
Buyang Huanwu Decoction blocks TGF-β/Smad Pathway by Inhibiting FUT8 Expression in IRI Mice
*Corresponding Author(s):
Lin HongliDepartment Of Nephrology, The First Affiliated Hospital, Dalian Medical University, Dalian, Liaoning, China
Email:Linhongli@vip.163.com
Wang Dapeng
Department Of Nephrology, The First Affiliated Hospital, Dalian Medical University, Dalian, Liaoning, China
Tel:+86 18098875653,
Email:bobowdp@163.com
Abstract
In order to elucidate the mechanism of Buyang-Huanwu decoction in TGF-β/Smad signaling pathway in AKI-to-CKD mice. We constructed animal model of AKI-to-CKD by renal ischemia-reperfusion injury (IRI). We grouped the mice, assessed renal function on the 30th day after modeling, tested the level of α-SMA and TGF-β/Smad pathways, and detected the changes of Fut8 and VEGF R2 levels and core fucosylation of TGF-βR II. The results show that Buyang-Huanwu decoction also down-regulates the level of Fut8 and core fucosylation of TGF-βRII, up-regulates the level of VEGF R2, inhibits the activation of TGF-β/Smad, and eases the process of IRI. These data indicate that Buyang-Huanwu decoction could inhibit the activation of TGF-β/Smad pathway by inhibiting the Fut8 and then block the pathological process of AKI-to-CKD.
Keywords
AKI-to-CKD; Buyang Huanwu decoction; Core fucosylation; Fut8; TGF-β/Smad; IRI
Renal Ischemia/Reperfusion Injury (IRI) refers to the injury that occurs when renal tissue becomes ischemic and then reverts to blood perfusion. IRI could cause Acute Kidney (AKI) injury and is also a risk factor for chronic kidney disease (CKD) and end-stage renal disease (ESRD) During IRI, the blood flow of Peritubular capillary network (PTC) decreases, which damages vascular endothelial cells, leading to the destruction of PTC and decrease of PTC density [1]. At the meantime, the decrease of PTC density would cause local hypoxia in renal tissue, and hypoxia could cause damage to renal tubular epithelial cells and activate surrounding fibroblasts, which results in obvious deposition of Extracellular Matrix (ECM) and proliferation of fibroblastsin in renal interstitium . AKI could also cause inflammatory cell infiltration, and ultimately lead to Renal Interstitial Fibrosis (RIF), which would destroy the normal structure of the kidney and impaire normal function of the kidney. At the same time, renal tubular epithelial cells are damaged and dysfunctional, which affects the secretion of Vascular Endothelial Growth Factor (VEGF) and the generation of PTC.All these aggravate tissue cell hypoxia, and promote the progression of CKD [2-4]. Grams, etc. [5] shows that AKI is closely related to the subsequent risk of ESKD when eGFR decline ≥30%. Therefore, it is of great significance to clarify the mechanism of the progression of AKI-to-CKD for the prevention and treatment of AKI-to-CKD.
AKI-to-CKD is characterized by myofibroblast proliferation. Pericytes are the main source of myofibroblasts in renal fibrosis, so inhibiting the conversion of pericytes to myofibroblasts is an anti-fibrosis method. Transforming Growth Factor (TGF) -β is an important pro-fibrotic factor. TGF -β initiates the repair of RIF by participating in the fibrotic formation process of endothelial cells and pericytes [6,7]. It could inhibit the aggregation and activation of pericytes by regulating the TGF-β/Smad signaling pathway [8]. Meanwhile, with the help of TGF-β, Smad3 participates in the regulation of α-SMA during differentiation of myofibroblast [9]. In conclusion, moderately reducing the expression of TGF-β/Smad pathway could slow down the process of renal fibrosis without affecting the normal renal function. Therefore, establishing further understanding of the mechanism of this signaling pathway would help to prevent and control the occurrence and development of AKI-to-CKD.
