
“Carney Complex” an often Missed Diagnosis - Case of Cerebrovascular Accident in a Young Patient with Atrial Myxoma and Myxoid Neurofibromas
*Corresponding Author(s):
Nkechi MbaebieDepartment Of Internal Medicine, Berkshire Medical Center, Pittsfield, Massachusetts, United States
Tel:+1 5045050690,
Fax:+91 1772831948
Email:nmbaebie@bhs1.org
Abstract
We present a case of nearly missed diagnosis of a rare disease entity in a 38 year old female with a past medical history of myxoid neurofibroma who presents with sudden onset of left hemiparesis, dysarthria and somnolence.
Physical examination revealed numerous ephelides and nevi mostly in the face, forearm and back. She was incidentally found to have left atrial myxoma as a source of her cardio-embolic stroke.
The constellation of her symptoms, physical examination and imaging findings was reviewed thus meeting the diagnosis of carney complex of the NAME subtype.
Keywords
BACKGROUND
Carney Complex (CNC) and its subsets NAME syndrome and LAMB syndrome are very rare multiple neoplastic syndromes, mostly inherited in an autosomal-dominant manner and associated with distinctive cutaneous lesions, myxomas both cardiac and cutaneous and endocrine over activity. It is due to an inactivating mutation in the protein kinase A type-1-alpha regulatory subunit (PRKAR1A) gene [1].
The NAME acronym stands for nevi, atrial myxoma, myxoidneurofibromas and ephelides while LAMB acronym stands for lentigenes, atrial myxomas and blue nevi and both are subsets of the carney complex [2]. CNC is a very rare disease with an unknown prevalence. 63% of patients with this disease entity are females while 37% are males [3]. Approximately 7% of all cardiac myxomas are associated with carney complex. About 750 cases have been described across different ethnic groups [1]. 70% of CNC cases had an affected parents and the remaining had no known affected relatives [4].
Diagnosis of CNC is made with the presence of two or more major clinical criteria and in patients in whom a pathogenetic variant is identified in PRKAR1A. The diagnostic criteria are illustrated in table 1. We present a case of a 45 year old female with history of myxoid neurofibromatosis who presented with multiple acute cerebral infarcts, found to have atrial myxoma with fair functional recovery post resection of the cardiac tumor.
|
Major diagnostic Criteria |
|
Spotty skin pigmentation with typical distribution (lips, conjunctiva and inner or outer canthi, vaginal and penile mucosa) |
|
Myxoma(cutaneous and mucosal) |
|
Cardiac myxoma |
|
Breast myxomatosis or fat-suppressed MRI findings suggestive of this diagnosis |
|
Acromegaly due to growth hormone-producing adenoma |
|
PPNAD or paradoxical positive response of urinary glucocorticoid excretion to dexamethasone administration during Liddle's test |
|
Thyroid carcinoma or multiple, hypoechoic nodules on thyroid ultrasound in a young patient |
|
LCCSCT or characteristic calcification on testicular ultrasound |
|
Blue nevus, epithelioid blue nevus |
|
Psammomatousmelanotic schwannomas |
|
Osteochondromyxoma |
|
Breast ductal adenoma |
|
Supplemental Criteria |
|
Affected first-degree relative |
|
Inactivating mutation of the PRKAR1A gene |
Table 1: CNC diagnostic criteria.
CASE PRESENTATION
38 year old female with history of myxoid neurofibromas confirmed histologically about twelve years ago presented with sudden onset of left sided weakness and slurred speech an hour prior to presentation and denies any other symptoms.
Physical examination revealed numerous spotty skin pigmentations in form of nevi, ephelides and innumerable neurofibromatosis distributed on the face, both forearms and back (Figure 1).
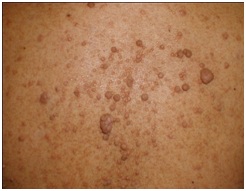
Figure 1: Innumerable myxoid neurofibromatosis distributed on the forearms and back.
Neurology exam revealed somnolence but easily arousable, able to follow two step commands, memory appeared intact; speech was dysarthric, left homonymous hemianopia, left facial droop and weakness. Motor examination revealed left flaccid hemiplegia, power of 3/5 in left upper and lower extremity, trace reflexes on left upper extremity and 2+ reflexes on the right upper and lower extremity, sensory examination showed extinction to pin in the left arm and leg and coordination was intact, gait was unable to be tested. National Institute of Health Stroke Scale (NIHSS) score was 14.
