
Brunner’s Gland Hamartoma: A Case Report, Meta-Analysis, and Algorithm Approach to Obstructing Duodenal Masses
*Corresponding Author(s):
Sujit KulkarniDivision Of Hepatobiliary And Pancreatic Surgery, LAC-USC And Keck Medical Center Of USC, Los Angeles, United States
Tel:+1 3234427172,
Email:sujit.kulkarni@med.usc.edu
Abstract
Background: Brunner’s Gland Hamartomas (BGH) are rare, benign lesions. First described by Cruveilhier in 1835, there are fewer than 200 cases reported in the English literature and no large characterizing studies.
Methods: We present a patient found to have a 6 cm hamartoma just distal to the pylorus. We also include a meta-analysis of 93 cases of BGH dating from 1952 through 2010.
Results: Sixty four percent of patients with BGH were male and the mean age was 52.6 years. The average size was 4.2 cm. 63.4% of hamartomas were in the first part of the duodenum, with 23.7% in the 2nd, and the rest distributed between the remaining small bowel. 56.6% of patients experienced abdominal pain and discomfort as a presenting symptom. 53% of patients reported melena, while 22.9% of patients reported fatigue, dizziness, weakness, or syncope. Nausea and vomiting were found in 14.5 and 26.6 percent of patients, respectively. 25.8% of lesions were removed endoscopically, while 65% were removed at laparotomy. There were no adverse outcomes reported.
Conclusion: BGH are most often found in men. They often come to physician attention through physical complaints of abdominal discomfort and/or GI bleeding, and can be removed endoscopically or surgically with excellent outcomes.
Introduction
Brunner’s glands are submucosal glands which extend from distal pylorus to ligament of Treitz. Dandalides, et al. found them most numerous in the second portion of the duodenum [1]. Their main function is to provide alkaline environment by secreting mucin, pepsinogen, and urogastrin in response to acid. Hamartomas of Brunner’s Glands (BGH) are relatively rare, benign neoplasms first described by Cruveilhier in 1835 [2]. There are less than 150 cases reported in the English literature, with the largest characterizing study including 27 patients [3]. There has been only one case reported where the lesion was suspected to have malignant potential [4].
The reported global incidence of all neoplasms of the duodenum is generally less than 1.0 per 100,000 [5]. However, the actual prevalence of benign tumors of the entire small bowel is difficult to determine due to their tendency to be asymptomatic, although two autopsy series including 22,810 and 2648 specimens found incidences of 0.15% and 0.83%, respectively [6,7]. Autopsy studies have also found that benign tumors account for up to 75% of small bowel tumors, making them far more prevalent than malignant neoplasms [8].
Here we present a patient with abdominal pain, nausea, vomiting, melena, and jaundice found to have a 6cm Brunner’s gland hamartoma just distal to the pylorus.
Case Report
A 35-year-old Hispanic male with no past medical history presented to LAC+USC Medical Center with abdominal pain and melena for two months. Pain was frequent and short-lasting, located in the epigastrium, and radiated to the lower quadrants of the abdomen. He reported an 8 pound weight loss in the previous two weeks. He drank three to four beers per week for ten years, and smoked one pack per day for one year.
On exam, he had epigastric tenderness without rebound or guarding. Digital rectal examination reveal maroon stool and positive guaiac. Labs were unremarkable except for a hemoglobin of 7.6.
Computed tomography scan showed a six centimeter mass in the second and third portions of the duodenum, which appeared to be a leading edge of severely thickened gastric mucosa prolapsing into the duodenum (Figure 1). Upper endoscopy showed a large pedunculated submucosal tumor in the duodenal bulb with a deformed antrum and pylorus. Biopsy of the mass showed ulceration with acute and chronic inflammation without any evidence of malignancy.
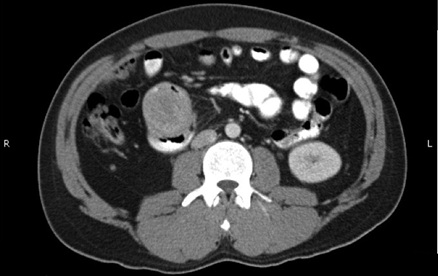 Figure 1: CT Abdomen with contrast.
Figure 1: CT Abdomen with contrast.
The patient underwent distal gastrectomy with partial duodenectomy and the histopathology showed a Brunner’s gland hamartoma. (Figures 2 and 3). Given the paucity of characterizing studies for BGH, we include a meta-analysis of 93 cases of BGH dating from 1952 through 2004 [3,4,9-81].