Glycosylation modification is an important post-translational modifications, which occurs in more than 50% of proteins [10]. Glycosylation modification has a significant effect on protein expression, and glycosylation modification mainly occurs in the extracellular environment [11]. Fucosyltransferases 8 (Fut8), also known as core fucosyltransferases, recognizes the core GlcNAc linked to asparagine in the chitobiose unit of N-glycan chain and catalyzes the formation of an α1, 6 glycosidic bond between the fucosyltransferases and the GlcNAc [12]. Fut8 regulates the structure of the N-glycan chain in conjunction with other glycosyltransferases, and is known to have a significant effect on protein expression due to its central position in the site of action, which plays an important role in physiological and pathological processes. Core fucosylation is catalyzed by Fut8 [13]. Core Fucosylation (CF) could affect fibrosis process by regulating TGF-βRII [14-16]. In addition, our previous experiments have found that regulating CF could reduce active expression of TGF-β/Smad pathway, renal fibrosis, and renal cell damage [17] in fibrosis cell model induced by UUO, peritoneal fibrosis and proteinuria. However, the effect of Fut8 in TGF-β/Smad pathway in IRI has not been studied, and there is a lack of effective therapeutic methods to interfere with Fut8.
Buyang Huanwu decoction is a classic prescription of traditional Chinese medicine, which is composed of Huangqi, Dilong, Honghua, etc. It was mainly used to treat cerebrovascular diseases in the past. And now, modern medical research has found that Buyang Huanwu decoction could protect capillaries [18,19]. Medical research shows that Buyang Huanwu decoction could reduce RIF , but the mechanism is unclear. Therefore, the purpose of this experiment is to explore whether Buyang Huanwu decoction could inhibit RIF and block AKI-to-CKD by regulating the expression of Fut8 and inhibiting the activation of TGF-β/Smad pathway.
Materials And Methods
Experimental animals and grouping
Healthy male C57BL/6 mice aged 8-12 weeks, weighing 22±28 g, were homed in standard experimental environment. The mice were randomly divided into 5 groups: control group, sham group, IRI group, IRI+L group and IRI+H group, with 5 mice in each group.
Drugs and reagents
Buyang Huanwu decoction: Huangqi 120g, Chishao 4.5g, Danggui 6g, Chuanxiong 3g,Taoren 3g, Honghua 3g, Dilong 3g. According to the body surface area conversion coefficient, the low and high dose concentrations of Buyang Huanwu decoction were 18.525g/kg and 37.05g/kg. Keep all decoction in the refrigerator at 4 ° C for later use. After modeling, keep the serum and kidney on the 30th day.
I/R AKI-to-CKD model
The IRI mice underwent bilateral IRI surgery, and the sham mice underwent the same procedure except that the renal pedicles were not clamped with atraumatic aneurysm clips.
Results
- Changes of renal function (Table 1)
|
Group |
CRE(μmol/L) |
BUN(mmol/L) |
|
con |
10.8±0.447 |
11.252±2.897 |
|
sham |
10±0.707 |
6.614±0.959 |
|
IRI |
33.5±4.950 |
29.435±0.955 |
|
IRI+L |
16.5±4.359 |
17.433±2.737 |
|
IRI+H |
15.667±3.215 |
11.57±1.410 |
Table 1: Test results of CRE and BUN contents of mice in each group (X ±S).
Compared with control group, the content of CRE in the serum of IRI group is increased(p < 0.01). Compared with IRI group, the serum levels of CRE in the IRI+L group and the IRI+H group decrease (p > 0.05, p < 0.05). The results are shown in figure 1 (A).
Compared with control group, the content of BUN in the serum of IRI group is increased (p < 0.0001). Compared with IRI group, the content of BUN in the IRI+L group and the IRI+H group decreased, and the difference was statistically significant (p < 0.001, p < 0.05). The results are shown in figure 1 (B).
