J Surg Oncol 29: 450-454.
- Guadagni S, Catarci M, Kinoshitá T, Valenti M, De Bernardinis G, et al. (1997) Causes of death and recurrence after surgery for early gastric cancer. World J Surg 21: 434-439.
- Rino Y, Okukawa T, Okada K, Kobayashi O, Sairenji M, et al. (1996) A case report of bone metastasis from early gastric cancer preliminary to the diagnosis of the primary lesion. Shokakigeka 19: 1493-1497.
- Batson OV (1940) The function of the vertebral veins and their role in the spread of metastases. Clin Orthop Relat Res 312: 4-9.
- Seto M, Tonami N, Koizumi K, Sui O, Hisada K (1983) Bone metastasis in gastric cancer--clinical evaluation of bone scintigrams. Kaku Igaku 20: 795-801.
- Ell PJ (1991) Skeletal imaging in metastatic disease. Curr Opin Radiol 3: 791-796.
- Hironaka SI, Boku N, Ohtsu A, Nagashima F, Sano Y, et al. (2000) Sequential methotrexate and 5-fluorouracil therapy for gastric cancer patients with bone metastasis. Gastric Cancer 3: 19-23.
- Yoshikawa K, Kitaoka H (1983) Bone metastasis of gastric cancer. Jpn J Surg 13: 173-176.
- Kobayashi T, Sasaki T, Ibuka T, Imai K, Monma K, et al. (1992) Sequential MTX and 5-FU therapy of gastric cancer with systemic bone metastasis and disseminated intravascular coagulation. Gan to Kagaku Ryoho 19: 69-74.
- Kusumoto H, Haraguchi M, Nozuka Y (2006) Characteristic features of disseminated carcinomatosis of the bone marrow due to gastric cancer: the pathogenesis of bone destruction. Oncol Rep 16: 735–740.
- Pasquini E, Gianni L, Aitini E, Nicolini M, Fattori PP, et al. (1995) Acute disseminated intravascular coagulation syndrome in cancer patients. Oncology 52: 505-508.
Citation: Ali BI, Hyun J, Bukhari KO (2020) Bone Scan in Gastric Cancer Patients. J Nucl Med Radiol Radiat Ther 5: 026
Copyright: © 2020 Bandar Idrees Ali, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.