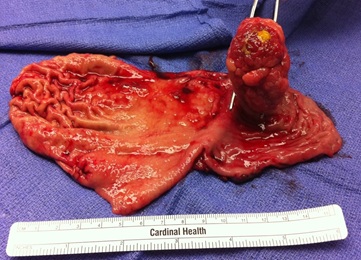 Figure 2: Gross specimen of Brunner’s Gland Hamartoma.
Figure 2: Gross specimen of Brunner’s Gland Hamartoma.
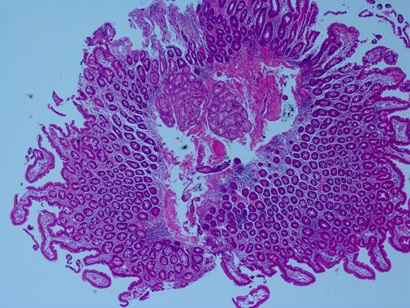 Figure 3: Microscopic specimen showing Brunner’s Glands.
Figure 3: Microscopic specimen showing Brunner’s Glands.
Methods
Cases of Brunner’s gland hamartoma were found using PubMed searches of the English literature. All available reports describing patient presentation and management were included in our study, for a total of 93 patients. Patient characteristics including age, sex, presenting symptoms, size and location of tumor, and treatment modality were extracted and analyzed. As articles varied in their method of describing tumor size, we used the largest single dimension reported in this study. The location of the tumor was characterized along anatomical boundaries, including the stomach, pylorus, first, second, third, and fourth portions of the duodenum, and the jejunum. Treatment modality was grouped by surgical removal, endoscopic removal, or other. Symptoms were analyzed by including all symptoms that the patient had experienced from the time of symptom onset until the time of presentation. Statistical analysis was done using Statistical Package for Social Sciences (SPSS Windows), version 17.0 (SPSS Inc, Chicago, IL).
Results
In 93 patients with Brunner’s Gland Hamartoma, the most common presenting symptom was abdominal pain or discomfort, which 56.6% of patients experienced (Table 1). 53% of patients reported melena, while 22.9% of patients reported fatigue, dizziness, weakness, or syncope and 21.7% were found to be anemic. Nausea and vomiting were found in 14.5 and 26.6 percent of patients, respectively.
|
Symptom |
Percent |
N |
|
Abdominal pain/discomfort |
56.6 |
47 |
|
Melena |
53 |
44 |
|
Vomiting |
26.5 |
22 |
|
Fatigue/dizziness/syncope |
22.9 |
19 |
|
Anemia |
21.7 |
18 |
|
Nausea |
14.5 |
12 |
|
Anorexia |
8.4 |
7 |
|
Shortness of breath |
6 |
5 |
|
Gas |
4.8 |
4 |
|
Weight loss |
4.8 |
4 |
|
Reflux |
2.4 |
2 |
|
Diarrhea |
2.4 |
2 |
|
Jaundice |
2.4 |
2 |
|
Hematochezia |
1.2 |
1 |
Table 1: Symptoms.
64.5% of patients with BGH were male and the mean age was 52.6 years old. The average lesion size was 4.2cm and ranged from 0.5 up to 12cm. 52.7% of tumors were pedunculated. 63.4% of hamartomas were in the first part of the duodenum, 23.7% were in the second, and the rest were distributed between the remaining duodenal segments, pyloric ring, stomach, and jejunum (Table 2).
|
Percent |
N |
||
|
Male |
64.5 |
60 |
|
|
Female |
35.5 |
33 |
|
|
Tumor location |
|||
|
D1 |
63.4 |
59 |
|
|
D2 |
23.7 |
22 |
|
|
D3 |
4.3 |
4 |
|
|
D4 |
1.1 |
1 |
|
|
Jejunum |
2.2 |
||
|
Pyloric ring |
3.2 |
3 |
|
|
Stomach |
1.1 |
1 |
|
|
Total |
100 |
93 |
|
|
Range |
Mean |
SD |
|
|
Patient age (years) |
14-82 |
52.6 |
16.2 |
|
Tumor size (cm) |
0.5-12 |
4.2 |
2.8 |
|
Yes (Percent) |
No (Percent) |
||
|
Pedunculated |
49 (52.7) |
44 (47.3) |
Table 2: Patient and tumor characteristics.
25.8% of lesions were removed endoscopically, while 65% were removed at laparotomy. One hamartoma detached spontaneously and was eliminated per rectum [71]. There was no size difference between tumors removed endoscopically versus surgically, with the average tumor size being 3.97cm and 4.21cm, respectively (p=0.71). There was only one case where the lesion was suspected to have malignant potential and there were no adverse outcomes reported (Table 3) [4].
|
Type of treatment |
Percent |
N |
|
Surgical removal |
69.9 |
65 |
|
Endoscopic removal |
25.8 |
24 |
|
Observation |
1.1 |
1 |
|
BGH eliminated per rectum |
1.1 |
1 |
|
Not specified |
2.2 |
2 |
|
Total |
100 |
93 |
Table 3: Treatment modality.