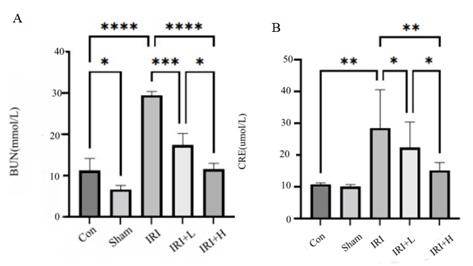 Figure 1: Changes of CRE and BUN of mice in each group.
Figure 1: Changes of CRE and BUN of mice in each group.
- Masson and PAS
There was no obvious blue-stained collagen fibers in control group. Compared with control group, the renal tubules in IRI group were dilated, and a large number of blue-stained collagen fibers appeared in the renal interstitium. Compared with IRI group, after Buyang Huanwu decoction intervention, the degree of renal tubular dilatation and RIF in the treatment group were reduced, and the results are shown in figure 2.
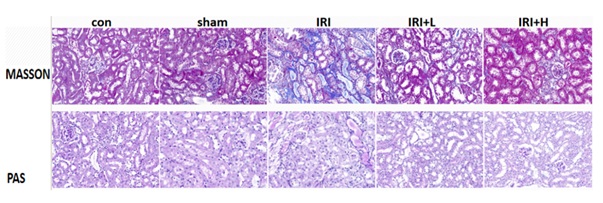 Figure 2: Masson and PAS of mice in each group.
Figure 2: Masson and PAS of mice in each group.
- Changes of α-SMA expression
Compared with control group, the expression of α-SMA in renal tissue of IRI group is increased (p < 0.0001). Compared with IRI group, the expression of α-SMA in renal tissue in IRI+L group decreases (p>0.05), but the difference was not statistically significant, and the expression of α-SMA in IRI+H group decreases (p < 0.05). The results are shown in figure 3.
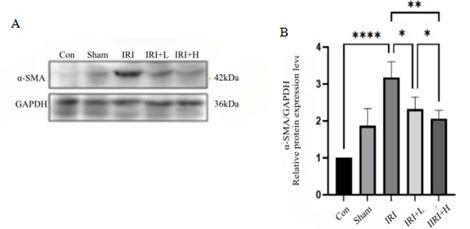 Figure 3: Changes in α-SMA expression of mice in each group.
Figure 3: Changes in α-SMA expression of mice in each group.
- Changes in the expression of α-SMA
The results show that the expression of α-SMA in IRI mice is significantly higher than that in control and sham groups. Compared with IRI group, the expression of α-SMA in IRI+L and IRI+H groups is significantly decreased, and the therapeutic effect of IRI+H group is more significant. The results are shown in figure 4.
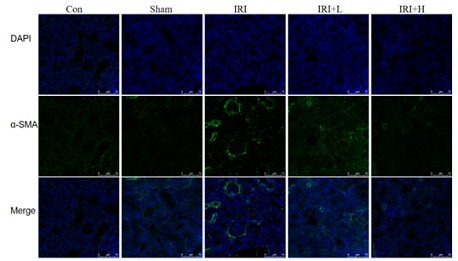 Figure 4: Changes in fluorescence staining of α-SMA of mice in each group.
Figure 4: Changes in fluorescence staining of α-SMA of mice in each group.
- Expression changes of Fut8
Compared with control group, the expression of Fut8 in the renal tissue of IRI group is increased (p < 0.0001). Compared with IRI group, the expression of Fut8 in renal tissue of IRI+L group and IRI+H group decreases (p < 0.05, p < 0.001), and the results are shown in figure 5.
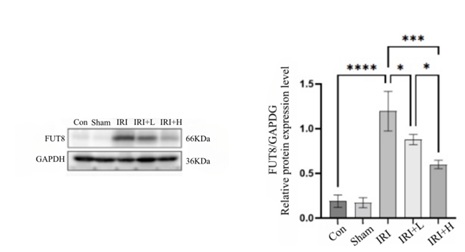 Figure 5: 11 Expression changes of Fut8 of mice in each group.