INVESTIGATION
Laboratory work up to include complete blood count, electrolytes, renal function test, liver functions and hormonal assays were all normal. ECG showed normal sinus rhythm.
Head Computed Tomography (CT) showed subtle hyperdense vessel sign concerning for thrombus within the proximal division of the right Middle Cerebral Artery (MCA) in the sylvian fissure and small chronic appearing infarct of the right inferior cerebellum in the posterior inferior cerebellar artery territory. No acute intracranial hemorrhage or mass (Figure 2). Computed Tomography Angiography (CTA) of the head showed proximal embolic occlusion of the M1 segment of the right middle cerebral artery with extension into the right MCA bifurcation. Possible non-occlusive thrombus in the A1 segment of the right anterior cerebral artery and likely early changes of acute right middle cerebral artery territory infarct in the right insula and temporal stem. No acute intracranial hemorrhage or mass (Figure 3). MRI revealed right middle cerebral infarct, left occipital and parietal lobe infarct, left posterior inferior cerebellar artery infarct. She was diagnosed with embolic ischemic stroke.
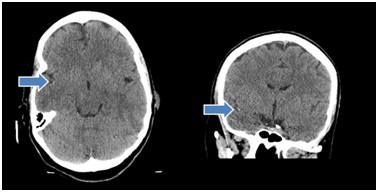
Figure 2: Hyperdensity in right Middle Cerebral Artery (MCA) concerning for thrombus.
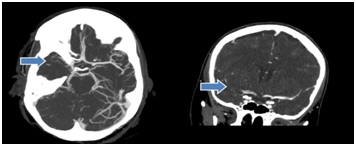
Figure 3: Axial MIP image from CT angiogram of brain demonstrating thrombus occluding the M1 segment and bifurcation of right MCA. Coronal MIP image from CT angiogram of brain demonstrating thrombus occluding the M1 segment and bifurcation of right MCA.
Echocardiogram revealed normal systolic function; ejection fraction was estimated to be 60%. There were no regional wall motion abnormalities. A Left Atrial (LA) mass measuring 50 mm x 26 mm on the left side of interatrial septum was seen, mass most likely represents a myxoma, is attached to the LA septum and appears to “bend over on itself” longitudinally. It is very mobile, seeing to remain inside the LA but close to prolapsing into the left ventricle across the mitral valve. It does not appear to obstruct the mitral valve at this time (Figures 4&5). Histolopathology of the left atrial mass showed atrial myxoma with the myxomas composed of oval, round, polygonal or stellate cells in loose myxoid matrix containing numerous mucopolysaccharides.
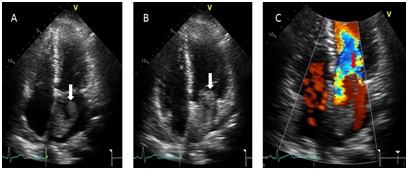
Figure 4: Apical four chamber images without and with color showing a large irregular echo density within the left atrium (white arrow image A {systole}) and seen prolapsing into the left ventricle through the mitral valve (white arrow image B {diastole}) with evidence of mitral in-flow obstruction during diastole (red arrow image C).
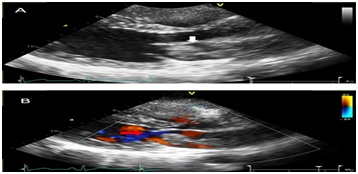
Figure 5: Parasternal long axis images without (A) and with color (B) showing a large irregular echo density within the left atrium (white arrow image A) with evidence mitral in-flow obstruction during diastole (red arrow image B).
DIFFERENTIAL DIAGNOSIS
Given the constellation of symptoms, physical examination of numerous ephelides, nevi and history of myxoid neurofibromas in the setting of incidental finding of atrial myxoma and stroke the following differentials were considered.
• Carney Complex- NAME versus LAMB subtypes
• Peutz-Jegher syndrome
• LEOPARD syndrome
• PTEN harmartoma tumor syndrome
• McCune-Albright syndrome
• Benign familial lentiginosis
• Acute cardioembolic stroke
• Acute thrombotic stroke
• Cerebral venous thrombosus
Patient with history of myxoid neurofibromatosis, found to have atrial myxoma, ephelides, nevi presenting with stroke meets 2 major criteria for diagnosis of CNC of NAME subtype table 1.
TREATMENT
Patient presented within the window for tissue Plasminogen Activator (tPA) administration, she received tPA after indications and contraindications as well as risk and benefits of this medication was reviewed with patient. She was then sent for thrombectomy of right MCA.