Discussion
Brunner’s gland hamartomas can present in a nonspecific and variable fashion. They can be removed endoscopically or surgically with excellent outcomes. The ratio of these treatment modalities is likely skewed in our study due the inclusion of cases prior to the use of endoscopy. We chose to remove the mass surgically secondary to an inability to snare the polyp endoscopically, as well as an inability to obtain a tissue diagnosis and with the malignant potential of duodenal lesions in mind. This provides an example of how the approach to obstructing duodenal tumors such as BGH can be difficult. The differential diagnosis is extensive and dependable endoscopic characteristics of individual polyps, with the exception of villous adenomas, are lacking [82].
Differental diagnosis
Benign tumors of the duodenum include leiomyoma, adenoma, lipoma, BGH, hemangioma, and nodular lymphoid hyperplasia. Neuroendocrine tumors include carcinoid, ganglioneuroma, gastrinoma, somatostatinoma, and VIPoma [83].
Adenocarcinoma, lymphoma, Gastrointestinal Stromal Tumor (GIST), sarcoma, leiomyosarcoma, and ampullary, adenocarcinoma make up the malignant tumors of the duodenum. Bilimoria et al., looked at 67483 primary malignant tumors of the duodenum found in the SEER and NCDB studies. 37.4% were carcinoid tumors, 36.9% were adenocarcinomas, stromal tumors accounted for 8.4%, and 17.3% were lymphomas [84]. During the study period, the incidence of carcinoid tumors increased 4-fold, making them currently the most common small bowel malignancy [84-86].
Patients at increased risk for adenocarcinoma of the duodenum include those with Hereditary Nonpolyposis Colon Cancer Syndrome (HNPCC), Peutz-Jegers syndrome, Familial Adenomatous Polyposis (FAP), Turcot’s syndrome, and Gardner’s syndrome. Additionally, those with FAP, Turcot’s syndrome, and Gardner’s syndrome are also at increased risk for desmoid tumors of the small bowel. Patients with neurofibromatosis type 1 are at higher risk of developing GISTs, which can be multiple [87].
Metastatic tumors of the duodenum outnumber primary tumors [8]. Common tumors metastatic to duodenum include melanoma, which is the most common, bronchogenic malignancy, and breast cancer [83]. Testicular and renal tumors can also metastasize to the duodenum, and direct invasion can occur from surrounding structures [8].
Features of duodenal polyps
To clarify the features of polyps in the duodenum, Matsui et al., looked at 263 patients with polyps in the duodenal bulb. 69.2% of the polyps revealed gastric tissue, 19.4% were cysts, and the remaining 11.4% were miscellaneous lesions such as tubular adenomas and BGH [88]. Reddy et al., looked at biopsies done in 38 patients with duodenal polyps found on duodenoscopy. In 19 patients, they found normal duodenal mucosa or chronic inflammation. In the remaining patients, they found eight adenomatous polyps, six villous adenomas, and two BGH. Two lipomas and one carcinoid tumor were also found [82].
Many duodenal tumors, both primary and metastatic, can be pedunculated. Pedunculated small bowel tumors reported in the literature include ampullary adenocarcinoma, villous adenoma, pleomorphic sarcoma, angiosarcoma, metastatic osteosarcoma, and direct invasion of an ovarian mature cystic teratoma [89-94]. Shiba et al., reported a case of a pedunculated ampullary adenocarcinoma where the atypia was limited to the mucosa [89].
Approach to suspected small bowel tumors
While adhesions are the most common cause of partial or complete small bowel obstruction, tumors are also a leading cause. Symptoms are similar, with nausea, vomiting, and abdominal pain, and were seen in our study as well [95]. An algorithm approach to diagnosis and management is shown in figure 4.
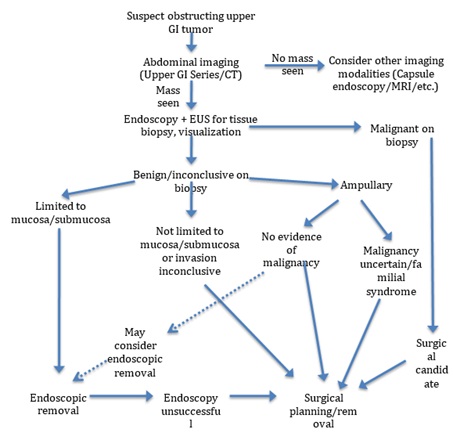 Figure 4: Algorithm for diagnosis and management of suspected obstructing duodenal mass.