Figure 5: 11 Expression changes of Fut8 of mice in each group.
- Changes of fibrosis indexes and signaling pathways in western blotting
1CF changes in TGF/Smad2/3 pathway: TGFβR II plays an important role in renal fibrosis, and there is concordance between renal pathologic damage and dynamic changes in Fut8 and LCA . The level of LCA in IRI+L and IRI+H group decrease (p < 0. 05, p < 0. 05). The level of LCA keeps consistent with the expression of Fut8 and TGFβR II during CKD. The results are shown in figure 6.
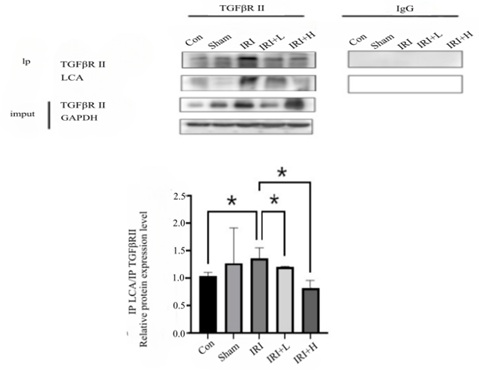 Figure 6: Changes in TGFβR II and LCA expression of mice in each group.
Figure 6: Changes in TGFβR II and LCA expression of mice in each group.
TGF/Smad2/3 pathway: Compared with control group, the expressions of TGF-βR II and Smad2/3 in the renal tissue of IRI group are increased (p < 0. 001, p < 0. 01). Compared with IRI group, the expression of TGF-βR II in IRI+L group is decreased (p < 0. 05), and the expression of TGF-βR II in IRI+H group is decreased (p < 0. 05). Compared with IRI group, the expression of p/Smad2/3/Smad2/3 in IRI+L group decreases (p < 0. 05), and the expression of p/Smad2/3/Smad2/3 in IRI+H group decreases (p < 0. 001), the differences are statistically significant, and the results are shown in figure 7.
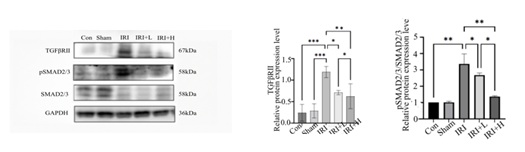 Figure 7: Changes in TGF/Smad2/3 pathway expression of mice in each group.
Figure 7: Changes in TGF/Smad2/3 pathway expression of mice in each group.
Immunofluorescence staining changes of VEGF R2: Compared with control group, the level of VEGF R2 of IRI group is increased (p < 0. 0001). Compared with IRI group, the level of VEGF R2 IRI+L group and IRI+H group decreases (p < 0. 001, p < 0. 05), and the results are shown in figure 8.
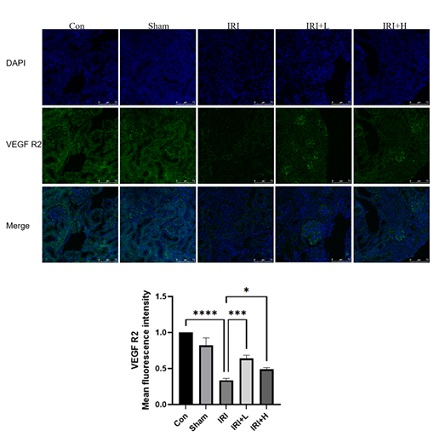 Figure 8: Changes in VEGF R2 level of mice in each group.
Figure 8: Changes in VEGF R2 level of mice in each group.
Discussion
IRI is an important factor in the occurrence and development of AKI, and there is a close relationship between AKI and CKD. In the process of IRI, there are PTC damage, tissue ischemia and hypoxia and inflammatory cell infiltration,which leads to cell damage, fibroblast activation, extracellular matrix deposition,etc.All these result in RIF, which further increases the risk of CKD and ESRD. Previous studies show that core fucosylation of key proteins plays an important role in the process of chronic renal interstitial fibrosis [20], but there is no satisfying data on the changes of core fucosylation modification profile during CKD.