Post clot retrieval, she was admitted into the stroke unit for supportive management. Cardiothoracic team was consulted given atrial myxoma, they recommended starting heparin drip, however repeat CT of head 7 days post admission revealed a hemorrhagic conversion (Figure 6) and heparin was held. Repeat Head CT after another 3 days revealed that the hemorrhagic conversion had stabilized and heparin was restarted.
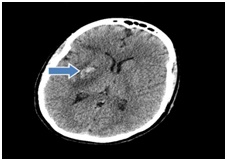
Figure 6: Focal hemorrhagic conversion of ischemic right MCA territory infarct within the lentiform nucleus.
The cardiothoracic team then recommended for 4 weeks of anticoagulation before an open heart surgery could be done. She eventually had the resection of the atrial myxoma and specimen was sent for histopathology which confirmed the mass to be atrial myxoma (Figure7).
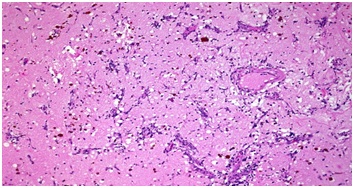
Figure 7: Histology showing atrial myxoma with the myxomas composed of oval, round, polygonal or stellate cells in loose myxoid matrix containing numerous mucopolysaccharides..
OUTCOME AND FOLLOW UP
In the immediate post-operative, she developed atrial fibrillation with rapid ventricular rate in the 140’s and was started on intravenous metoprolol 5 mg every 5 minutes with a total dose of 15 mg in 15 minutes with conversion to normal sinus rhythm after about 2 hours, she was then initially placed on metoprolol tartrate 50 mg twice daily and then transitioned to metoprolol succinate 100 mg daily. She was also anticoagulated with warfarin 5 mg daily and received warfarin for 3 months with close monitoring of International Normalization Ratio (INR) with goal of 2 to 3. She is currently on 81 mg aspirin daily.
Repeat echocardiography revealed normal systolic function with ejection fraction estimated in the range of 55% to 65%. There were no regional wall motion abnormalities, atrial myxoma resected and no remnant was noted. Patient and her immediate family was tested for the gene mutation and was found to be positive for the PRKAR1A gene mutation, her mother and sister was also positive for this gene mutation. She is continued on surveillance with six monthly echocardiogram which has since remained normal. Her neurological symptoms had completely resolved by the sixth month visit.
DISCUSSION
This complex was first described in 1985 by J Aidan Carney as the complex of myxomas, spotting skin pigmentations and endocrine over activity [5]. It was however renamed CNC by Bain and in 1994 as Carney syndrome by Mendelian inheritance in man [1].
The diagnosis of CNC should be suspected in patients presenting with characteristic cutaneous findings such as lentiginosis with periorificial distribution, lentigines located in the lid margins or lacrimal caruncle, multiple blue nevi, cutaneous myxomas, cardiac myxomas, myxoid neurofibromatosis and endocrine diseases e.g., Cushing’s syndrome, gigantism/acromegaly [6].
The most recent diagnosis of CNC is established when any of the following are present: two or more major criteria are present (Table 1) or pathogenic variant is identified in the protein kinase A-type 1-alpha regulatory subunit or one major criteria is present and a first degree relative has CNC or an inactivating mutation of PRKAR1A [6]. Important to note that genetic testing is not indicated for diagnosis but may be needed for potentially affected family members of patients with CNC. As follow up, our patient was found to be positive for this gene mutation as well as her mother and sister. Clinical surveillance is advised for at risk family members even when PRKAR1A pathogenic variant is not identified.
Our patient presented with a history of innumerable myxoid neurofibromatosis diagnosed twelve years ago by histopathology, multiple ephelides, nevi distributed in her face and incidental finding of atrial myxoma presenting with its complication of cardio-embolic stroke. She meets three major criteria needed for this diagnosis namely spotty skin pigmentation with typical distribution, cutaneous myxoid neurofibromatosis and cardiac myxoma.
Atrial myxoma occurs in about 7% of patient with CNC, although our patient has a cutaneous manifestation that could have led to the suspicion of this disease entity twelve years ago, it was rather not explored. It may have been possible that if further work-up were done at that time, atrial myxoma may have been identified and its subsequent complication of stroke averted.