Figure 4: Algorithm for diagnosis and management of suspected obstructing duodenal mass.
When a small bowel tumor is suspected, imaging should be performed first. An upper GI series may show a filling defect and will characterize whether the lesion is completely obstructing with high sensitivity. These studies will not, however, help in characterizing the etiology and as a result, many surgeons recommend using Computerized Tomography (CT) scan as the initial test of choice [96].
CT may be helpful in diagnosing duodenal tumors, incuding BGH. Hur et al., correlated the CT images with histopathologic diagnosis of BGH in 9 patients and found that the masses generally display internal cystic change, are located in the submucosa, and are hypoattenuated. 67% of the masses also displayed peripheral rimlike enhancement in the early phase, and most enhanced homogenously in the later phase [97]. Their study looked at smaller tumors with a mean size of 1.9cm, so larger tumors or those that intussuscept may be less suggestive of BGH on CT.
If a mass is not detected on UGI or CT but a tumor is still suspected, other imaging modalities such as capsule endoscopy or MRI can be utilized.
If CT scan does show a mass, an endoscopy with or without Endoscopic Ultrasound (EUS) is often helpful in visualization of the tumor. Although diagnosis at endoscopy is difficult, EUS may be helpful as it can provide information about the origin of the tumor as well as vascularity and echogenicity [98-102]. Endoscopy can also be used as a treatment modality [100]. Endosonographic features of BGH, characterized by Hizawa et al., were found to be heterogenous solid and/or cystic masses within the submucosa. They concluded that the tumor could be safely removed by endoscopic resection after confirmation that the tumor origin is within the submucosa [101].
Treatment
If the tumor is benign or inconclusive on biopsy, gross features of the tumor can aid in management. If the tumor is limited to the mucosa or submucosa, endoscopic snare removal can be attempted. Abbass et al., managed 59 patients with duodenal polyps endoscopically with a 98% removal success rate and an average polyp size was 1.72cm. They found, however, that 37% of polyps recurred within a mean follow-up time of 26 months and that recurrence was more common in polyps greater than 2cm [102]. If endoscopic removal fails, surgical removal should be considered.
The management of benign ampullary tumors is more controversial. Historically, management was surgical but recently, endoscopic management has become an additional treatment modality in those with no evidence of invasive cancer. Often, multiple endoscopic attempts were necessary and success varied widely. It has been recommended that endoscopic removal be performed by an experienced endoscopist and patients should undergo post-procedure surveillance [103,104].
For lesions that are invasive beyond the submucosa, or for which invasion cannot be determined, surgical management should be considered. When biopsy of the mass shows malignancy, the patient should be evaluated for surgical and/or chemotherapeutic candidacy as indicated for the type of tumor found.
Conclusion
Brunner’s Gland Hamartoma is a rare, benign lesion of the duodenum and presents with a wide variety of symptoms. BGH may have characteristic findings on CT and EUS. It can be removed endoscopically or surgically with excellent outcomes. In the workup of suspected BGH, a systematic approach should be taken given the potential for malignancy among the wide differential diagnosis.
Acknowledgement
The Division of Hepatobiliary and Pancreatic Surgery, Keck Medical Center of the University of Southern California.
Sources of Funding for Research/Publication
Division of Hepatobiliary and Pancreatic Surgery, LAC-USC and Keck Hospital of USC, Los Angeles, CA
References
- Leeson TS, Leeson CR (1968) The fine structure of Brunner's glands in man. J Anat 103: 263-276.
- Dandalides SM, Carey WD, Petras R, Achkar E (1989) Endoscopic small bowel mucosal biopsy: A controlled trial evaluating forceps size and biopsy location in the diagnosis of normal and abnormal mucosal architecture. Gastrointest Endosc 35: 197-200.
- Chattopadhyay P, Kundu AK, Bhattacharyya S, Bandyopadhyay A (2008) Diffuse nodular hyperplasia of Brunner's gland presenting as upper gastrointestinal haemorrhage. Singapore Med J 49: 81-83.
- Levine JA, Burgart LJ, Batts KP, Wang KK (1995) Brunner's gland hamartomas: Clinical presentation and pathological features of 27 cases. Am J Gastroenterol 90: 290-294.
- Brookes MJ, Manjunatha S, Allen CA, Cox M (2003) Malignant potential in a Brunner's gland hamartoma. Postgrad Med J 79: 416-417.
- Schottenfeld D, Beebe-Dimmer JL, Vigneau FD (2009) The epidemiology and pathogenesis of neoplasia in the small intestine. Ann Epidemiol 19: 58-69.
- Shandalow SL (1955) Benign tumors of the small intestine. AMA Arch Surg 71: 761-767.