We established IRI mouse model to study the changes from AKI to CKD. We observe the disease progression and curative effect, and measure the indicators on the 30th day after modeling. The results show that IRI induced obvious interstitial fibrosis and renal function damage on the 30th day. The expression level of FUT8 and LCA (a lectin that binds specifically to the N-glycan core fucose structure) showes a consistent trend, and the core fucosylation modification is significantly increased in IRI group. These data suggest that changes in the expression level of core fucosylation modification in the CKD stage in IRI models may closely relate to the occurrence and development of renal interstitial fibrosis. Our research group has previously found that core fucosylation is involved in the paracrine regulation of renal interstitial fibrosis induced by proteinuria [21], which is consistent with the results of this experiment.
Renal interstitial fibrosis is an important cause of chronic kidney disease. We investigated the TGF-β/Smad pathway in IRI mice. TGF-β/Smad is a signaling pathway that induces renal fibrosis. TGF-β1 induces RIF by activating the classical signaling pathway based on Smad. This process involves activation of fibroblasts and massive deposition of ECM [22]. Compared with control group, the expression of TGF-βR II and Smad2/3 IRI group is increased on the 30th day (p < 0.001, p < 0.01). Compared with IRI group, intervene groups significantly reduce the expression of TGF-β/Smad signaling pathway in a dose-dependent manner, suggesting that Buyanghuanwu decoction could inhibit the excessive activation of TGF-β/Smad pathway and then slow down the process of renal fibrosis, thereby blocking the pathological process of AKI-to-CKD. Wu et al. found that Buyang Huanwu decoction could inhibit TGF-β1/Smad3, NF-κB pathway and Arkadia and restore renal Smad7, thus reducing STZ-induced diabetic kidney fibrosis and inflammation [23], which is consistent with our results.
IRI could cause damage or loss of PTC, which makes the renal interstitium into a state of chronic hypoxia, leading to renal fibrosis and promoting the progression of AKI-to-CKD. We observed the effect of Buyang Huanwu decoction on CKD. First, the expression of α-SMA in IRI group is significouldtly increased in Western blot and immunofluorescence,which indicates fibrosis-like changes appear. The level of α-SMA in intervene group is significouldtly reduced in a dose-dependent manner. It is suggested that Buyang Huanwu decoction could improve the pathological condition of renal interstitial fibrosis. Han Miaoru's research results showes that Buyang Huanwu decoction could reduce Scr, α-SMA and the degree of renal interstitial collagen fiber accumulation in UUO rats, which is consistent with our research results [24]. Zhang Xuanming et al. 's research showes that Buyang Huanwu decoction could improve renal function and renal tubulointerstitial fibrosis in rats with unilateral ureteral ligation which could reduce the renal interstitial damage index and the expression of α-SMA, and reduce the severity of renal interstitial fibrosis [25].
VEGF plays a significant role in many physiological and pathophysiological processes. VEGF could affect angiogenesis by regulating endothelial cell proliferation and repair damaged capillary networks. VEGF sustainably expresses in endothelia of glomeruli and in peritubular capillaries in human and mouse kidney, which is essential for maintaining the function of endothelia [26]. VEGF plays a role in angiogenesis mainly with the help of VEGF R2. Therefore, we select VEGF R2 as an indicator to observe PTC function. The results show that IRI could reduce the level of VEGF R2 and impair PTC function in mice. After the treatment of Buyang-Huanwu decoction, the level of VEGF R2 increases, which means the capillary density also increases. Dimke et al. find that VEGFA and its receptors express along the Nephron and in Renal Microvasculature segmentally, and the specific deletion of VEGFA from Tubular Epithelia could impair development of peritubular capillary networks [27], which is consistent with our findings.