Our patient presented with stroke in the young. Ischemic strokes in the young patient are often due to hypercoagulability, arteriopathies unrelated to atherosclerosis, illicit drug use and emboli emanating from structural cardiac abnormalities [7]. Stroke is usually the initial presentation in 50% of cases of atrial myxoma and in 75% of these cases, it occurs in the left atrium [8]. Mechanism of embolic stroke in atrial myxoma are due to fragments of the tumor or thrombus formation on the surface of the tumor, thus the diagnosis of embolic stroke in the young should warrant further work up utilizing neuroimaging and echocardiography even in the absence of remarkable auscultatory and ECG findings [9].
Management of CNC entails treatment of systemic manifestations and complications. This index patient presented with stroke and atrial myxoma warranting clot retrieval and eventual atrial myxoma resection, early resection of atrial myxoma carries only an operative mortality risk of 5% and non-treatment could lead to debilitating outcomes [10]. The hallmark of care of patients with this condition is ongoing surveillance to assess extent of disease and early resection of any suspecting lesion.
Prognosis of this condition is good; however the greatest risk of mortality in CNC is associated with cardiac diseases and its complications. Other major cause of mortality includes metastatic or intracranial psammomatous melanotic schwnnoma, carcinoma of metastatic tumor and non-cardiac postoperative complications [11].
LEARNING POINT/TAKE HOME MESSAGE
• CNC should be suspected in any patient presenting with any myxoid cutaneous manifestations
• Diagnosis of CNC is made when two major criteria are present and it is important that this diagnosis is made as it increases the surveillance rate of patients with such diagnosis
• Initial presentation of CNC could be a stroke in the young; hence stroke in young patient should warrant a diagnostic work up
• Ongoing surveillance is the hallmark of care after this diagnosis is made
• Early surgical resection remains the best measure to prevent embolization and further neurological sequelae
PATIENTS PERSPECTIVE
It is interesting how this disease process developed, who would have imagined that my skin condition diagnosed several years ago could have been a pointer to more severe illness in the future. I am grateful that this heart tumor was taken off and will continue to monitor myself for any new symptoms and present promptly to my doctors.
REFERENCES
- Correa R, Salpea P, Stratakis CA (2015) Carney complex: An update. Eur J Endocrinol 173: 85-97.
- Stratakis CA, Carney JA (2009) The triad of paragangliomas, gastric stromal tumors and pulmonary chondromas (Carney triad) and the dyad of paragangliomas and gastric stromal sarcomas (Carney-Stratakis syndrome): Molecular genetics and clinical implications. J Intern Med 266: 43-52.
- Bertherat J, Horvarth A, Groussin L, Grabar S, Boikos S, et al. (2009) Mutations in regulatory subunit type 1A of cyclic adenosisne 5’-monophosphate-dependent protein kinase (PRKAR1A): Phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab 94: 2085-2091.
- Stratakis CA, Kirschner LS, Carney JA (2001) Clinical and molecular features of the Carney complex: Diagnostic criteria and recommendations for patient evaluation. J Clin Endocrinol Metab 86: 4041-4046.
- Atherton DJ, Pitcher DW, Wells RS, MacDonald DM (1980) A syndrome of various cutaneous pigmented lesions, myxoid neurofibromata and atrial myxoma: The NAME syndrome. Br J Dermatol 103: 421-429.
- Cowen E, Hand J, Corona R (2017) Carney Complex. UptoDate, USA.
- 7. Warlow C (1996) The organization of stroke services. In: Warlow CO, Denis MS (eds.). Stroke: A practical guide to management. Blackwell Science, New Jersey, USA. Pg no: 664.
- Reynen K (1995) Cardiac myxomas. N Engl J Med 333: 1610-1617.
- Negi RC, Chauhan V, Sharma B, Bhardwaj R, Thakur S (2013) Atrial Myxoma: A Rare Cause of Ischemic Stroke. J Assoc Physicians India 61: 280-282.
- Novendstern SL, Silliman SL, Booth RP (2001) Cerebrovascular Complications of Atrial Myxoma. Hospital physician 39-42.
- Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, et al. (2000 Mutations of the gene encoding the protein kinase A 1-alpha regulatory subunit in patients with Carney complex. Nat Genet 26: 89-92.
Citation: Mbaebie N, Veeranna V (2018) “Carney Complex” an often Missed Diagnosis - Case of Cerebrovascular Accident in a Young Patient with Atrial Myxoma and Myxoid neurofibromas. J Clin Stud Med Case Rep 5: 053.
Copyright: © 2018 Nkechi Mbaebie, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