- Myhre J (1963) Diagnosis of small-bowel tumors. Am J Dig Dis 8: 916-922.
- Paski SC, Semrad CE (2009) Small bowel tumors. Gastrointest Endosc Clin N Am 19: 461-479.
- Adeonigbagbe O, Lee C, Karowe M, Feeney M, Wallack M, et al. (1999) A Brunner's gland adenoma as a cause of anemia. J Clin Gastroenterol 29: 193-196.
- Alper EI, Haubrich WS (1973) Duodenoscopic removal of a Brunner's gland adenoma. Gastrointest Endosc 20: 73.
- Babich JP, Klein J, Friedel DM (2010) Endoscopic removal of a brunneroma with EUS guidance. South Med J 103: 250-252.
- Barnhart GR, Maull KI (1979) Brunner's gland adenomas: Clinical presentation and surgical management. South Med J 72: 1537-1539.
- Bastounis E, Pikoulis E, Leppäniemi A, Tsetis D, Tsetis A (1999) Polypoid hamartoma of Brunner's gland of the duodenum. Dig Surg 16: 431-433.
- Bayan K, Tüzün Y, Yilmaz S, Yilmaz G, Bilici A (2009) Pyloric giant Brunner's gland hamartoma as a cause of both duodenojejunal intussusception and obscure gastrointestinal bleeding. Turk J Gastroenterol 20: 52-56.
- Becker SA, Ziv-Sokolovskya N (2004) Brunner's gland hamartoma of the duodenum (Brunneroma). Isr Med Assoc J 6: 702-703.
- Block KP, Frick TJ, Warner TF (2000) Gastrointestinal bleeding from a Brunner's gland hamartoma: Characterization by endoscopy, computed tomography, and endoscopic ultrasound. Am J Gastroenterol 95: 1581-1583.
- Changchien CS, Hsu CC, Hu TH (2001) Endosonographic appearances of Brunner's gland hamartomas. J Clin Ultrasound 29: 243-246.
- Chattopadhyay P, Kundu AK, Bhattacharyya S, Bandyopadhyay A (2008) Diffuse nodular hyperplasia of Brunner's gland presenting as upper gastrointestinal haemorrhage. Singapore Med J 49: 81-83.
- Chen Y, Su W, Soon M, Yen H (2005) Hemoclip-assisted polypectomy of large duodenal Brunner's gland hamartoma. Dig Dis Sci 51: 1670-1672.
- Chuang JH, Chen WJ (1991) Duodenojejunal intussusception secondary to hamartomatous polyp of Brunner's glands. J Pediatr Gastroenterol Nutr 13: 96-100.
- Coppola D, Karl RC (1997) Brunner's gland hamartoma: Is it just a morphologic curiosity?: This regular feature presents special issues in oncologic pathology. Cancer Control 4: 359-363.
- Dandalides SM, Carey WD, Petras R, Achkar E (1989) Endoscopic small bowel mucosal biopsy: A controlled trial evaluating forceps size and biopsy location in the diagnosis of normal and abnormal mucosal architecture. Gastrointest Endosc 35: 197-200.
- De Castella H (1966) Brunner's gland adenoma. An unusual cause of intestinal bleeding. Br J Surg 53: 153-156.
- de Nes LCF, Ouwehand F, Peters SHA, Boom MJ (2007) A large Brunner's gland hamartoma causing gastrointestinal bleeding and obstruction. Dig Surg 24: 450-452.
- Deren MD, Henry PD (1956) Adenoma of Brunner's glands. Ann Intern Med 44: 180-187.
- Earlam RJ, Cowan WK (1996) Adenoma of Brunner's glands. Br J Surg 53: 736-738.
- East JE, Dickinson RJ (2003) Giant Brunner's gland hamartoma. Gastrointest Endosc 57: 384.
- Fadare O (2002) Images in pathology: Brunner's gland hamartoma. Int J Surg Pathol 10: 294.
- Farkas I, Patko A, Kovacs L, Koller O, Preisich P (1980) The brunneroma, the adenomatous hyperplasia of the Brunner's glands. Acta Gastroenterol Belg 43: 179-186.
- Fujimaki E, Nakamura S, Sugai T, Takeda Y (2000) Brunner's gland adenoma with a focus of p53-positive atypical glands. J Gastroenterol 35: 155-158.
- Gao Y, Zhu J, Zheng W (2004) Brunner's gland adenoma of duodenum: A case report and literature review. World J Gastroenterol 10: 2616-2617.
- George B, Srikureja W, Shah A, Chacko A (2008) Endonography findings in brunneroma. Indian J Gastroenterol 27: 45.
- Gourtsoyiannis NC, Zarifi M, Gallis P, Mouchtouris A, Livaditou A (1990) Radiologic appearances of Brunner's gland adenoma: A case report. Eur J Radiol 11: 188-190.