To further clarify the role of CF, we measure the expression level of total Fut8 in renal tissue by Western blot. The results showe that the expression level of total Fut8 in renal tissue is significantly increased in IRI group, while the expression level of Fut8 in renal tissue is significantly decreased in intervene group. Further CO-IP results show that, Buyang Huanwu decoction could significantly reduce the expression level of CF in TGF-β II, thereby inhibiting the activation of TGF-β/Smad pathway. Previous studies have found that TGF-β is modified by CF catalyzed by Fut8, and CF has a role in regulating the activation of signaling pathway [21], which is consistent with our results. Buyanghuanwu Decoction could inhibit the expression of Fut8 and reduce the fucose modification level of TGF-βRII, thereby inhibiting the excessive activation of downstream pathways and blocking the process of AKI-to-CKD.
In our study, interventions targeting relevant mechanisms have demonstrated the potential to ease fibrosis in CKD, highlighting the significance of further exploring effective repair mechanisms and identifying novel targets to prevent AKI-to-CKD. This study also has some limitations. This study only focus on the TGF-β/Smad signaling pathway and dose not examine other pathways that may be involved in the process of AKI-to-CKD. In the future, we would further improve the above.
Conclusion
Buyanghuanwu Decoction could inhibit Fut8 and core fucosylation of TGF-βR II, block the activation of TGF-β/Smad pathway, slow down the process of IRI injury, and block the pathological process of AKI-to-CKD
in IRI mice.
References
- Yongji Z (2020) Relationship between peritubular capillary and acute kidney injury. Chinese Journal of Nephrology 36: 73-76.
- Tanaka S, Tanaka T, Nangaku M (2014) Hypoxia as a key player in the AKI-to-CKD transition. Am J Physiol Renal Physiol 307: 1187-1195.
- Yang Q, Xie HL (2016) Mechanisms of progression of acute kidney injury to chronic kidney disease. Chinese Journal of Nephrology, Dialysis & Transplantation 25: 274-278.
- Zhong X, Tang T-T, Shen A-R, Cao J-Y, Jing J, et al. (2022) Tubular epithelial cells-derived small extracellular vesicle-VEGF-A promotes peritubular capillary repair in ischemic kidney injury. npj 7: 73.
- Macedo E, Lima C (2019) Comprehensive Assessment of Kidney Health in Acute Kidney Injury: Can It Be Achieved? Nephron 143: 188-192.
- Gwon MG, An HJ, Kim JY, Kim WH, Gu H, et al. (2020) Anti-fibrotic effects of synthetic TGF-β1 and Smad oligodeoxynucleotide on kidney fibrosis in vivo and in vitro through inhibition of both epithelial dedifferentiation and endothelial-mesenchymal transitions. FASEB J 34: 333-349.
- Wang M, Xu H, Li Y, Cao C, Zhu H, et al. (2020) Exogenous bone marrow derived-putative endothelial progenitor cells attenuate ischemia reperfusion-induced vascular injury and renal fibrosis in mice dependent on pericytes. Theranostics 10: 12144-12157.
- Liu YL, Shi G, Cao DW, Wan YG, Wu W, et al. (2018) Pathomechanisms of pericyte-myofibroblast transition in kidney and interventional effects of Chinese herbal medicine. Zhongguo Zhong Yao Za Zhi 43: 4192-4197.
- Hu B, Wu Z, Phan SH (2003) Smad3 mediates transforming growth factor-beta-induced alpha-smooth muscle actin expression. Am J Respir Cell Mol Biol 29: 397-404.
- Uozumi N, Teshima T, Yamamoto T, Nishikawa A, Gao YE, et al. (1996) A fluorescent assay method for GDP-L-Fuc:N-acetyl-beta-D-glucosaminide alpha 1-6fucosyltransferase activity, involving high performance liquid chromatography. J Biochem 120: 385-392.
- Lichtenstein RG, Rabinovich GA (2013) Glycobiology of cell death: when glycans and lectins govern cell fate. Cell Death Differ 20: 976-986.