- Hirasaki S, Kubo M, Inoue A, Miyake Y, Oshiro H (2009) Pedunculated Brunner's gland hamartoma of the duodenum causing upper gastrointestinal hemorrhage. World J Gastroenterol 15: 373-375.
- Hol JW, Stuifbergen WNHM, Teepen JLJM, van Laarhoven CJHM (2007) Giant Brunner's hamartomas of the duodenum and obstructive jaundice. An overview of the literature and suspicion of malignancy in a case. Dig Surg 24: 452-455.
- Huang CH, Perng CL, Yang YH, et al. (1999) Endoscopic removal of a huge duodenal Brunner's gland adenoma: A new technique. Gastrointest Endosc 50: 868-869.
- Hur S, Han JK, Kim M, Bae J, Choi BI (2010) Brunner's gland hamartoma: Computed tomographic findings with histopathologic correlation in 9 cases. J Comput Assist Tomogr 34: 543-547.
- Hyatt P, Resnick M, Habr F, Baffy G (2006) Brunner's gland hamartoma. Clin Gastroenterol Hepatol 4: 26.
- Iusco D, Roncoroni L, Violi V, Donadei E, Sarli L (2005) Brunner's gland hamartoma: 'Over-treatment' of a voluminous mass simulating a malignancy of the pancreatic-duodenal area. JOP 6: 348-353.
- Jain KL, Tayeb AA (1968) Adenoma of Brunner's gland--A report of two cases. Br J Surg 55: 119-120.
- Jansen JM, Stuifbergen WNHM, van Milligen de Wit AWM (2002) Endoscopic resection of a large Brunner's gland adenoma. Neth J Med 60: 253-255.
- Jeong YS, Chung JP, Lee DY, Ji SW, Lee SJ, et al. (2004) Pyloric Brunner's gland hamartoma. Gastrointest. Endosc 59: 76.
- Kaufman DJ, Al Kharrat H, Weiss S, Robert M, Topazian M (2003) EUS-guided endoscopic removal of a large Brunner's gland hamartoma. Gastrointest Endosc 58: 313-314.
- Kim YS, Jung IS, Cheon GJ, Cho JY, Lee JS, et al. (2007) Endoscopic removal of giant Brunneroma presenting as a large pedunculated polyp. Endoscopy 1: 72.
- Laarman GJ, van der Wall EE, Muller JW, Eggink HD, Hoekstra JB (1988) Extreme adenomatous hyperplasia of Brunner's glands in the proximal jejunum. Neth J Med 32: 20-26.
- Lin J, Hsieh T, Chan D (2009) Brunner's gland hamartoma. Gastroenterology 137: 789.
- Lingawi SS, Filipenko JD (1998) Brunner's gland hamartoma causing gastric outlet obstructive symptoms. South Med J 91: 964-965.
- Maglinte DD, Mayes SL, Ng AC, Pickett RD (1982) Brunner's gland adenoma: Diagnostic considerations. J Clin Gastroenterol 4: 127-131.
- Moffat F, Anderson W (1966) Adenoma of Brunner's gland. Br J Surg 43: 106-107.
- Mukherjee S, Mainzer TA, Murthy UK (1999) Endoscopic injection and polypectomy for bleeding Brunner's gland hamartoma. Gastrointest Endosc 50: 597-598.
- Mumtaz R, Shah IA, Ramirez FC (2002) Brunner's gland hamartoma simulating a pancreatic mass with duodenal obstruction. Gastrointest Endosc 56: 932-934.
- Nagendran T, Imm F, Butler KL (1987) Brunneroma. A case report. Ala Med 57: 27-28.
- Nakanishi T, Takeuchi T, Hara K, Sugimoto A (1984) A great Brunner's gland adenoma of the duodenal bulb. Dig Dis Sci 29: 81-85.
- Nielsen OF, Whitaker EG, Roberts FM (1965) Adenoma of Brunner's glands. Am J Surg 110: 977-980.
- Park CH, Lee SJ, Park JH, Park JH, Lee WS, et al. (2004) A case of Brunner's gland hamartoma presenting as obscure gastrointestinal hemorrhage. Korean J Gastroenterol 43: 211-214.
- Park BJ, Kim MJ, Lee JH, Park SS, Sung DJ, et al. (2004) Cystic Brunner's gland hamartoma in the duodenum: A case report. World J Gastroenterol 15: 4980-4983.
- Ponka JL, Shaalan AK (1964) Massive Gastrointestinal hemorrhage secondary to tumors of brunner's glands. Am J Surg 108: 51-56.