- Kurimoto A, Kitazume S, Kizuka Y, Nakajima K, Oka R, et al. (2014) The absence of core fucose up-regulates GnT-III and Wnt target genes: a possible mechanism for an adaptive response in terms of glycan function. J Biol Chem 289: 11704-11714.
- Zhao YY, Takahashi M, Gu JG, Miyoshi E, Matsumoto A, et al. (2008) Functional roles of N-glycans in cell signaling and cell adhesion in cancer. Cancer Sci 99: 1304-1310.
- Wang X, Gu J, Miyoshi E, Honke K, Taniguchi N (2006) Phenotype changes of Fut8 knockout mouse: core fucosylation is crucial for the function of growth factor receptor. Methods Enzymol 417: 11-22.
- Whitman M (1998) Smads and early developmental signaling by the TGFbeta superfamily. Genes Dev 12: 2445-2462.
- Li L, Shen N, Wang N, Wang W, Tang Q, et al. (2018) Inhibiting core fucosylation attenuates glucose-induced peritoneal fibrosis in rats. Kidney Int 93: 1384-1396.
- Shen N, Lin H, Wu T, Wang D, Wang W, et al. (2013) Inhibition of TGF-beta1-receptor posttranslational core fucosylation attenuates rat renal interstitial fibrosis. Kidney Int 84: 64-77.
- Duan JY (1989) Anti-inflammatory and immunopharmacological effects of Buyang Huanwu decoction. Chinese Journal of Integrated Traditional and Western Medicine 1989: 164-166.
- Dong XG (2008) 70 cases of diabetic nephropathy in the early stages of Modified Buyang Huanwu decoction. Journal of Practical Traditional Chinese Internal Medicine 22: 38-39.
- Shen N, Lin H, Wu T, Wang D, Wang W, et al. (2013) Inhibition of TGF-β1-receptor posttranslational core fucosylation attenuates rat renal interstitial fibrosis. Kidney Int 84: 64-77.
- Liu A, Wang X, Hu X, Deng Y, Wen X, et al. (2022) Core fucosylation involvement in the paracrine regulation of proteinuria-induced renal interstitial fibrosis evaluated with the use of a microfluidic chip. Acta Biomater 142: 99-112.
- Meng XM, Nikolic-Paterson DJ, Lan HY (2016) TGF-β: the master regulator of fibrosis. Nat Rev Nephrol 12: 325-338.
- Wu W, Wang Y, Li H, Chen H, Shen J (2021) Buyang Huanwu Decoction protects against STZ-induced diabetic nephropathy by inhibiting TGF-β/Smad3 signaling-mediated renal fibrosis and inflammation. Chin Med 16: 118.
- Han MR (2022) Exploring the mechanism of Buyang Huanwu decoction against renal interstitial fibrosis by "Bu-Qi-Huo-Xue". Tianjin University of Traditional Chinese Medicine, China.
- Xuan-ming Z (2011) The Effect of Buyang Huanwu Decoction on α-SMA in Renal Tubulointerstitial Fibrosis Rats. China Journal of Experimental Traditional Medical Formulae 17: 136-140.
- Simon M, Gröne HJ, Jöhren O, Kullmer J, Plate KH, et al. (1995) Expression of vascular endothelial growth factor and its receptors in human renal ontogenesis and in adult kidney. Am J Physiol 268: 240-250.
- Dimke H, Sparks MA, Thomson BR, Frische S, Coffman TM, et al. (2015) Tubulovascular cross-talk by vascular endothelial growth factor a maintains peritubular microvasculature in kidney. J Am Soc Nephrol 26: 1027-1038.
Citation: Qingsi W, Qing G, Jianguo X, Yue Z, Yan S, et al. (2024) Buyang Huanwu Decoction blocks TGF-β/Smad Pathway by Inhibiting FUT8 Expression in IRI Mice. J Altern Complement Integr Med 10: 467.
Copyright: © 2024 Wen Qingsi, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