- Pricolo VE, Lee KC, Van Zuiden PE, Vezeridis MP (1987) Brunner's gland adenoma. South Med J 80: 1572-1574.
- Rienhoff WF (1963) Adenoma of brunner's gland. South Med J 56: 1381-1383.
- Rieth DG, Abbott GF, Gray G (1977) Duodenal intussusception secondary to Brunner's gland hamartoma. A case report. Gastrointest Radiol 2: 13-16.
- Rocco A, Borriello P, Compare D, Colibus PD, Pica L, et al. (2006) Large Brunner's gland adenoma: Case report and literature review. World J Gastroenterol 12: 1966-1968.
- Rüfenacht H, Kasper M, Heitz PU, Streule K, Harder F (1986) "Brunneroma": Hamartoma or tumor? Pathol Res Pract 181: 107-111.
- Saida Y, Matsueda K, Itai Y (2002) Distal migration of duodenal tumors: Simple prolapse or intussusception? Abdom Imaging 27: 9-14.
- Shemesh E, Horin SB, Barshack I, Bar-Meir S (2000) Brunner's gland hamartoma presenting as a large duodenal polyp. Gastrointest Endosc 52: 435-436.
- Stermer E, Elias N, Keren D, Rainis T, Goldstein O, et al. (2006) Acute pancreatitis and upper gastrointestinal bleeding as presenting symptoms of duodenal Brunner's gland hamartoma. Can J Gastroenterol 20: 541-542.
- Stewart ZA, Hruban RH, Fishman EF, Wolfgang CL (2009) Surgical management of giant Brunner's gland hamartoma: Case report and literature review. World J Surg Oncol 7: 68.
- Stolpman DR, Hunt GC, Sheppard B, Huang H, Gopal DV (2002) Brunner's gland hamartoma: A rare cause of gastrointestinal bleeding-case report and review of the literature. Can J Gastroenterol 16: 309-313.
- Suda K, Hamada S, Takahashi M, Kumashiro Y, Yao T, et al. (2007) A true Brunner's gland adenoma. Endoscopy 39: 37-38.
- Tai M, Yoshikawa I, Kume K, Murata I, Otsuki M (2001) A large Brunner's gland hamartoma resected by endoscopic polypectomy. Gastrointest Endosc 53: 207-208.
- Tan Y, Wong W (2002) Giant Brunneroma as an unusual cause of upper gastrointestinal hemorrhage: Report of a case. Surg Today 32: 910-912.
- Tomikashi K, Fukuda M, Nakano K, Takami S, Imamura M, et al. (2006) Gastrointestinal obstruction caused by the spontaneously detached Brunner's gland hamartoma of the duodenum. J Gastroenterol Hepatol 21: 1502-1505.
- Urganci N, Arapoglu M, Akyildiz B, Calay Z, Nuhoglu A (2005) Brunner's gland adenoma: A rare cause of vomiting. Acta Paediatr 94: 631-633.
- Varma D, Prakash K, Augustine P, Mahadevan P, Ramesh H (2001) Brunner's gland adenoma with circumferential duodenal involvement. Indian J Gastroenterol 20: 243-244.
- Wagholikar GD, Dhingra S, Krishnani N, Kapoor VK (2002) Large Brunneroma presenting with bleeding. Indian J Gastroenterol 21: 201-202.
- Walden DT, Marcon NE (1998) Endoscopic injection and polypectomy for bleeding Brunner's gland hamartoma: Case report and expanded literature review. Gastrointest Endosc 47: 403-407.
- Weisselberg B, Melzer E, Liokumovich P, Kurnik D, Koller M, et al. (1997) The endoscopic ultrasonographic appearance of Brunner's gland hamartoma. Gastrointest Endosc 46: 176-178.
- Woharndee P, Sornmayura P, Bunyaratvej S (2005) Brunner's gland adenoma: A report of two cases. J Med Assoc Thai 88: 841-844.
- Wolk DP, Knapper WH, Farr GH (1973) Brunner's gland cystadenoma of the duodenum. Am J Surg 126: 439-440.
- Yadav D, Hertan H, Pitchumoni CS (2001) A giant Brunner's gland adenoma presenting as gastrointestinal hemorrhage. J Clin Gastroenterol 32: 448-450.
- Yamakawa M, Murata I, Yamao T, Kawai K, Kohno S (2003) Cystic Brunner's gland hamartoma. Gastrointest. Endosc 57: 919.
- Zangara J, Kushner H, Drachenberg C, B Daly, Flowers J, Fantry G, et al. Iron deficiency anemia due to a Brunner's gland hamartoma. J Clin Gastroenterol 27: 353-356.
- Reddy RR, Schuman BM, Priest RJ (1981) Duodenal polyps: Diagnosis and management. J Clin Gastroenterol 3: 139-147.
- Gill SS, Heuman DM, Mihas AA (2001) Small intestinal neoplasms. J Clin Gastroenterol 33: 267-282.
- Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, et al. (2009) Small bowel cancer in the United States: Changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg 249: 63-71.
- Miettinen M, Fetsch JF, Sobin LH, Lasota J (2006) Gastrointestinal stromal tumors in patients with neurofibromatosis 1: A clinicopathologic and molecular genetic study of 45 cases. Am J Surg Pathol 30: 90-96.
- Matsui K, Kitagawa M (1993) Biopsy study of polyps in the duodenal bulb. Am J Gastroenterol 88: 253-257.
- Shiba H, Misawa T, Wakiyama S, Iida T, Ishida Y, et al. (2010) Pedunculated early ampullary carcinoma treated by ampullectomy: Report of a case. J Gastrointest Cancer 41: 138-140.
- Greco S, Cassinotti A, Massari A, Bossi I, Trabucchi E, et al. (2008) Isolated ampullary adenoma causing biliary obstruction. J Gastrointestin Liver Dis 17: 329-332.
- Agaimy A, Gaumann A, Schroeder J, Dietmaier W, Hartmann A, et al. (2007) Primary and metastatic high-grade pleomorphic sarcoma/malignant fibrous histiocytoma of the gastrointestinal tract: An approach to the differential diagnosis in a series of five cases with emphasis on myofibroblastic differentiation. Virchows Arch 451: 949-957.
- Panizo-Santos A, Sola I, Lozano M, de Alava E, Pardo J (2000) Metastatic osteosarcoma presenting as a small-bowel polyp. A case report and review of the literature. Arch Pathol Lab Med 124: 1682-1684.
- Ohara Y, Oka K, Pak S, Sando N, Matsumoto T, et al. (2007) A case of ovarian mature cystic teratoma presenting as a pedunculated ileal tumor. Pathol Res Pract 203: 45-51.
- Sou S, Nomura H, Takaki Y, Nagahama T, Matsubara F, et al. (2006) Hemorrhagic duodenal lipoma managed by endoscopic resection. J Gastroenterol Hepatol 21: 479-481.
- Brolin RE, Krasna MJ, Mast BA (1987) Use of tubes and radiographs in the management of small bowel obstruction. Ann Surg 206: 126-133.
- Peck JJ, Milleson T, Phelan J (1999) The role of computed tomography with contrast and small bowel follow-through in management of small bowel obstruction. Am J Surg 177: 375-378.
- Hur S, Han JK, Kim M, Bae J, Choi BI (2010) Brunner's gland hamartoma: Computed tomographic findings with histopathologic correlation in 9 cases. J Comput Assist Tomogr 34: 543-547.
- Puli SR, Singh S, Hagedorn CH, Reddy J, Olyaee M (2007) Diagnostic accuracy of EUS for vascular invasion in pancreatic and periampullary cancers: A meta-analysis and systematic review. Gastrointest Endosc 65: 788-797.
- Boyce GA, Sivak MV (1990) Endoscopic ultrasonography in the diagnosis of pancreatic tumors. Gastrointest Endosc 36: 28-32.
- Norton ID, Jones DB (2003) Endoscopic ultrasound: Diagnostic and therapeutic applications. Intern Med J 33: 26-32.
- Puli S, Reddy J, Bechtold ML, Antillon D, Ibdah JA, et al. (2008) Staging accuracy of esophageal cancer by endoscopic ultrasound: A meta-analysis and systematic review. World J Gastroenterol 14: 1479-1490.
- Puli SR, Reddy JBK, Bechtold ML, Antillon MR, Ibdah JA (2008) How good is endoscopic ultrasound for TNM staging of gastric cancers? A meta-analysis and systematic review. World J Gastroenterol 14: 4011-4019.
- Hizawa K, Iwai K, Esaki M, Suekane H, Inuzuka S, et al. (2002) Endosonographic features of Brunner's gland hamartomas which were subsequently resected endoscopically. Endoscopy 34: 956-958.
- Abbass R, Rigaux J, Al-Kawas FH (2010) Nonampullary duodenal polyps: Characteristics and endoscopic management. Gastrointest Endosc 71: 754-759.
- Adler DG, Qureshi W, Davila R, Gan SI, Lichtenstein D, et al. (2006) The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc 64: 849-854.
Citation: Joseph MN, Laird DJ, Petrosyan M, Selby R, Kulkarni S (2021) Brunner’s Gland Hamartoma: A Case Report, Meta-Analysis, and Algorithm Approach to Obstructing Duodenal Masses. Archiv Surg S Educ 3: 031.
Copyright: © 2021 Melissa N Joseph, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

